

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22925 ((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of human erythrocytic adenosine deaminase. | J Med Chem 26: 1478-82 (1983) Checked by Author BindingDB Entry DOI: 10.7270/Q29Z95GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483336 (CHEMBL1651153 | GRL-0476) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM13925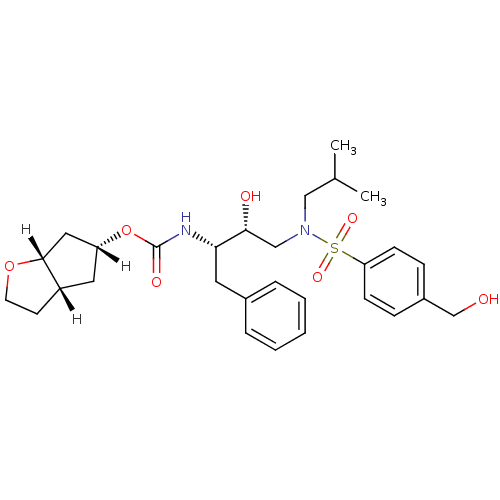 ((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00450 | -64.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 49: 5252-61 (2006) Article DOI: 10.1021/jm060561m BindingDB Entry DOI: 10.7270/Q23R0R41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483338 (CHEMBL1651155) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481584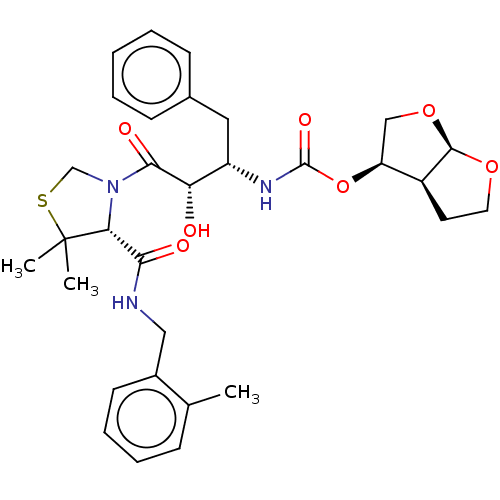 (CHEMBL589988 | GRL-0355) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem Lett 20: 1241-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.123 BindingDB Entry DOI: 10.7270/Q2CJ8HB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483334 (CHEMBL1651160) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM50367032 (COFORMYCIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of human erythrocytic adenosine deaminase | J Med Chem 26: 1478-82 (1983) Checked by Author BindingDB Entry DOI: 10.7270/Q29Z95GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0140 | -62.0 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 49: 5252-61 (2006) Article DOI: 10.1021/jm060561m BindingDB Entry DOI: 10.7270/Q23R0R41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9236 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0150 | -61.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 12: 1993-6 (2002) Article DOI: 10.1016/s0960-894x(02)00300-1 BindingDB Entry DOI: 10.7270/Q2SJ1HS5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem Lett 20: 1241-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.123 BindingDB Entry DOI: 10.7270/Q2CJ8HB8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483337 (CHEMBL1651154) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483335 (CHEMBL1651161) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483341 (CHEMBL1651159) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM13924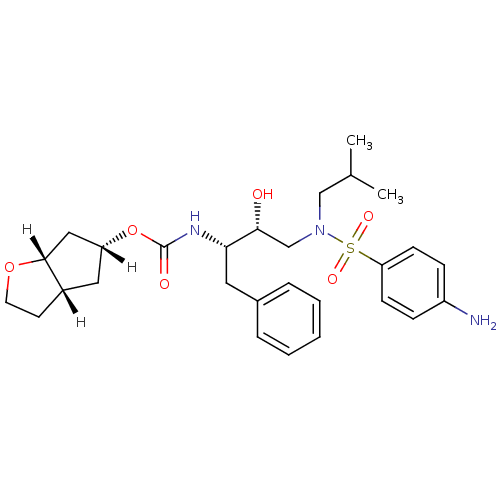 ((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | -56.2 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 49: 5252-61 (2006) Article DOI: 10.1021/jm060561m BindingDB Entry DOI: 10.7270/Q23R0R41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481586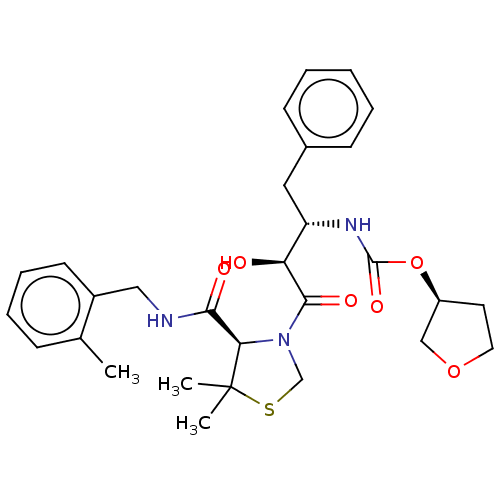 (CHEMBL604931) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem Lett 20: 1241-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.123 BindingDB Entry DOI: 10.7270/Q2CJ8HB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481579 (CHEMBL601049) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem Lett 20: 1241-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.123 BindingDB Entry DOI: 10.7270/Q2CJ8HB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481582 (CHEMBL601052) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem Lett 20: 1241-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.123 BindingDB Entry DOI: 10.7270/Q2CJ8HB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522184 (CHEMBL4444017) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481583 (CHEMBL601050) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem Lett 20: 1241-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.123 BindingDB Entry DOI: 10.7270/Q2CJ8HB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9264 ((2R)-2-hydroxy-2-[(10S)-16-hydroxy-12-oxo-3,4,5,6,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | J Med Chem 48: 3576-85 (2005) Article DOI: 10.1021/jm050019i BindingDB Entry DOI: 10.7270/Q2NS0S39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481587 (CHEMBL599785) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem Lett 20: 1241-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.123 BindingDB Entry DOI: 10.7270/Q2CJ8HB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9262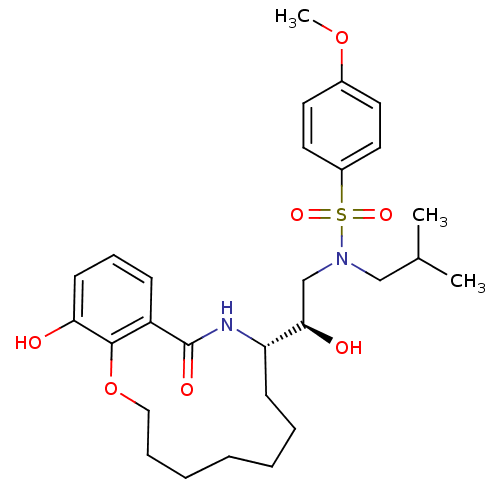 ((2R)-2-hydroxy-2-[(9S)-15-hydroxy-11-oxo-2,3,4,5,6...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | J Med Chem 48: 3576-85 (2005) Article DOI: 10.1021/jm050019i BindingDB Entry DOI: 10.7270/Q2NS0S39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481581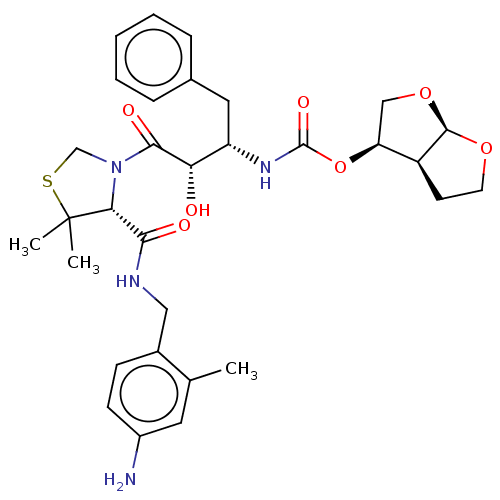 (CHEMBL601051) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem Lett 20: 1241-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.123 BindingDB Entry DOI: 10.7270/Q2CJ8HB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9270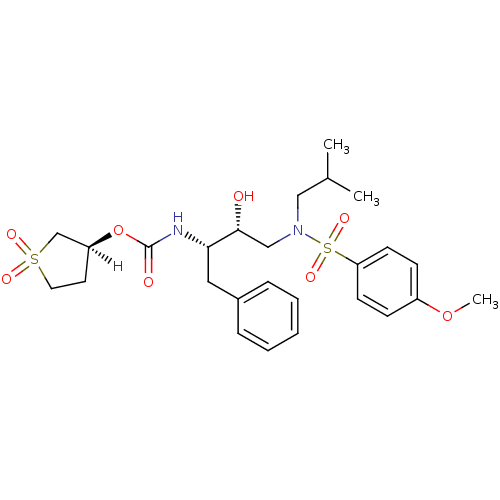 ((3S)-1,1-dioxo--thiolan-3-yl N-[(2S,3R)-3-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 8: 687-90 (1998) Article DOI: 10.1016/s0960-894x(98)00098-5 BindingDB Entry DOI: 10.7270/Q2J101DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9277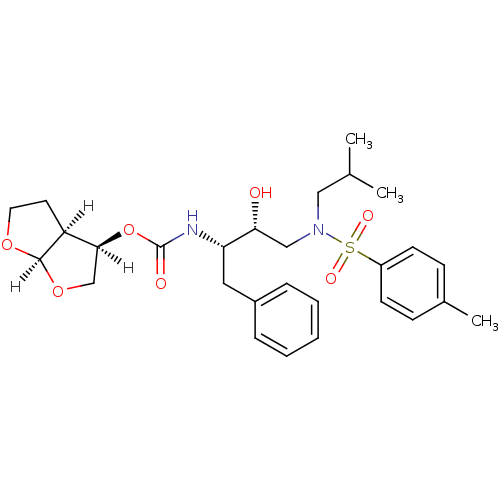 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 8: 687-90 (1998) Article DOI: 10.1016/s0960-894x(98)00098-5 BindingDB Entry DOI: 10.7270/Q2J101DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9271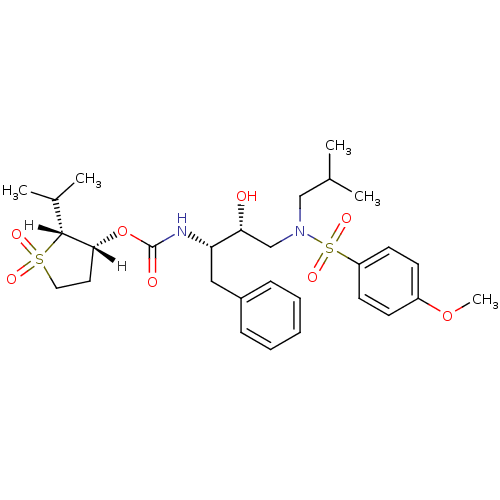 ((2R,3R)-1,1-dioxo-2-(propan-2-yl)--thiolan-3-yl N-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | -50.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 8: 687-90 (1998) Article DOI: 10.1016/s0960-894x(98)00098-5 BindingDB Entry DOI: 10.7270/Q2J101DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60607 Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against HIV protease | Bioorg Med Chem Lett 8: 979-82 (1999) BindingDB Entry DOI: 10.7270/Q2D79BXD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483342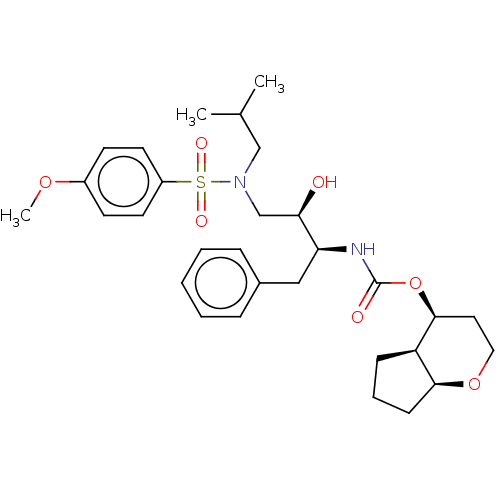 (CHEMBL1651156) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9273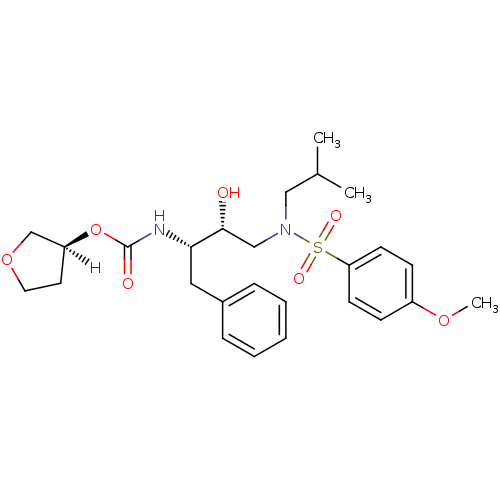 ((3S)-oxolan-3-yl N-[(2S,3R)-3-hydroxy-4-[(4-methox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | -50.4 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 8: 687-90 (1998) Article DOI: 10.1016/s0960-894x(98)00098-5 BindingDB Entry DOI: 10.7270/Q2J101DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9272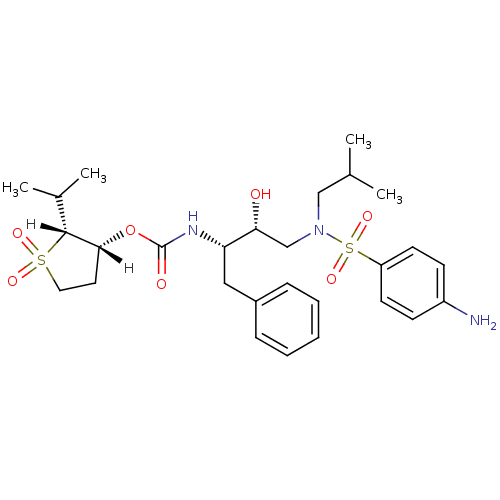 ((2R,3R)-1,1-dioxo-2-(propan-2-yl)--thiolan-3-yl N-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | -50.4 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 8: 687-90 (1998) Article DOI: 10.1016/s0960-894x(98)00098-5 BindingDB Entry DOI: 10.7270/Q2J101DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9266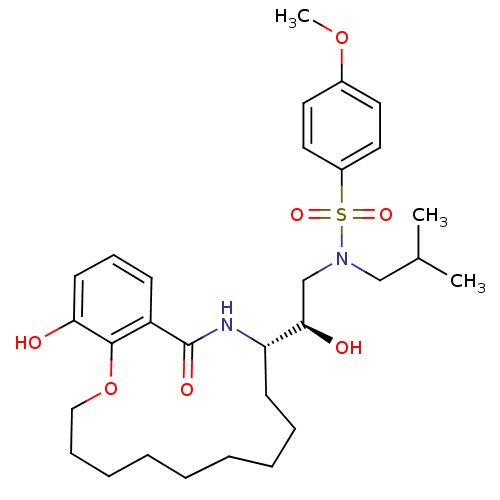 ((2R)-2-hydroxy-2-[(11S)-17-hydroxy-13-oxo-2,3,4,5,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | J Med Chem 48: 3576-85 (2005) Article DOI: 10.1021/jm050019i BindingDB Entry DOI: 10.7270/Q2NS0S39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481578 (CHEMBL589989) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem Lett 20: 1241-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.123 BindingDB Entry DOI: 10.7270/Q2CJ8HB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9263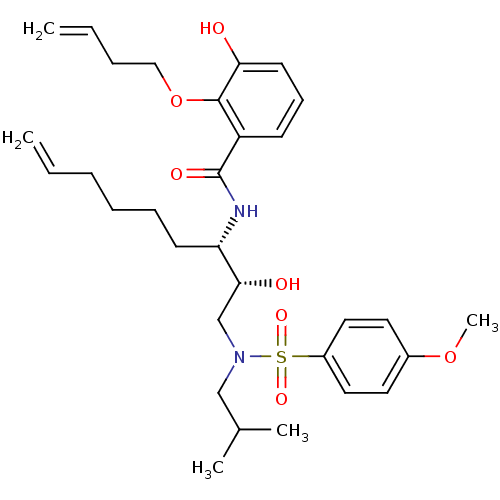 (2-(but-3-en-1-yloxy)-3-hydroxy-N-[(2R,3S)-2-hydrox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | J Med Chem 48: 3576-85 (2005) Article DOI: 10.1021/jm050019i BindingDB Entry DOI: 10.7270/Q2NS0S39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50417494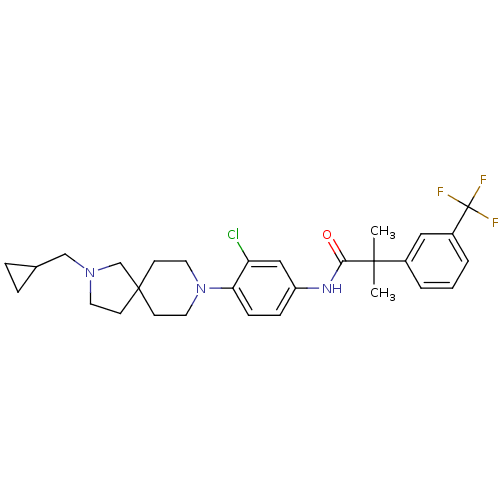 (CHEMBL1288285) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human NPY Y2 receptor in KAN-TS cells by [35]GTPgammaS assay | Bioorg Med Chem Lett 20: 7341-4 (2010) Article DOI: 10.1016/j.bmcl.2010.10.065 BindingDB Entry DOI: 10.7270/Q2BV7HWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM563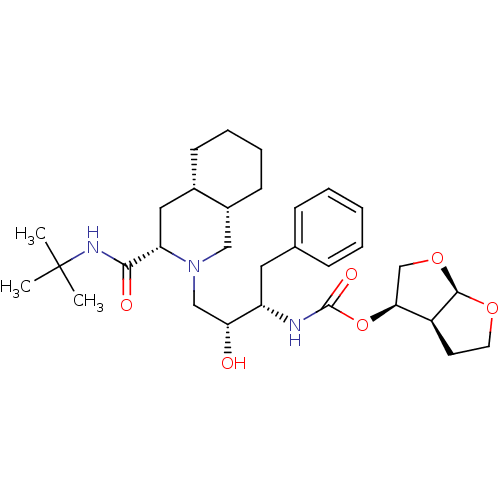 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl (1S,2R...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 12: 1993-6 (2002) Article DOI: 10.1016/s0960-894x(02)00300-1 BindingDB Entry DOI: 10.7270/Q2SJ1HS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9260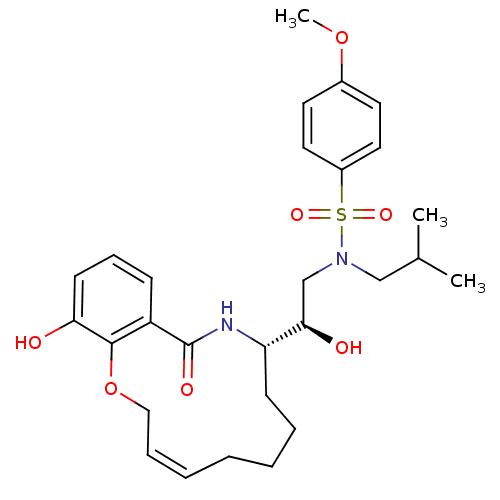 ((2R)-2-hydroxy-2-[(9S)-15-hydroxy-11-oxo-2,5,6,7,8...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | J Med Chem 48: 3576-85 (2005) Article DOI: 10.1021/jm050019i BindingDB Entry DOI: 10.7270/Q2NS0S39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9278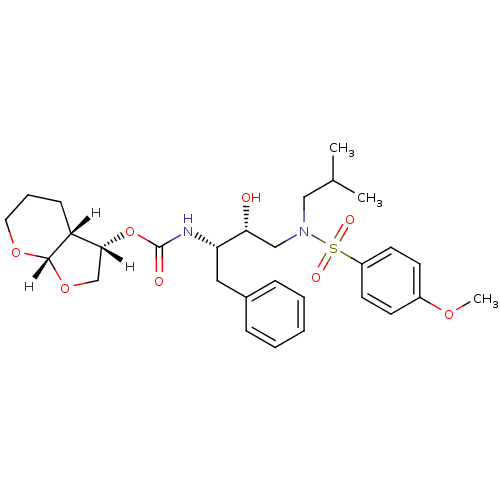 ((3S,3aR,7aS)-hexahydro-2H-furo[2,3-b]pyran-3-yl N-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | -49.4 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 8: 687-90 (1998) Article DOI: 10.1016/s0960-894x(98)00098-5 BindingDB Entry DOI: 10.7270/Q2J101DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9269 ((3S)-thiolan-3-yl N-[(2S,3R)-3-hydroxy-4-[(4-metho...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | -49.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 8: 687-90 (1998) Article DOI: 10.1016/s0960-894x(98)00098-5 BindingDB Entry DOI: 10.7270/Q2J101DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50414466 (CHEMBL550583) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human neuropeptide Y2 receptor in KAN-TS cells by [35]GTPgammaS assay | Bioorg Med Chem Lett 19: 4022-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.035 BindingDB Entry DOI: 10.7270/Q2B27VBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481580 (CHEMBL601048) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem Lett 20: 1241-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.123 BindingDB Entry DOI: 10.7270/Q2CJ8HB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9268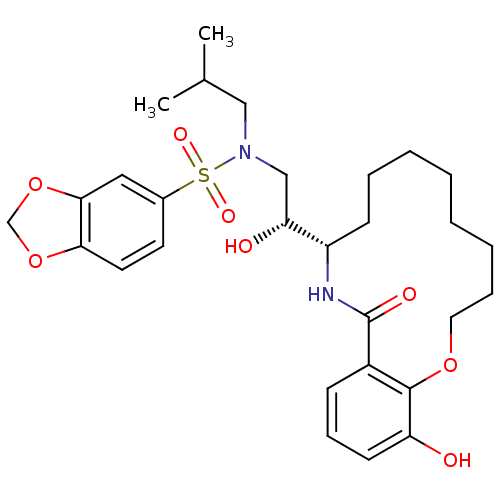 ((2R)-N-(2H-1,3-benzodioxol-5-yl)-2-hydroxy-2-[(10S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | J Med Chem 48: 3576-85 (2005) Article DOI: 10.1021/jm050019i BindingDB Entry DOI: 10.7270/Q2NS0S39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50414465 (CHEMBL550559) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human neuropeptide Y2 receptor in KAN-TS cells by [35]GTPgammaS assay | Bioorg Med Chem Lett 19: 4022-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.035 BindingDB Entry DOI: 10.7270/Q2B27VBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522181 (CHEMBL4456725) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9282 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.5 | -47.6 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 16: 1869-73 (2006) Article DOI: 10.1016/j.bmcl.2006.01.011 BindingDB Entry DOI: 10.7270/Q2D798N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9279 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.5 | -47.6 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 16: 1869-73 (2006) Article DOI: 10.1016/j.bmcl.2006.01.011 BindingDB Entry DOI: 10.7270/Q2D798N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9285 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 16: 1869-73 (2006) Article DOI: 10.1016/j.bmcl.2006.01.011 BindingDB Entry DOI: 10.7270/Q2D798N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50414470 (CHEMBL551311) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human neuropeptide Y2 receptor in KAN-TS cells by [35]GTPgammaS assay | Bioorg Med Chem Lett 19: 4022-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.035 BindingDB Entry DOI: 10.7270/Q2B27VBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483339 (CHEMBL1651158) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM13926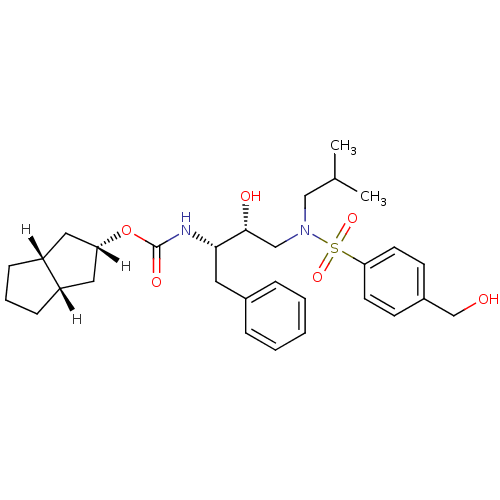 ((2R,3aR,6aS)-octahydropentalen-2-yl N-[(2S,3R)-3-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.30 | -47.2 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 49: 5252-61 (2006) Article DOI: 10.1021/jm060561m BindingDB Entry DOI: 10.7270/Q23R0R41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3690 total ) | Next | Last >> |