

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456223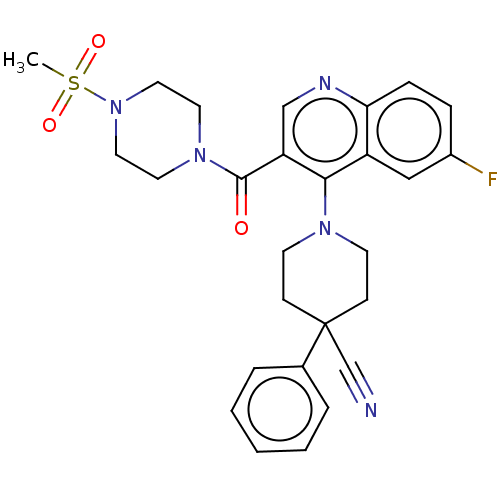 (CHEMBL4206892) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using propionaldehyde as substrate and varied concentration of NAD+ as cofactor preincubated for 15 mins followed by subs... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456222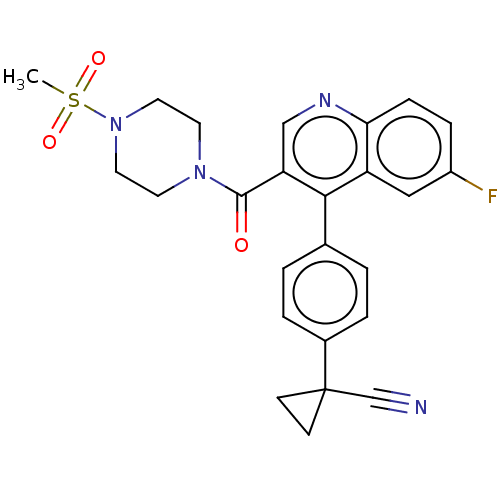 (CHEMBL4206272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using propionaldehyde as substrate and varied concentration of NAD+ as cofactor preincubated for 15 mins followed by subs... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456222 (CHEMBL4206272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+ as cofactor and varied concentration of propionaldehyde as substrate preincubated for 15 mins followed by subs... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456223 (CHEMBL4206892) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+ as cofactor and varied concentration of propionaldehyde as substrate preincubated for 15 mins followed by subs... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50257088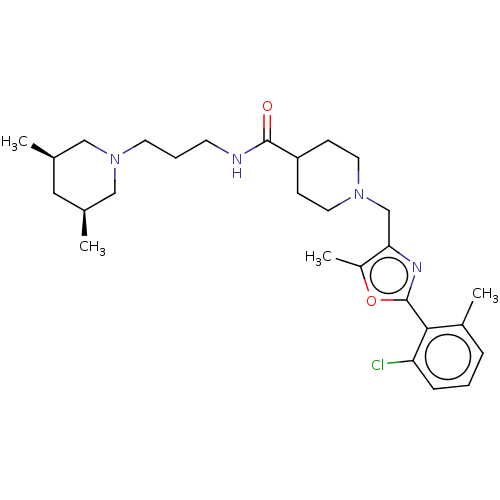 (CHEMBL4066784) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 415 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health , 10 Center Drive, Bethesda, Maryland 20892-1800, United States. Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2B receptor expressed in HEK cell membranes after 90 mins by scintillation counting method | J Med Chem 60: 6364-6383 (2017) Article DOI: 10.1021/acs.jmedchem.7b00561 BindingDB Entry DOI: 10.7270/Q2DR2XXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50257088 (CHEMBL4066784) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 443 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health , 10 Center Drive, Bethesda, Maryland 20892-1800, United States. Curated by ChEMBL | Assay Description Displacement of [3H]Cimetidine from human H2 receptor expressed in HEK cell membranes after 90 mins by scintillation counting method | J Med Chem 60: 6364-6383 (2017) Article DOI: 10.1021/acs.jmedchem.7b00561 BindingDB Entry DOI: 10.7270/Q2DR2XXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50257088 (CHEMBL4066784) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health , 10 Center Drive, Bethesda, Maryland 20892-1800, United States. Curated by ChEMBL | Assay Description Displacement of [3H]Rauwolscine from human alpha2C receptor expressed in MDCK cell membranes after 90 mins by scintillation counting method | J Med Chem 60: 6364-6383 (2017) Article DOI: 10.1021/acs.jmedchem.7b00561 BindingDB Entry DOI: 10.7270/Q2DR2XXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50257088 (CHEMBL4066784) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health , 10 Center Drive, Bethesda, Maryland 20892-1800, United States. Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from KOR receptor (unknown origin) expressed in HEK cell membranes after 90 mins by scintillation counting method | J Med Chem 60: 6364-6383 (2017) Article DOI: 10.1021/acs.jmedchem.7b00561 BindingDB Entry DOI: 10.7270/Q2DR2XXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50257088 (CHEMBL4066784) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health , 10 Center Drive, Bethesda, Maryland 20892-1800, United States. Curated by ChEMBL | Assay Description Displacement of [3H]Nisoxetine from human NET receptor expressed in HEK cell membranes after 90 mins by scintillation counting method | J Med Chem 60: 6364-6383 (2017) Article DOI: 10.1021/acs.jmedchem.7b00561 BindingDB Entry DOI: 10.7270/Q2DR2XXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50257088 (CHEMBL4066784) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health , 10 Center Drive, Bethesda, Maryland 20892-1800, United States. Curated by ChEMBL | Assay Description Displacement of [3H]Rauwolscine from human alpha2A receptor expressed in MDCK cell membranes after 90 mins by scintillation counting method | J Med Chem 60: 6364-6383 (2017) Article DOI: 10.1021/acs.jmedchem.7b00561 BindingDB Entry DOI: 10.7270/Q2DR2XXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50257088 (CHEMBL4066784) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health , 10 Center Drive, Bethesda, Maryland 20892-1800, United States. Curated by ChEMBL | Assay Description Displacement of [3H]QNB from human M5 receptor expressed in CHO cell membranes after 90 mins by scintillation counting method | J Med Chem 60: 6364-6383 (2017) Article DOI: 10.1021/acs.jmedchem.7b00561 BindingDB Entry DOI: 10.7270/Q2DR2XXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50257088 (CHEMBL4066784) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health , 10 Center Drive, Bethesda, Maryland 20892-1800, United States. Curated by ChEMBL | Assay Description Displacement of [3H]Pentazocine from guinea pig Sigma1 receptor after 90 mins by scintillation counting method | J Med Chem 60: 6364-6383 (2017) Article DOI: 10.1021/acs.jmedchem.7b00561 BindingDB Entry DOI: 10.7270/Q2DR2XXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50257088 (CHEMBL4066784) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health , 10 Center Drive, Bethesda, Maryland 20892-1800, United States. Curated by ChEMBL | Assay Description Displacement of [3H]Citalopram from human SERT receptor expressed in HEK cell membranes after 90 mins by scintillation counting method | J Med Chem 60: 6364-6383 (2017) Article DOI: 10.1021/acs.jmedchem.7b00561 BindingDB Entry DOI: 10.7270/Q2DR2XXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50527623 (CHEMBL4443378) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50527619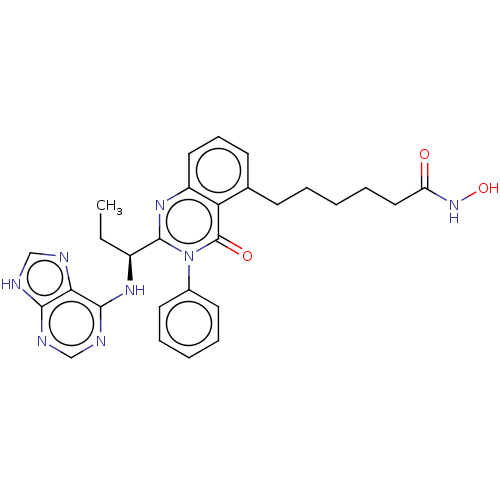 (CHEMBL4451623) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50527624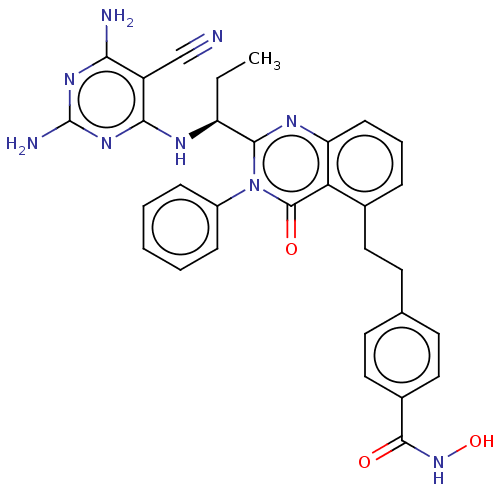 (CHEMBL4450613) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50527616 (CHEMBL4464003) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50527622 (CHEMBL4550648) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50527617 (CHEMBL4522666) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50527620 (CHEMBL4563838) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50527623 (CHEMBL4443378) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kgamma assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50527605 (CHEMBL4526673) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kgamma assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50527624 (CHEMBL4450613) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kgamma assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50527610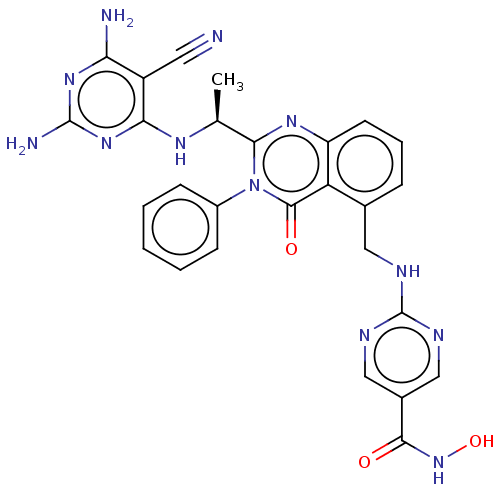 (CHEMBL4583074) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged HDAC6 (1 to 1125 residues) expressed in baculovirus infected insect cells using fluorogenic pep... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged HDAC6 (1 to 1125 residues) expressed in baculovirus infected insect cells using fluorogenic pep... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50527634 (CHEMBL4456827) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged HDAC6 (1 to 1125 residues) expressed in baculovirus infected insect cells using fluorogenic pep... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50527610 (CHEMBL4583074) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM25045 (3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50527622 (CHEMBL4550648) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kgamma assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50527629 (CHEMBL4459679) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged HDAC6 (1 to 1125 residues) expressed in baculovirus infected insect cells using fluorogenic pep... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50527625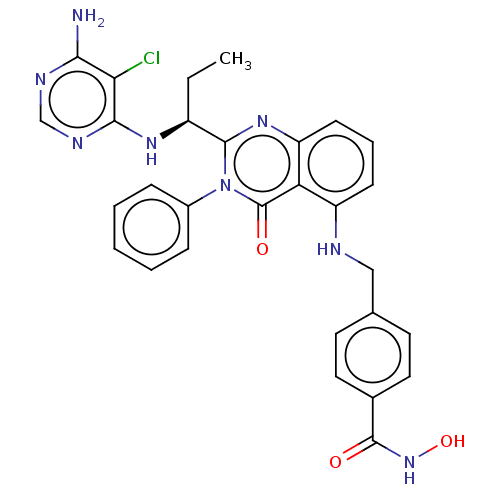 (CHEMBL4467927) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged HDAC6 (1 to 1125 residues) expressed in baculovirus infected insect cells using fluorogenic pep... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM25045 (3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kalpha assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50527633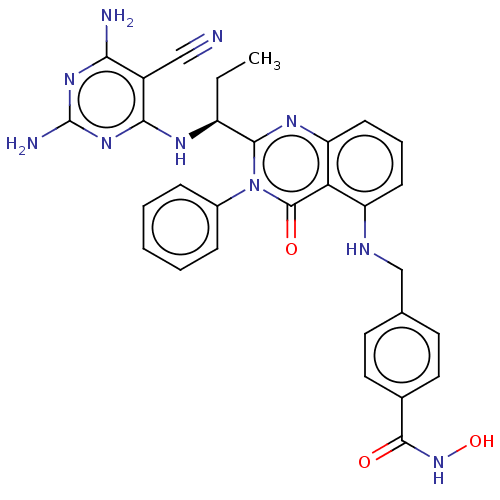 (CHEMBL4464812) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged HDAC6 (1 to 1125 residues) expressed in baculovirus infected insect cells using fluorogenic pep... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50527613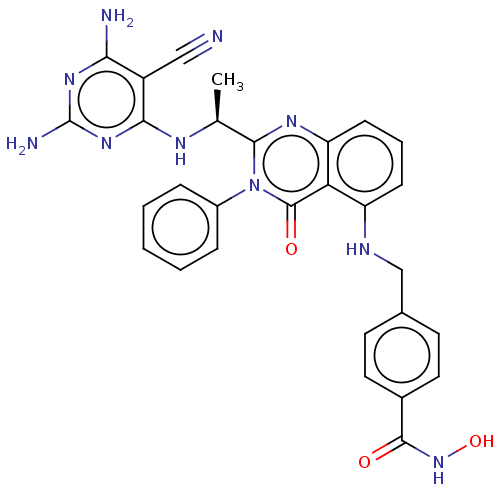 (CHEMBL4460447) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50527605 (CHEMBL4526673) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50527613 (CHEMBL4460447) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kgamma assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50527604 (CHEMBL4578757) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged HDAC6 (1 to 1125 residues) expressed in baculovirus infected insect cells using fluorogenic pep... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456309 (CHEMBL4207222) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50527627 (CHEMBL4516095) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged HDAC6 (1 to 1125 residues) expressed in baculovirus infected insect cells using fluorogenic pep... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50527628 (CHEMBL4555432) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50527608 (CHEMBL4445342) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50527611 (CHEMBL4544187) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50527619 (CHEMBL4451623) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged HDAC6 (1 to 1125 residues) expressed in baculovirus infected insect cells using fluorogenic pep... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456228 (CHEMBL4215704) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456249 (CHEMBL4212671) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456224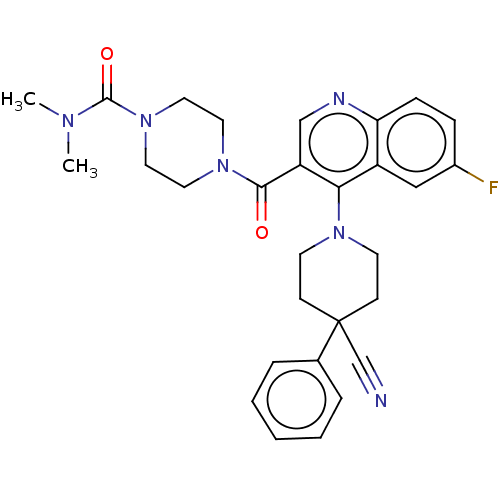 (CHEMBL4207514) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50527613 (CHEMBL4460447) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged HDAC6 (1 to 1125 residues) expressed in baculovirus infected insect cells using fluorogenic pep... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456233 (CHEMBL4211904) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50527602 (CHEMBL4581057) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged HDAC6 (1 to 1125 residues) expressed in baculovirus infected insect cells using fluorogenic pep... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 BindingDB Entry DOI: 10.7270/Q2KS6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456223 (CHEMBL4206892) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALDH1A1 in human SKOV3TR cells assessed as potentiation of paclitaxel-mediated cytotoxicity by measuring paclitaxel IC50 at 30 uM after... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 603 total ) | Next | Last >> |