

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4552 (4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | Bioorg Med Chem Lett 12: 2011-4 (2002) Article DOI: 10.1016/s0960-894x(02)00302-5 BindingDB Entry DOI: 10.7270/Q2PC30KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM6113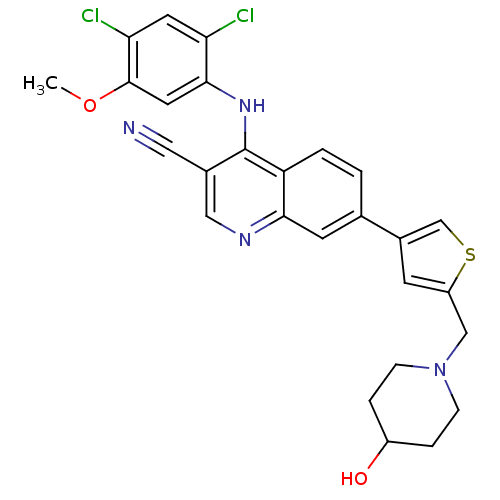 (4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-{5-[(4-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | Bioorg Med Chem Lett 12: 2011-4 (2002) Article DOI: 10.1016/s0960-894x(02)00302-5 BindingDB Entry DOI: 10.7270/Q2PC30KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM6115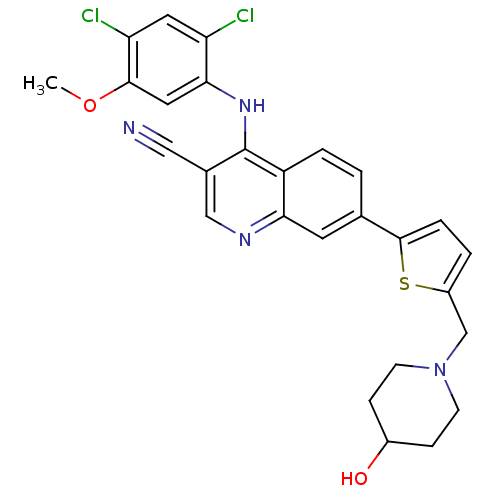 (4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-{5-[(4-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | Bioorg Med Chem Lett 12: 2011-4 (2002) Article DOI: 10.1016/s0960-894x(02)00302-5 BindingDB Entry DOI: 10.7270/Q2PC30KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM6108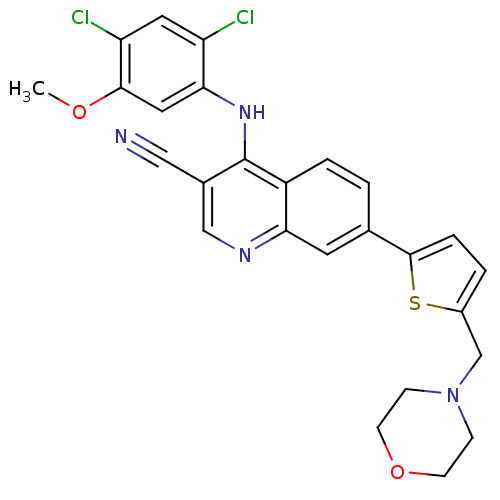 (4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-[5-(morp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | Bioorg Med Chem Lett 12: 2011-4 (2002) Article DOI: 10.1016/s0960-894x(02)00302-5 BindingDB Entry DOI: 10.7270/Q2PC30KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM6107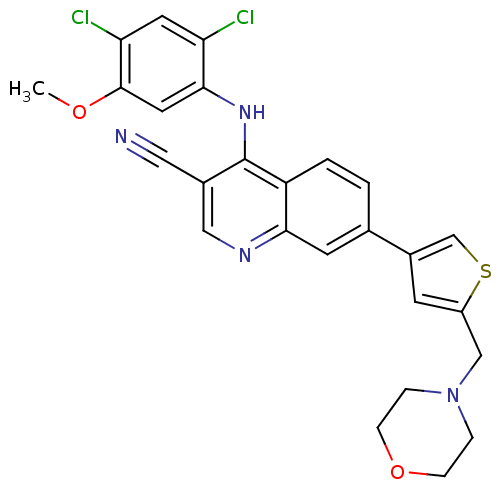 (4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-[5-(morp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | Bioorg Med Chem Lett 12: 2011-4 (2002) Article DOI: 10.1016/s0960-894x(02)00302-5 BindingDB Entry DOI: 10.7270/Q2PC30KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM6112 (3-quinolinecarbonitrile analog 2a | 4-[(2,4-dichlo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | Bioorg Med Chem Lett 12: 2011-4 (2002) Article DOI: 10.1016/s0960-894x(02)00302-5 BindingDB Entry DOI: 10.7270/Q2PC30KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM6114 (3-quinolinecarbonitrile analog 2b | 4-[(2,4-dichlo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | Bioorg Med Chem Lett 12: 2011-4 (2002) Article DOI: 10.1016/s0960-894x(02)00302-5 BindingDB Entry DOI: 10.7270/Q2PC30KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM6116 (4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-[5-(pipe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | Bioorg Med Chem Lett 12: 2011-4 (2002) Article DOI: 10.1016/s0960-894x(02)00302-5 BindingDB Entry DOI: 10.7270/Q2PC30KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM6117 (4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-[5-(thio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | Bioorg Med Chem Lett 12: 2011-4 (2002) Article DOI: 10.1016/s0960-894x(02)00302-5 BindingDB Entry DOI: 10.7270/Q2PC30KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM6109 (4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-[4-(morp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | Bioorg Med Chem Lett 12: 2011-4 (2002) Article DOI: 10.1016/s0960-894x(02)00302-5 BindingDB Entry DOI: 10.7270/Q2PC30KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Rattus norvegicus) | BDBM50254432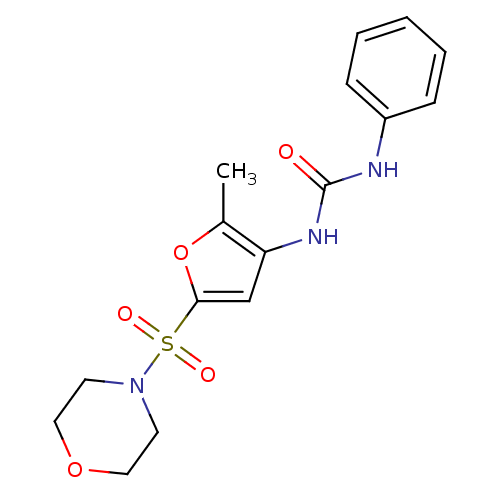 (1-(2-methyl-5-(morpholinosulfonyl)furan-3-yl)-3-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Lipoprotein lipase from adipose tissue of rat | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Rattus norvegicus) | BDBM50254434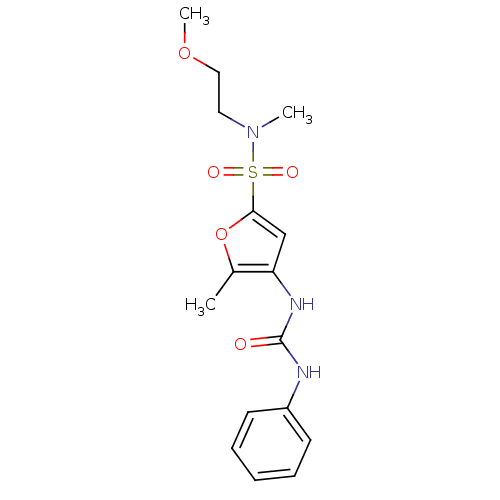 (5-Methyl-4-(3-phenyl-ureido)-furan-2-sulfonic acid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Lipoprotein lipase from adipose tissue of rat | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271950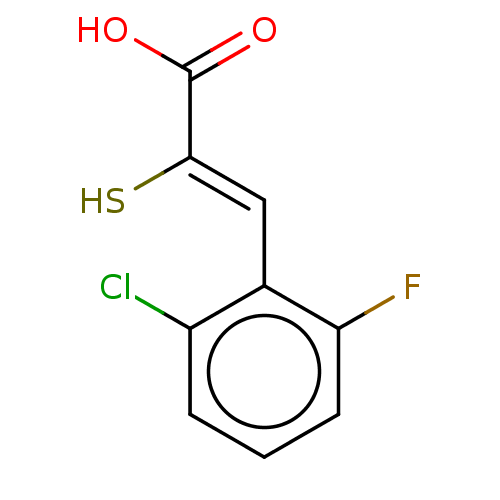 (CHEMBL4127821) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50254343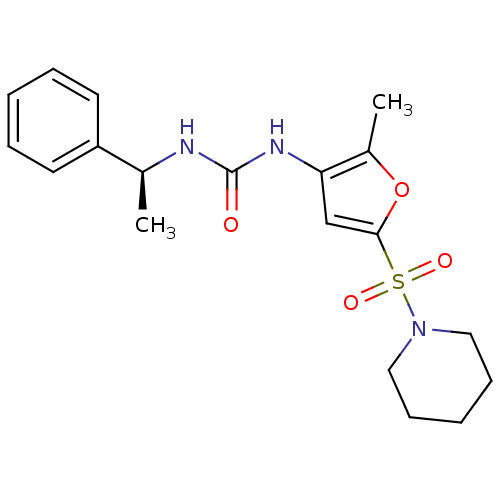 ((S)-1-(2-methyl-5-(piperidin-1-ylsulfonyl)furan-3-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of endothelial lipase (unknown origin) | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271847 (CHEMBL4127736) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271833 (CHEMBL3792857) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lipoprotein lipase (Rattus norvegicus) | BDBM50254290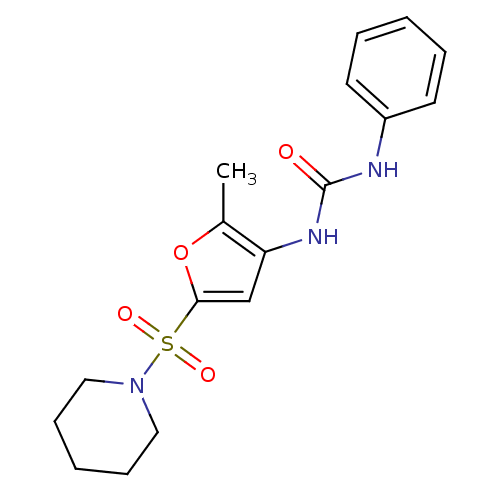 (1-(2-methyl-5-(piperidin-1-ylsulfonyl)furan-3-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Lipoprotein lipase from adipose tissue of rat | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271834 (CHEMBL3234727) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271878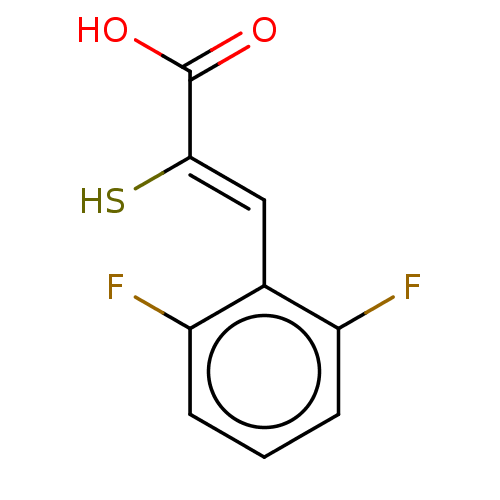 (CHEMBL4125829) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Rattus norvegicus) | BDBM50254436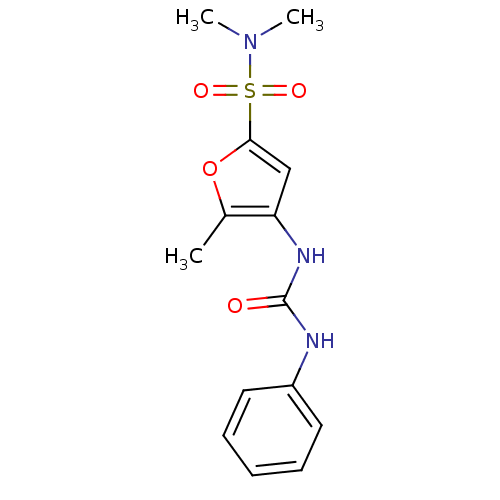 (5-Methyl-4-(3-phenyl-ureido)-furan-2-sulfonic acid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Lipoprotein lipase from adipose tissue of rat | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Rattus norvegicus) | BDBM50254339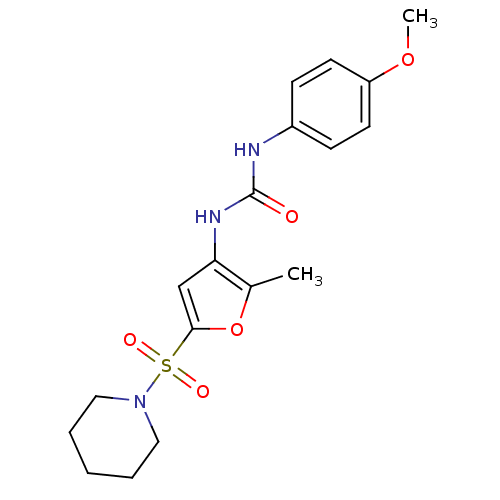 (1-(4-methoxyphenyl)-3-(2-methyl-5-(piperidin-1-yls...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Lipoprotein lipase from adipose tissue of rat | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50254432 (1-(2-methyl-5-(morpholinosulfonyl)furan-3-yl)-3-ph...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of endothelial lipase (unknown origin) | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Rattus norvegicus) | BDBM50254340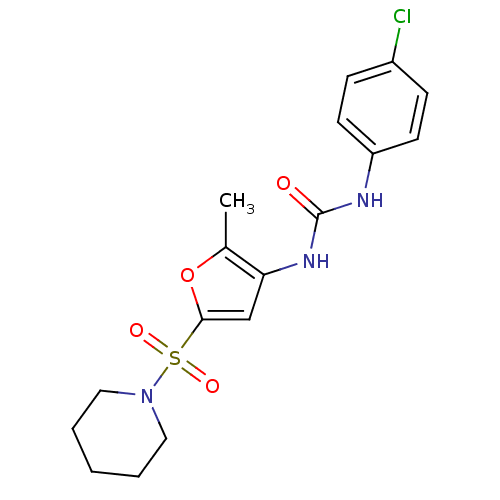 (1-(4-chlorophenyl)-3-(2-methyl-5-(piperidin-1-ylsu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Lipoprotein lipase from adipose tissue of rat | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50254339 (1-(4-methoxyphenyl)-3-(2-methyl-5-(piperidin-1-yls...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of endothelial lipase (unknown origin) | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50254341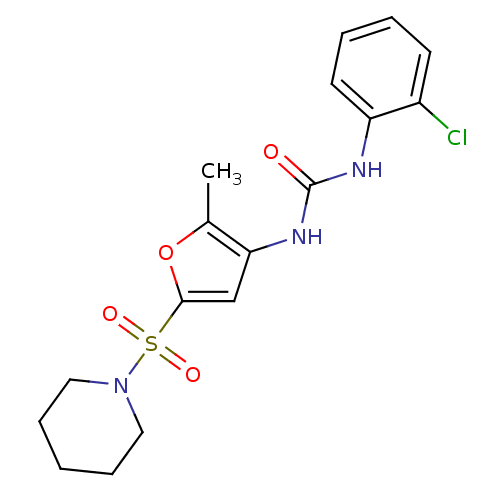 (1-(2-chlorophenyl)-3-(2-methyl-5-(piperidin-1-ylsu...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of endothelial lipase (unknown origin) | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50254290 (1-(2-methyl-5-(piperidin-1-ylsulfonyl)furan-3-yl)-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of endothelial lipase (unknown origin) | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50254393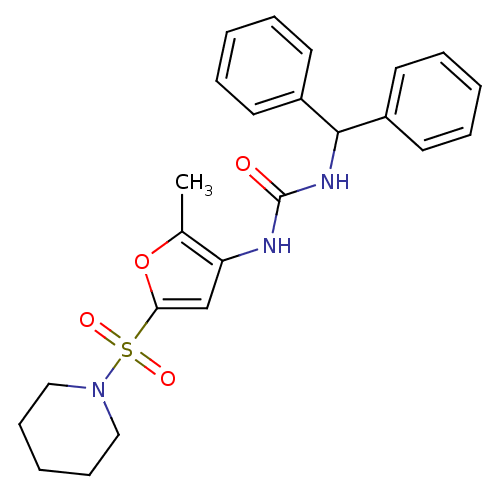 (1-benzhydryl-3-(2-methyl-5-(piperidin-1-ylsulfonyl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of endothelial lipase (unknown origin) | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50254340 (1-(4-chlorophenyl)-3-(2-methyl-5-(piperidin-1-ylsu...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of endothelial lipase (unknown origin) | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271893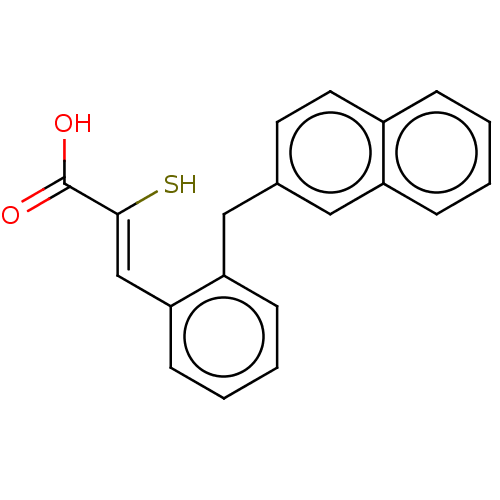 (CHEMBL4128221) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271889 (CHEMBL4129411) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271963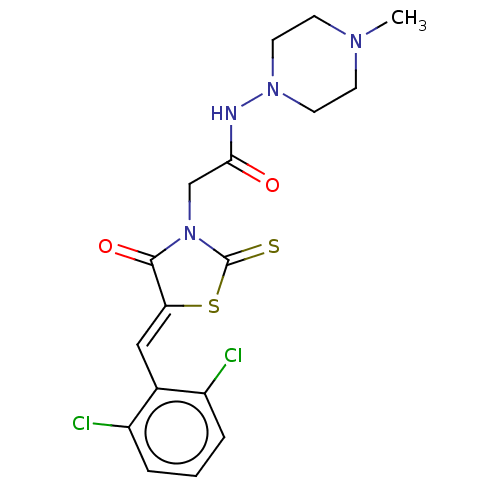 (CHEMBL1559342) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Aeromonas hydrophila) | BDBM50271833 (CHEMBL3792857) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Aeromonas hydrophila CphA using fluorogenic cephalosporin as substrate | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM6111 (4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-[4-(morp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | Bioorg Med Chem Lett 12: 2011-4 (2002) Article DOI: 10.1016/s0960-894x(02)00302-5 BindingDB Entry DOI: 10.7270/Q2PC30KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50254434 (5-Methyl-4-(3-phenyl-ureido)-furan-2-sulfonic acid...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of endothelial lipase (unknown origin) | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM6118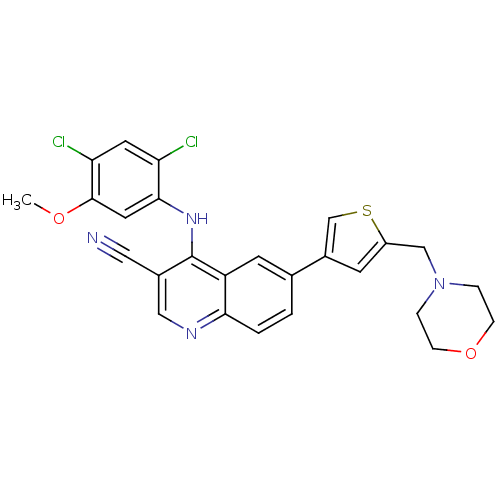 (4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-[5-(morp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | Bioorg Med Chem Lett 12: 2011-4 (2002) Article DOI: 10.1016/s0960-894x(02)00302-5 BindingDB Entry DOI: 10.7270/Q2PC30KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50254436 (5-Methyl-4-(3-phenyl-ureido)-furan-2-sulfonic acid...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of endothelial lipase (unknown origin) | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271952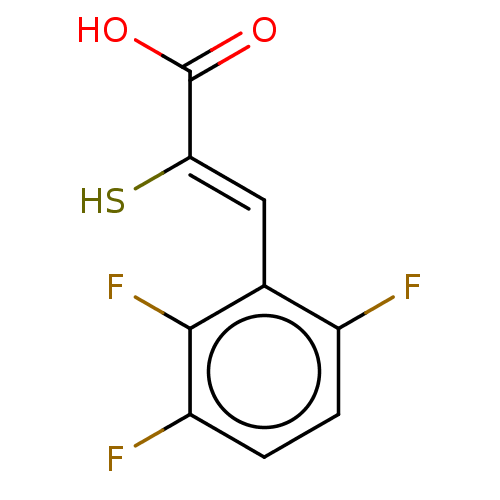 (CHEMBL4126465) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50247639 (CHEMBL4068716) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Rattus norvegicus) | BDBM50254341 (1-(2-chlorophenyl)-3-(2-methyl-5-(piperidin-1-ylsu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Lipoprotein lipase from adipose tissue of rat | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Rattus norvegicus) | BDBM50254343 ((S)-1-(2-methyl-5-(piperidin-1-ylsulfonyl)furan-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Lipoprotein lipase from adipose tissue of rat | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Rattus norvegicus) | BDBM50254435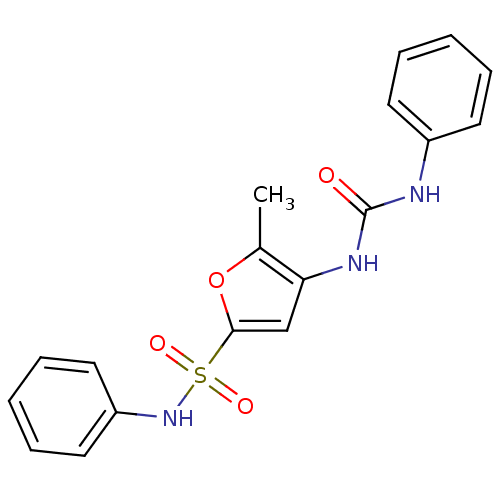 (5-Methyl-4-(3-phenyl-ureido)-furan-2-sulfonic acid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Lipoprotein lipase from adipose tissue of rat | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM6110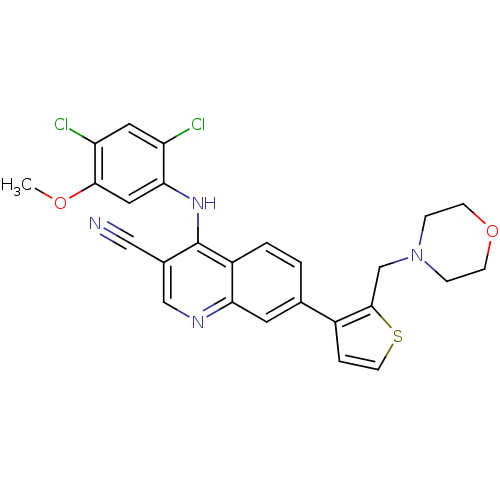 (4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-[2-(morp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | Bioorg Med Chem Lett 12: 2011-4 (2002) Article DOI: 10.1016/s0960-894x(02)00302-5 BindingDB Entry DOI: 10.7270/Q2PC30KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Rattus norvegicus) | BDBM50254433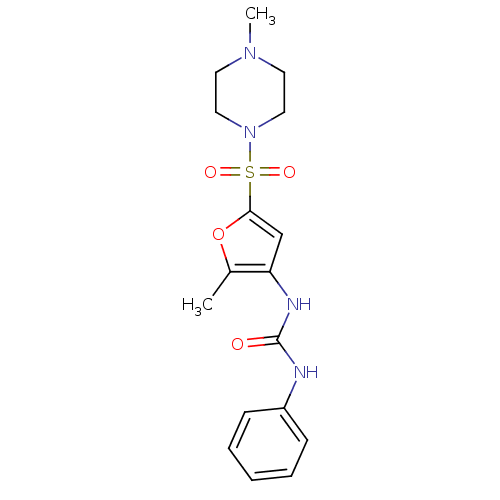 (1-(2-methyl-5-(4-methylpiperazin-1-ylsulfonyl)fura...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Lipoprotein lipase from adipose tissue of rat | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271891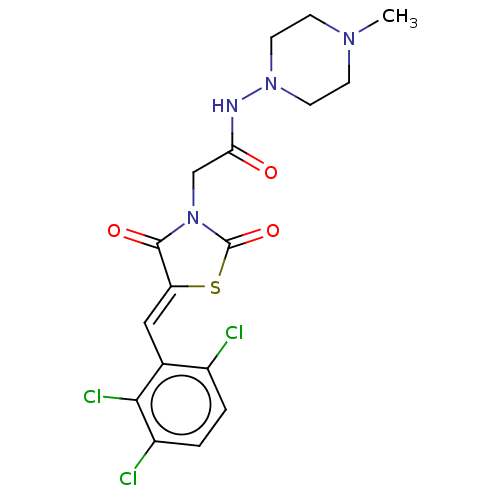 (CHEMBL4129450) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271824 (CHEMBL4129233) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271835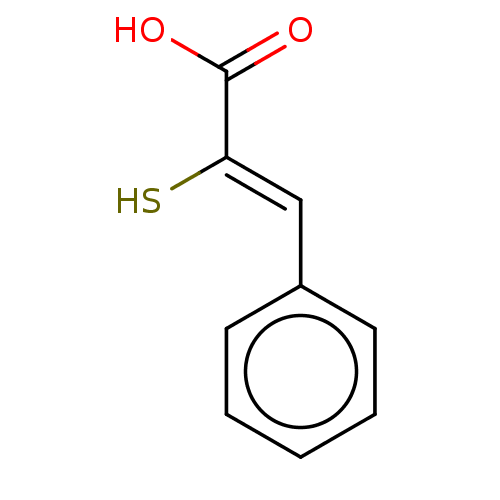 (CHEMBL4095898) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271798 (CHEMBL3221923) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271868 (CHEMBL4125695) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50254435 (5-Methyl-4-(3-phenyl-ureido)-furan-2-sulfonic acid...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of endothelial lipase (unknown origin) | Bioorg Med Chem Lett 19: 27-30 (2008) Article DOI: 10.1016/j.bmcl.2008.11.033 BindingDB Entry DOI: 10.7270/Q2BP02NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271867 (CHEMBL4128976) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 154 total ) | Next | Last >> |