

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50491169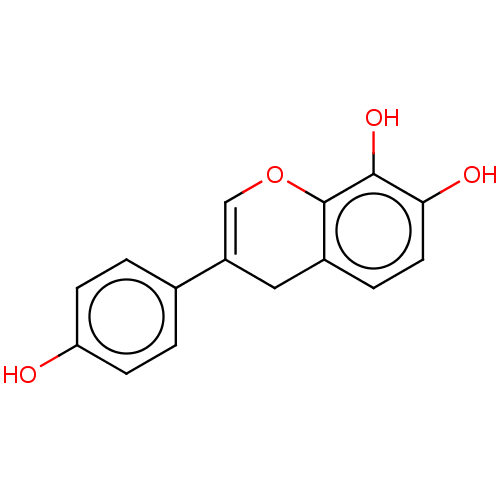 (CHEMBL2377164) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 641 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Competitive inhibition of Helicobacter pylori ATCC 43504 urease using urea as substrate by Lineweaver-Burk/Dixon plot analysis | Eur J Med Chem 63: 685-95 (2013) Article DOI: 10.1016/j.ejmech.2013.03.016 BindingDB Entry DOI: 10.7270/Q2D79FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50233808 (CHEMBL4086701 | US11021454, Compound 6e) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type competitive inhibition of Xanthine oxidase (unknown origin) assessed as enzyme-inhibitor complex preincubated for 3 mins followed by xanth... | Eur J Med Chem 124: 637-648 (2016) Article DOI: 10.1016/j.ejmech.2016.08.019 BindingDB Entry DOI: 10.7270/Q2SJ1NVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50468297 (CHEMBL4282897) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of bovine xanthine oxidase using xanthine as substrate preincubated for 3 mins followed by substrate addition by Lineweaver-Bu... | Eur J Med Chem 151: 849-860 (2018) Article DOI: 10.1016/j.ejmech.2018.01.096 BindingDB Entry DOI: 10.7270/Q2VT1VT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50468297 (CHEMBL4282897) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Uncompetitive inhibition of bovine xanthine oxidase using xanthine as substrate preincubated for 3 mins followed by substrate addition by Lineweaver-... | Eur J Med Chem 151: 849-860 (2018) Article DOI: 10.1016/j.ejmech.2018.01.096 BindingDB Entry DOI: 10.7270/Q2VT1VT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0610 | n/a | n/a | n/a | n/a | n/a | 25 |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli incubated for 20 to 30 mins at room temperature using (Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-I... | Bioorg Med Chem Lett 25: 1880-3 (2015) Article DOI: 10.1016/j.bmcl.2015.03.047 BindingDB Entry DOI: 10.7270/Q2FT8NQ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50001450 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of tachykinin 1 (NK1) receptor in rat brain synaptosomal membranes using [125I]-BH-SP as radioligand | Bioorg Med Chem Lett 3: 447-450 (1993) Article DOI: 10.1016/S0960-894X(01)80229-8 BindingDB Entry DOI: 10.7270/Q2R2119Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincub... | Bioorg Med Chem Lett 29: 357-361 (2019) Article DOI: 10.1016/j.bmcl.2018.12.040 BindingDB Entry DOI: 10.7270/Q2GX4FZQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50067593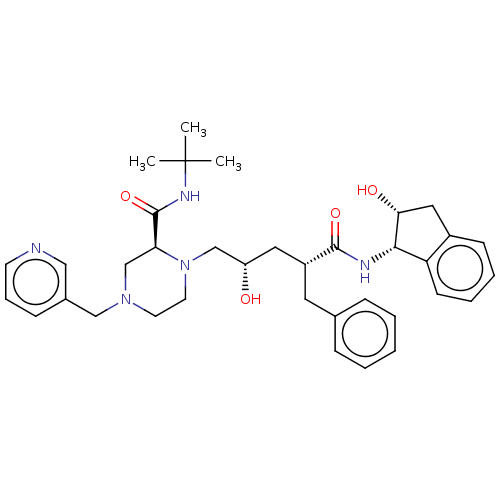 (CHEBI:44032 | Crixivan | Indinavir | L-735524 | MK...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | 25 |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli incubated for 20 to 30 mins at room temperature using (Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-I... | Bioorg Med Chem Lett 25: 1880-3 (2015) Article DOI: 10.1016/j.bmcl.2015.03.047 BindingDB Entry DOI: 10.7270/Q2FT8NQ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50449782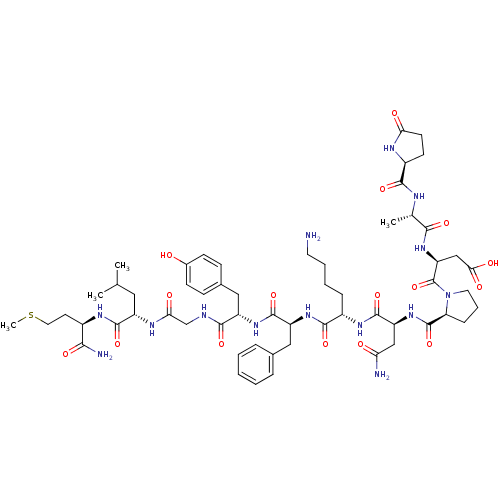 (CHEMBL2370874) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of tachykinin 1 (NK1) receptor in rat brain synaptosomal membranes using [125I]-BH-SP as radioligand | Bioorg Med Chem Lett 3: 447-450 (1993) Article DOI: 10.1016/S0960-894X(01)80229-8 BindingDB Entry DOI: 10.7270/Q2R2119Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrat... | Bioorg Med Chem Lett 29: 1541-1545 (2019) Article DOI: 10.1016/j.bmcl.2019.03.049 BindingDB Entry DOI: 10.7270/Q20P13BM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50067658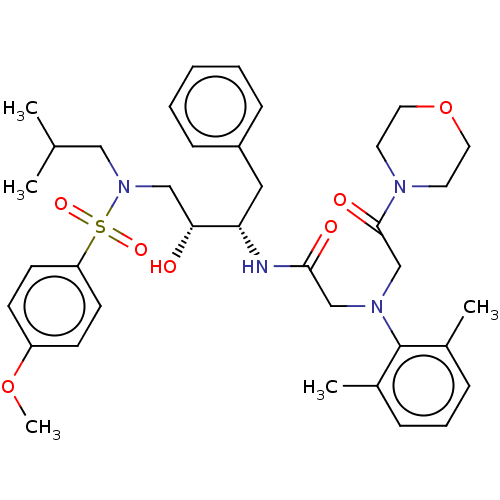 (CHEMBL3400155) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | 25 |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli incubated for 20 to 30 mins at room temperature using (Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-I... | Bioorg Med Chem Lett 25: 1880-3 (2015) Article DOI: 10.1016/j.bmcl.2015.03.047 BindingDB Entry DOI: 10.7270/Q2FT8NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50449783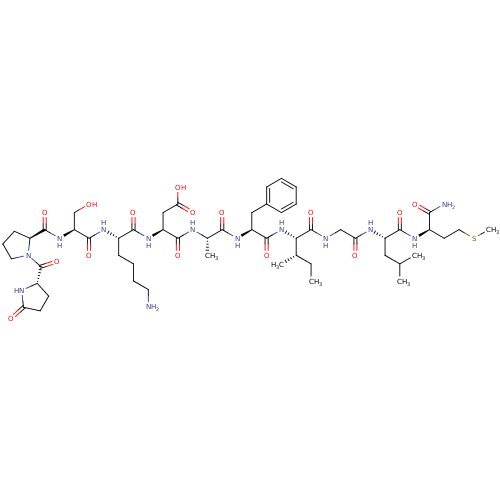 (CHEMBL2370873) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of tachykinin 1 (NK1) receptor in rat brain synaptosomal membranes using [125I]-BH-SP as radioligand | Bioorg Med Chem Lett 3: 447-450 (1993) Article DOI: 10.1016/S0960-894X(01)80229-8 BindingDB Entry DOI: 10.7270/Q2R2119Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50001447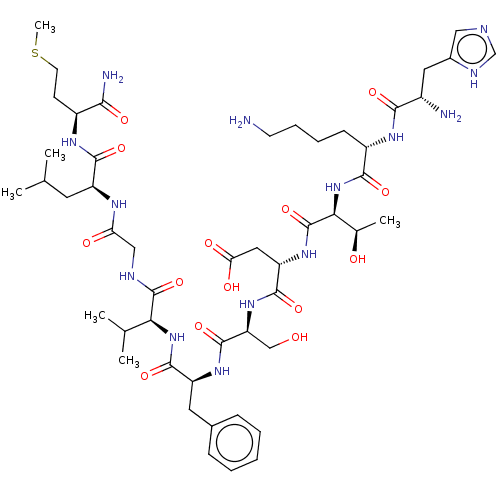 (CHEMBL217406 | His-Lys-Thr-Asp-Ser-Phe-Val-Gly-Leu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of tachykinin 1 (NK1) receptor in rat brain synaptosomal membranes using [125I]-BH-SP as radioligand | Bioorg Med Chem Lett 3: 447-450 (1993) Article DOI: 10.1016/S0960-894X(01)80229-8 BindingDB Entry DOI: 10.7270/Q2R2119Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50510472 (CHEMBL4550588) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrat... | Bioorg Med Chem Lett 29: 1541-1545 (2019) Article DOI: 10.1016/j.bmcl.2019.03.049 BindingDB Entry DOI: 10.7270/Q20P13BM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50510461 (CHEMBL4590327) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrat... | Bioorg Med Chem Lett 29: 1541-1545 (2019) Article DOI: 10.1016/j.bmcl.2019.03.049 BindingDB Entry DOI: 10.7270/Q20P13BM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50541389 (CHEMBL4648367) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Jinzhou Medical University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127019 BindingDB Entry DOI: 10.7270/Q2M61PS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50541372 (CHEMBL4644061) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Jinzhou Medical University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127019 BindingDB Entry DOI: 10.7270/Q2M61PS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50541375 (CHEMBL4641584) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Jinzhou Medical University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127019 BindingDB Entry DOI: 10.7270/Q2M61PS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50067616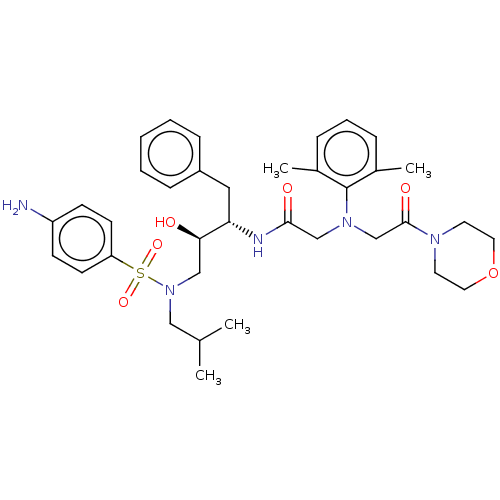 (CHEMBL3400718) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | 25 |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli incubated for 20 to 30 mins at room temperature using (Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-I... | Bioorg Med Chem Lett 25: 1880-3 (2015) Article DOI: 10.1016/j.bmcl.2015.03.047 BindingDB Entry DOI: 10.7270/Q2FT8NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50510462 (CHEMBL4574760) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrat... | Bioorg Med Chem Lett 29: 1541-1545 (2019) Article DOI: 10.1016/j.bmcl.2019.03.049 BindingDB Entry DOI: 10.7270/Q20P13BM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50067650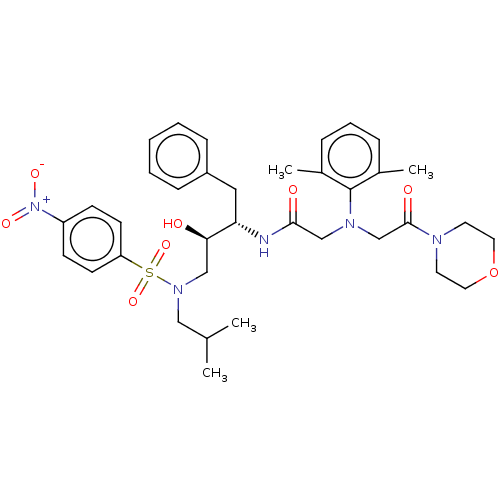 (CHEMBL3400712) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | 25 |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli incubated for 20 to 30 mins at room temperature using (Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-I... | Bioorg Med Chem Lett 25: 1880-3 (2015) Article DOI: 10.1016/j.bmcl.2015.03.047 BindingDB Entry DOI: 10.7270/Q2FT8NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50067598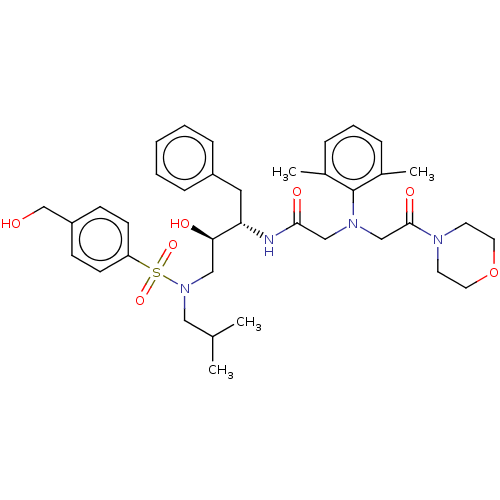 (CHEMBL3400724) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | 25 |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli incubated for 20 to 30 mins at room temperature using (Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-I... | Bioorg Med Chem Lett 25: 1880-3 (2015) Article DOI: 10.1016/j.bmcl.2015.03.047 BindingDB Entry DOI: 10.7270/Q2FT8NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50281562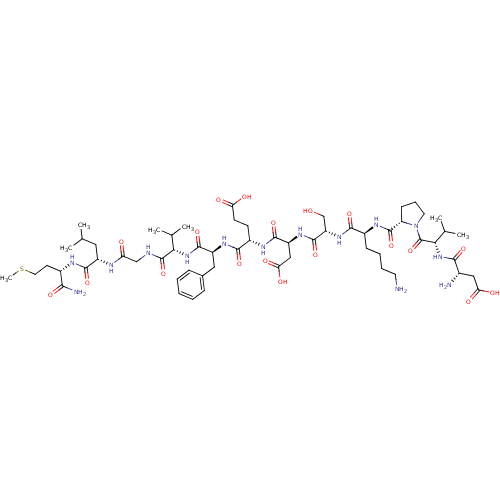 (Asp-Val-Pro-Lys-Ser-Asp-Gln-Phe-Val-Gly-Leu-Met-NH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of tachykinin 1 (NK1) receptor in rat brain synaptosomal membranes using [125I]-BH-SP as radioligand | Bioorg Med Chem Lett 3: 447-450 (1993) Article DOI: 10.1016/S0960-894X(01)80229-8 BindingDB Entry DOI: 10.7270/Q2R2119Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50510466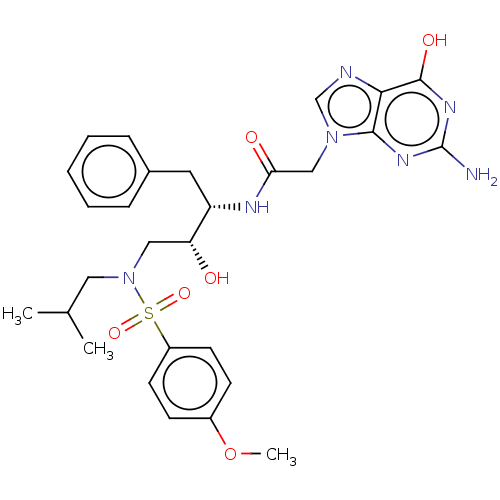 (CHEMBL4545918) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrat... | Bioorg Med Chem Lett 29: 1541-1545 (2019) Article DOI: 10.1016/j.bmcl.2019.03.049 BindingDB Entry DOI: 10.7270/Q20P13BM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50541374 (CHEMBL4637416) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Jinzhou Medical University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127019 BindingDB Entry DOI: 10.7270/Q2M61PS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50541373 (CHEMBL4642712) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Jinzhou Medical University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127019 BindingDB Entry DOI: 10.7270/Q2M61PS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50518684 (CHEMBL4448278) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincub... | Bioorg Med Chem Lett 29: 357-361 (2019) Article DOI: 10.1016/j.bmcl.2018.12.040 BindingDB Entry DOI: 10.7270/Q2GX4FZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50067617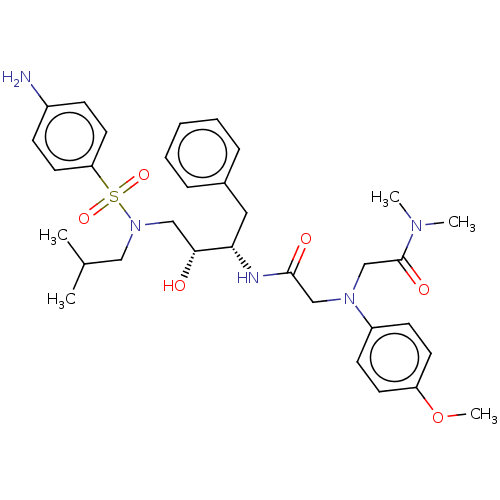 (CHEMBL3400717) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | 25 |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli incubated for 20 to 30 mins at room temperature using (Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-I... | Bioorg Med Chem Lett 25: 1880-3 (2015) Article DOI: 10.1016/j.bmcl.2015.03.047 BindingDB Entry DOI: 10.7270/Q2FT8NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50510453 (CHEMBL4522803) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrat... | Bioorg Med Chem Lett 29: 1541-1545 (2019) Article DOI: 10.1016/j.bmcl.2019.03.049 BindingDB Entry DOI: 10.7270/Q20P13BM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50518700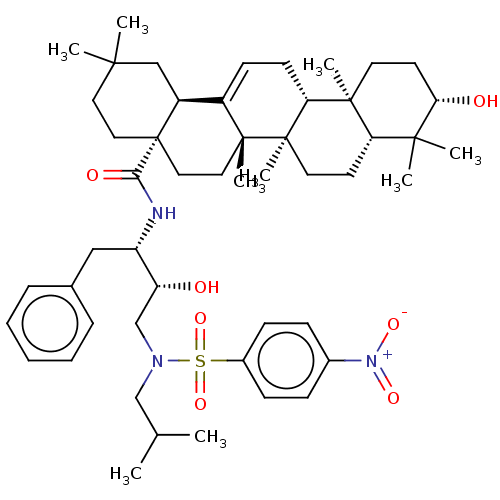 (CHEMBL4444407) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincub... | Bioorg Med Chem Lett 29: 357-361 (2019) Article DOI: 10.1016/j.bmcl.2018.12.040 BindingDB Entry DOI: 10.7270/Q2GX4FZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50518687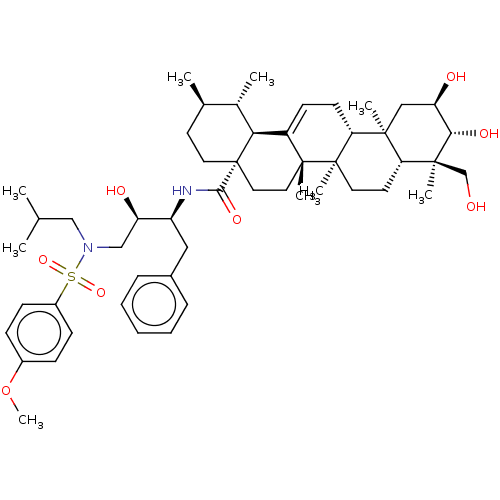 (CHEMBL4530869) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincub... | Bioorg Med Chem Lett 29: 357-361 (2019) Article DOI: 10.1016/j.bmcl.2018.12.040 BindingDB Entry DOI: 10.7270/Q2GX4FZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50067603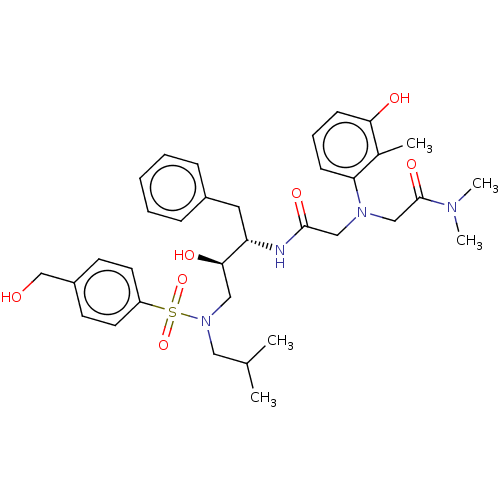 (CHEMBL3400721) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | 25 |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli incubated for 20 to 30 mins at room temperature using (Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-I... | Bioorg Med Chem Lett 25: 1880-3 (2015) Article DOI: 10.1016/j.bmcl.2015.03.047 BindingDB Entry DOI: 10.7270/Q2FT8NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50518686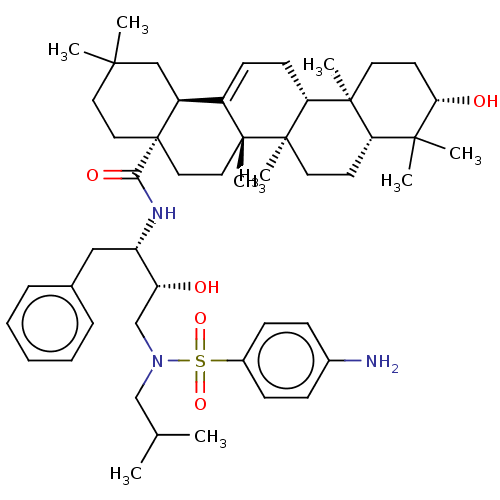 (CHEMBL4444031) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincub... | Bioorg Med Chem Lett 29: 357-361 (2019) Article DOI: 10.1016/j.bmcl.2018.12.040 BindingDB Entry DOI: 10.7270/Q2GX4FZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50541396 (CHEMBL4643837) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Jinzhou Medical University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127019 BindingDB Entry DOI: 10.7270/Q2M61PS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50518701 (CHEMBL4443902) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincub... | Bioorg Med Chem Lett 29: 357-361 (2019) Article DOI: 10.1016/j.bmcl.2018.12.040 BindingDB Entry DOI: 10.7270/Q2GX4FZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50510452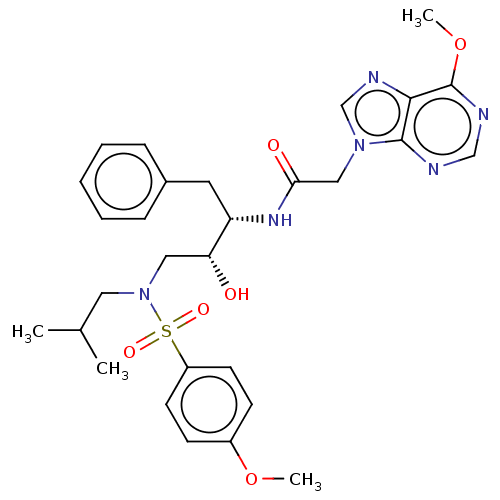 (CHEMBL4527349) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrat... | Bioorg Med Chem Lett 29: 1541-1545 (2019) Article DOI: 10.1016/j.bmcl.2019.03.049 BindingDB Entry DOI: 10.7270/Q20P13BM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50541392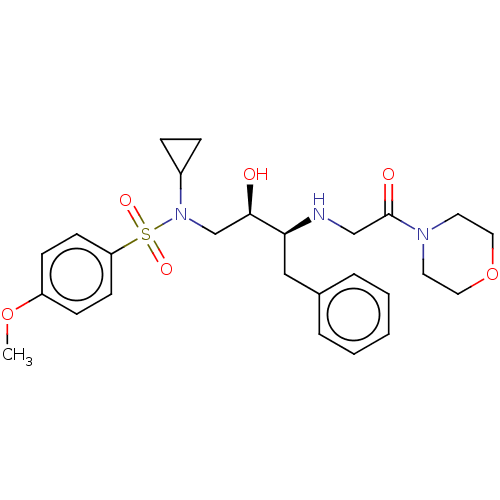 (CHEMBL4639173) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Jinzhou Medical University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127019 BindingDB Entry DOI: 10.7270/Q2M61PS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50067657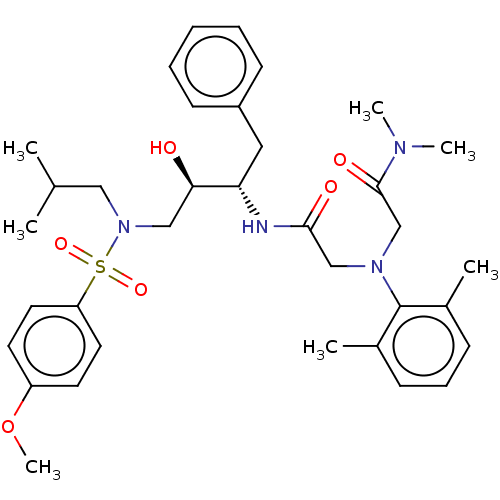 (CHEMBL3400707) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | 25 |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli incubated for 20 to 30 mins at room temperature using (Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-I... | Bioorg Med Chem Lett 25: 1880-3 (2015) Article DOI: 10.1016/j.bmcl.2015.03.047 BindingDB Entry DOI: 10.7270/Q2FT8NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50510449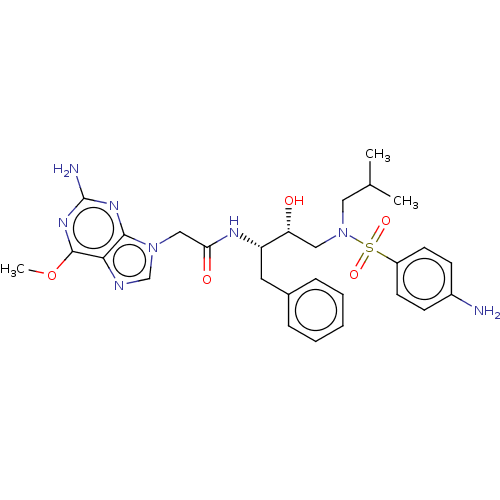 (CHEMBL4588514) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrat... | Bioorg Med Chem Lett 29: 1541-1545 (2019) Article DOI: 10.1016/j.bmcl.2019.03.049 BindingDB Entry DOI: 10.7270/Q20P13BM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50541376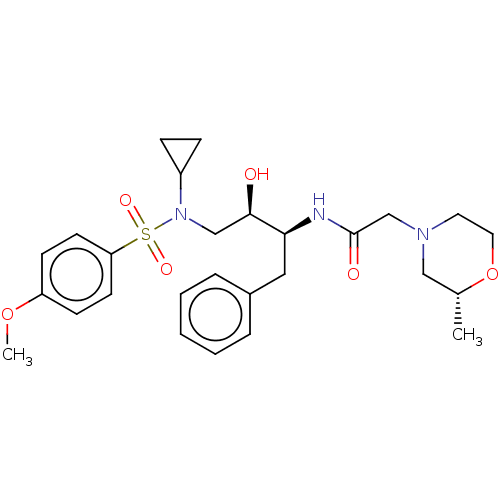 (CHEMBL4634015) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Jinzhou Medical University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127019 BindingDB Entry DOI: 10.7270/Q2M61PS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50067651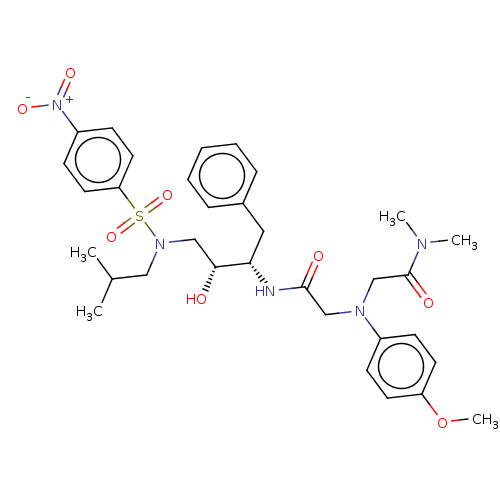 (CHEMBL3400711) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | 25 |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli incubated for 20 to 30 mins at room temperature using (Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-I... | Bioorg Med Chem Lett 25: 1880-3 (2015) Article DOI: 10.1016/j.bmcl.2015.03.047 BindingDB Entry DOI: 10.7270/Q2FT8NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50518690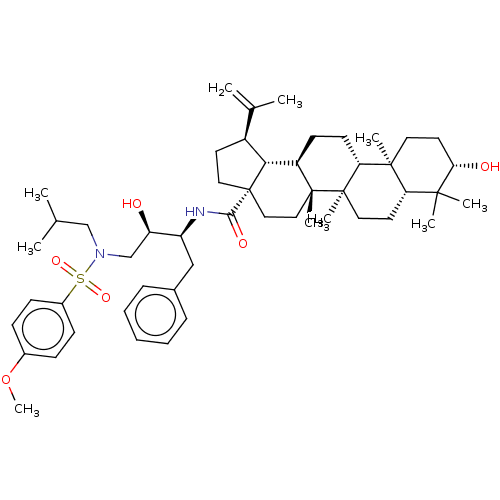 (CHEMBL4561381) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincub... | Bioorg Med Chem Lett 29: 357-361 (2019) Article DOI: 10.1016/j.bmcl.2018.12.040 BindingDB Entry DOI: 10.7270/Q2GX4FZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50067660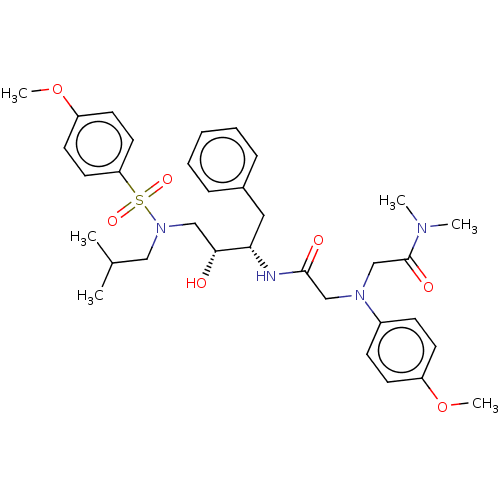 (CHEMBL3400706) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | 25 |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli incubated for 20 to 30 mins at room temperature using (Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-I... | Bioorg Med Chem Lett 25: 1880-3 (2015) Article DOI: 10.1016/j.bmcl.2015.03.047 BindingDB Entry DOI: 10.7270/Q2FT8NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50518688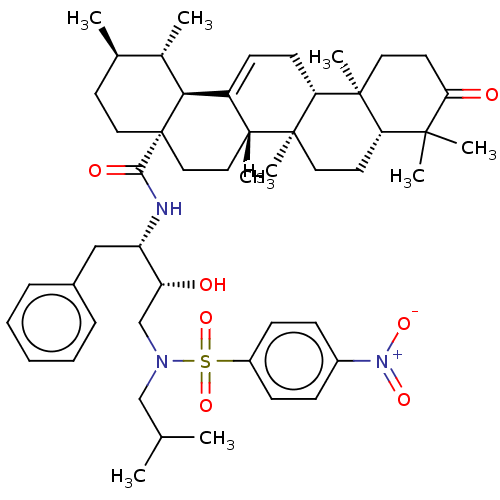 (CHEMBL4447543) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincub... | Bioorg Med Chem Lett 29: 357-361 (2019) Article DOI: 10.1016/j.bmcl.2018.12.040 BindingDB Entry DOI: 10.7270/Q2GX4FZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50518685 (CHEMBL4452115) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincub... | Bioorg Med Chem Lett 29: 357-361 (2019) Article DOI: 10.1016/j.bmcl.2018.12.040 BindingDB Entry DOI: 10.7270/Q2GX4FZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50510474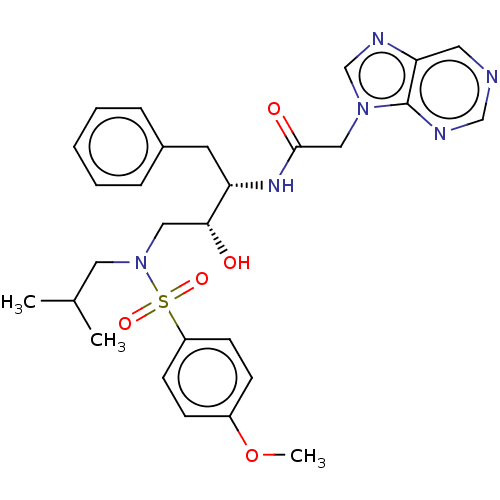 (CHEMBL4525077) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrat... | Bioorg Med Chem Lett 29: 1541-1545 (2019) Article DOI: 10.1016/j.bmcl.2019.03.049 BindingDB Entry DOI: 10.7270/Q20P13BM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50541388 (CHEMBL4635436) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Jinzhou Medical University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127019 BindingDB Entry DOI: 10.7270/Q2M61PS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50067664 (CHEMBL3400705) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | 25 |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli incubated for 20 to 30 mins at room temperature using (Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-I... | Bioorg Med Chem Lett 25: 1880-3 (2015) Article DOI: 10.1016/j.bmcl.2015.03.047 BindingDB Entry DOI: 10.7270/Q2FT8NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50067596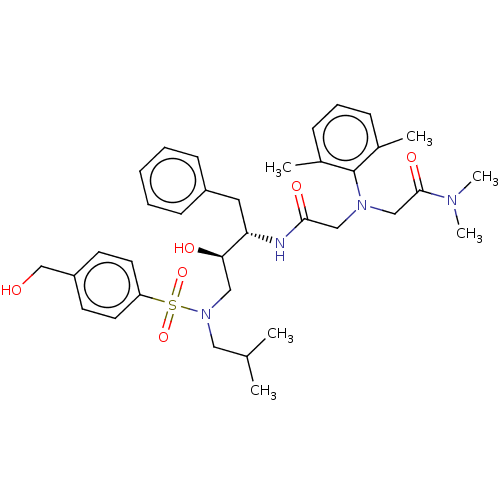 (CHEMBL3400725) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | 25 |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli incubated for 20 to 30 mins at room temperature using (Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-I... | Bioorg Med Chem Lett 25: 1880-3 (2015) Article DOI: 10.1016/j.bmcl.2015.03.047 BindingDB Entry DOI: 10.7270/Q2FT8NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50067648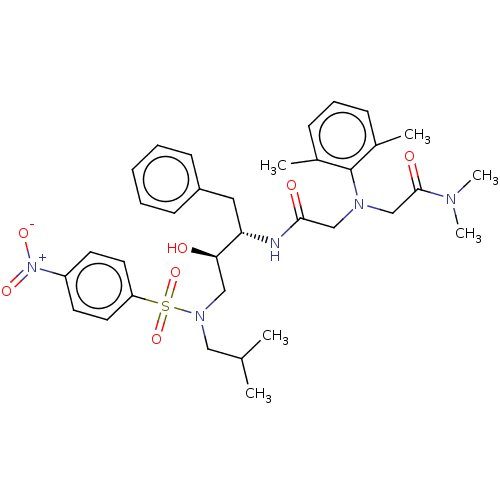 (CHEMBL3400713) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | 25 |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli incubated for 20 to 30 mins at room temperature using (Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-I... | Bioorg Med Chem Lett 25: 1880-3 (2015) Article DOI: 10.1016/j.bmcl.2015.03.047 BindingDB Entry DOI: 10.7270/Q2FT8NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 227 total ) | Next | Last >> |