

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase TEM (Escherichia coli) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518879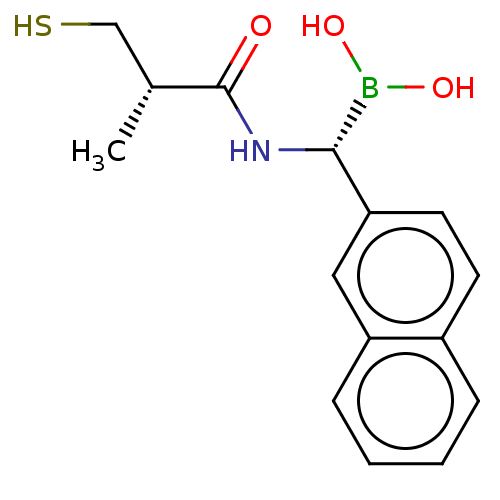 (CHEMBL4450911) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518902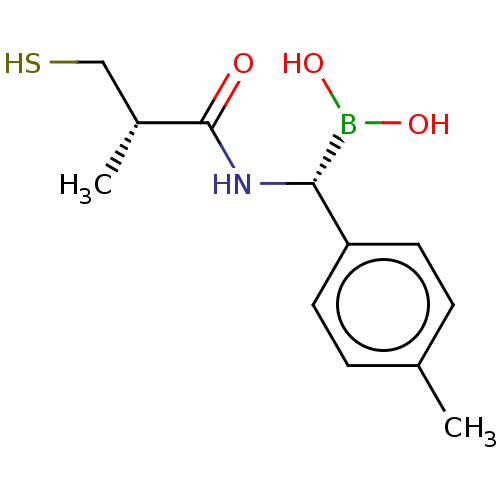 (CHEMBL4458011) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518878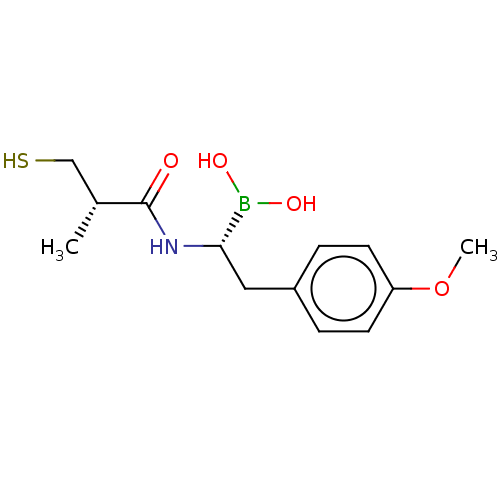 (CHEMBL4464588) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518889 (CHEMBL4560468) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518901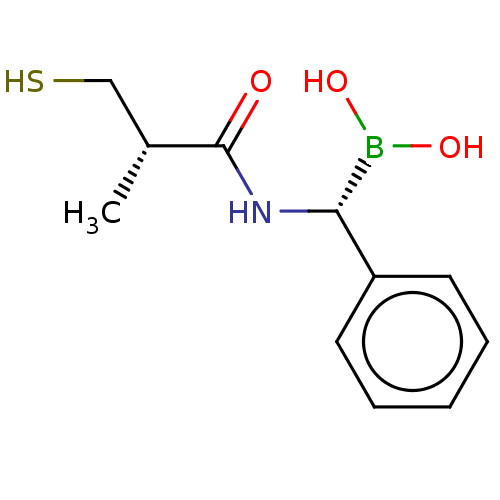 (CHEMBL4468952) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518882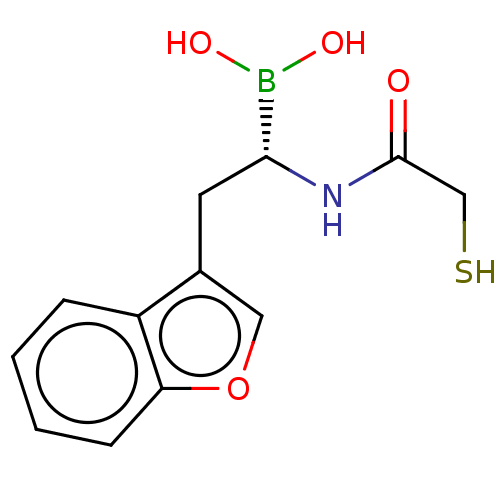 (CHEMBL4471138) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518890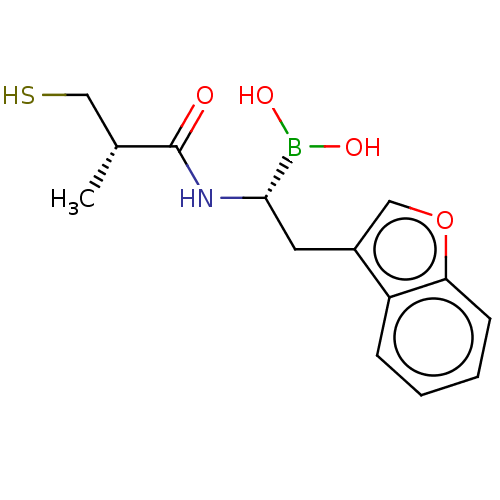 (CHEMBL4439726) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518907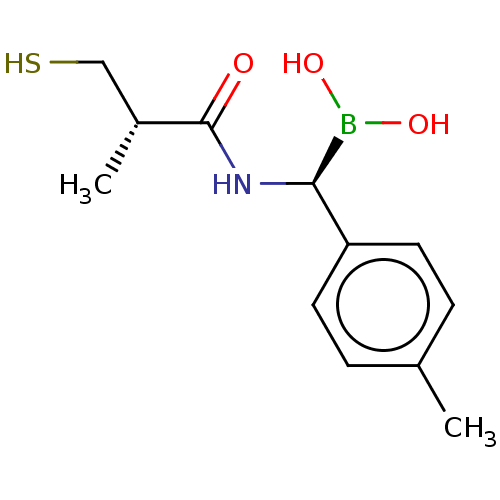 (CHEMBL4472399) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518892 (CHEMBL4453017) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518895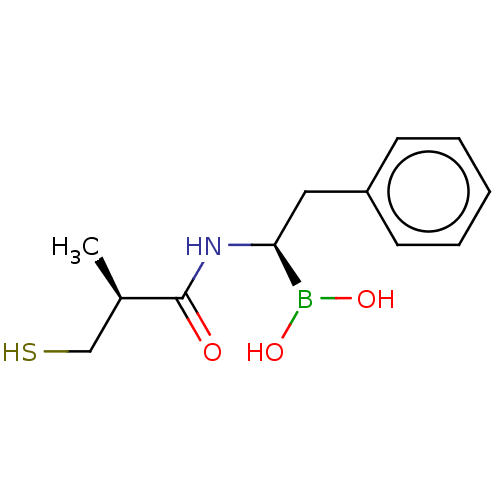 (CHEMBL4454286) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518887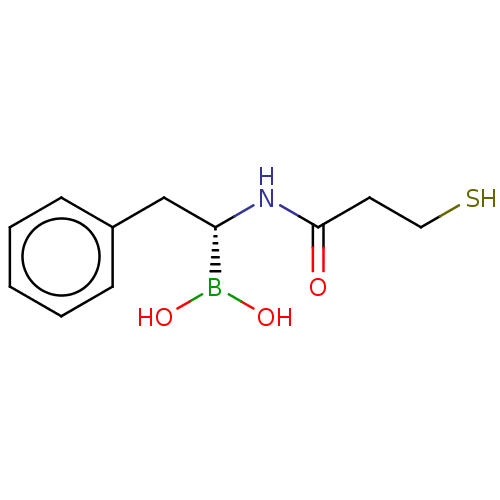 (CHEMBL4527309) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518906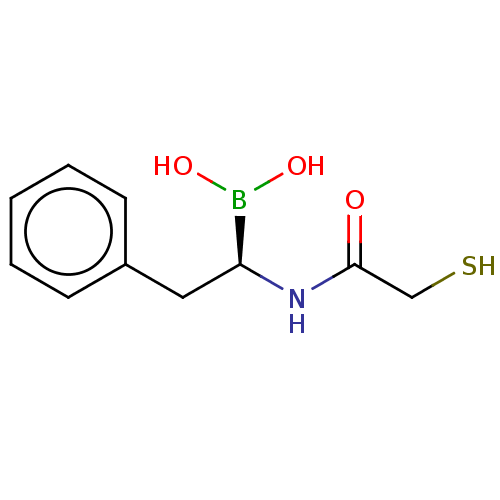 (CHEMBL4444728) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518899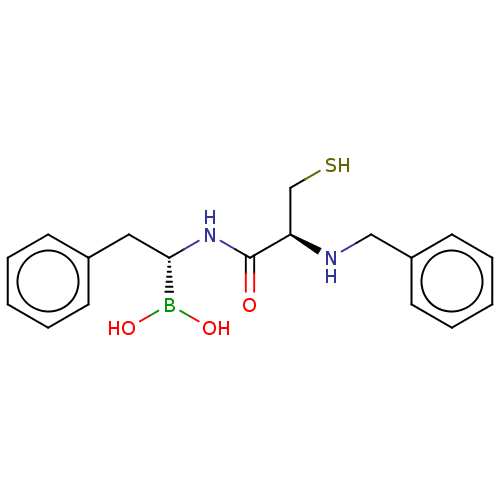 (CHEMBL4460555) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518891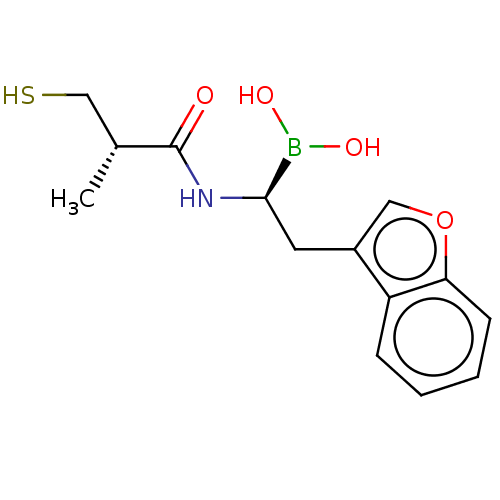 (CHEMBL4460127) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 3.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518886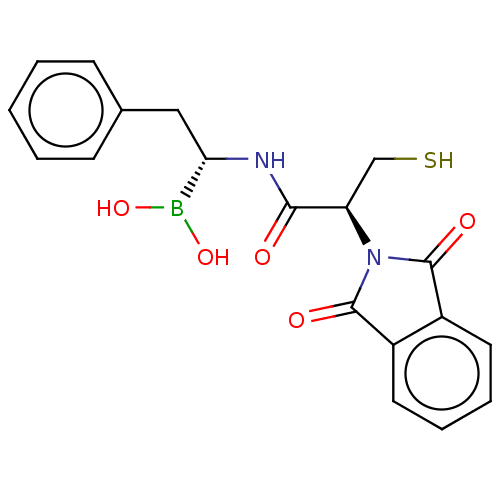 (CHEMBL4471673) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518881 (CHEMBL4455145) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518884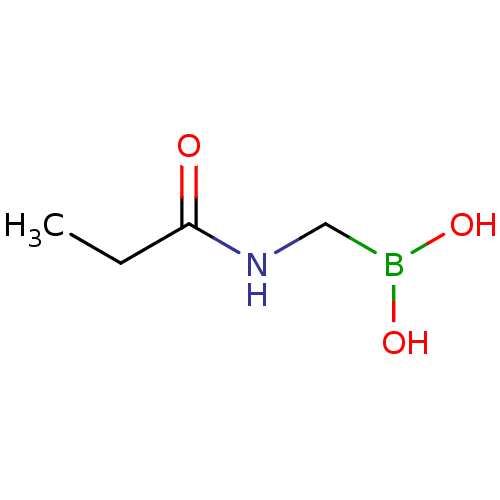 (CHEMBL4588584) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518897 (CHEMBL4450628) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518893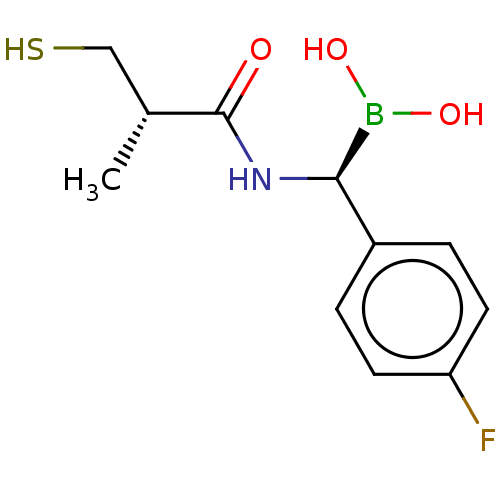 (CHEMBL4545947) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 6.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518896 (CHEMBL4517893) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518877 (CHEMBL4546207) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518908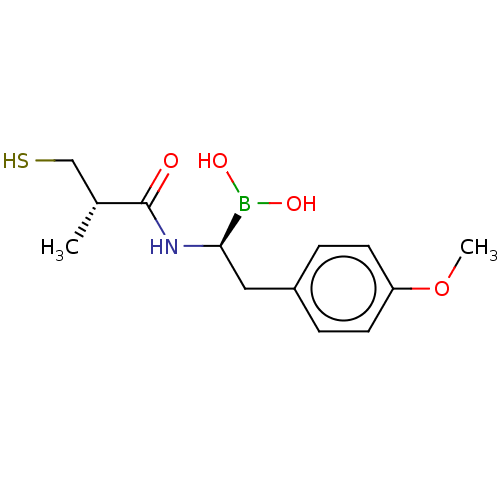 (CHEMBL4543432) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518883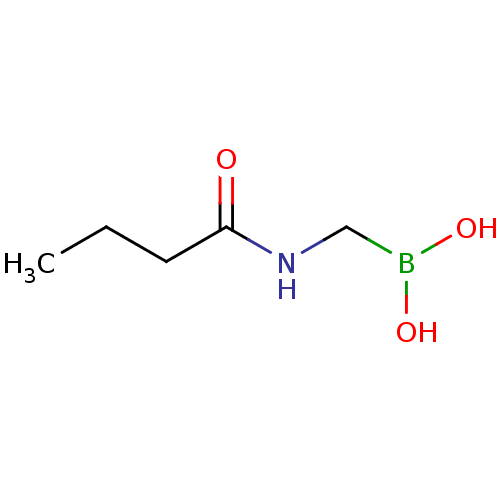 (CHEMBL4577981) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518885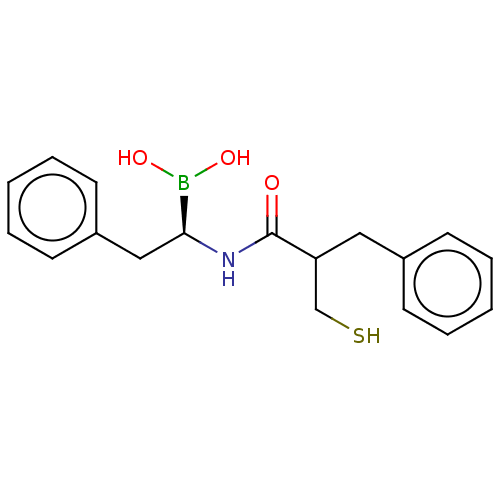 (CHEMBL4451026) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518903 (CHEMBL4445648) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.66E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518905 (CHEMBL4469059) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.71E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518900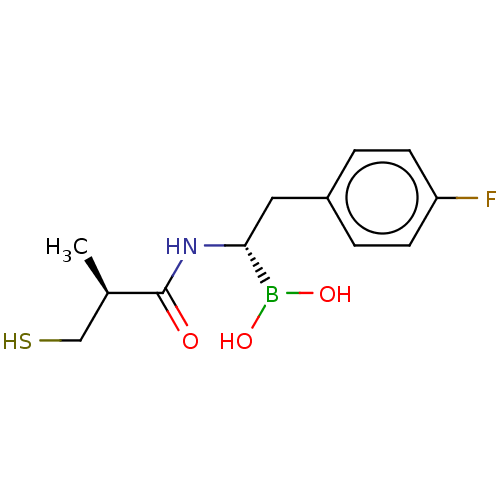 (CHEMBL4545274) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518894 (CHEMBL4444926) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518898 (CHEMBL4546893) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518904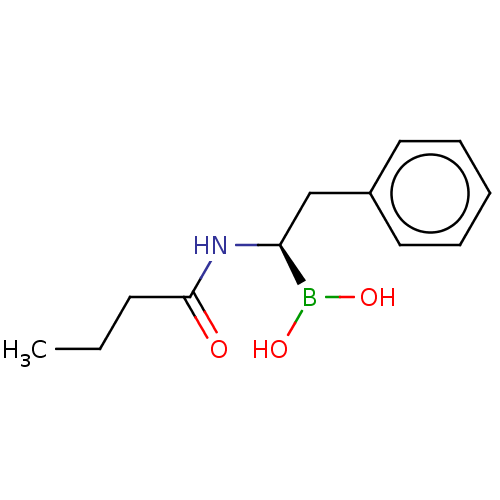 (CHEMBL4544829) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518880 (CHEMBL4438924) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518909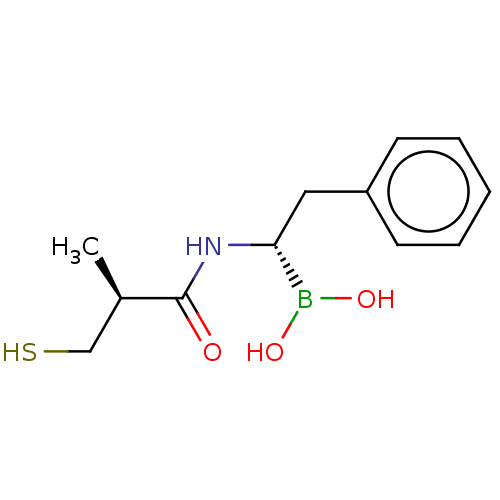 (CHEMBL4464521) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50518888 (CHEMBL4466256) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... | J Med Chem 62: 7160-7184 (2019) Article DOI: 10.1021/acs.jmedchem.9b00735 BindingDB Entry DOI: 10.7270/Q22R3W2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50456168 (CHEMBL1800685) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01632 BindingDB Entry DOI: 10.7270/Q26D5Z20 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50351665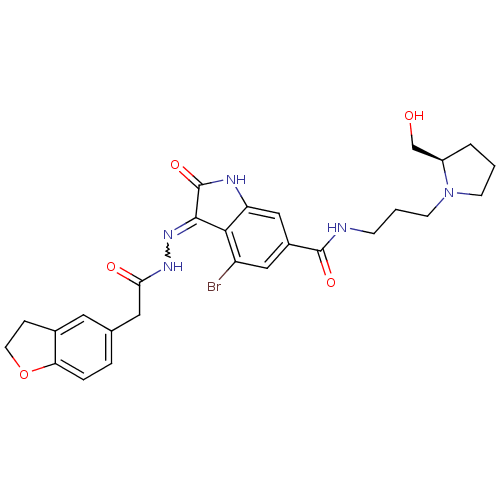 (CHEMBL1822369) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human c-MET | Eur J Med Chem 46: 3675-80 (2011) Article DOI: 10.1016/j.ejmech.2011.05.031 BindingDB Entry DOI: 10.7270/Q2891676 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50351664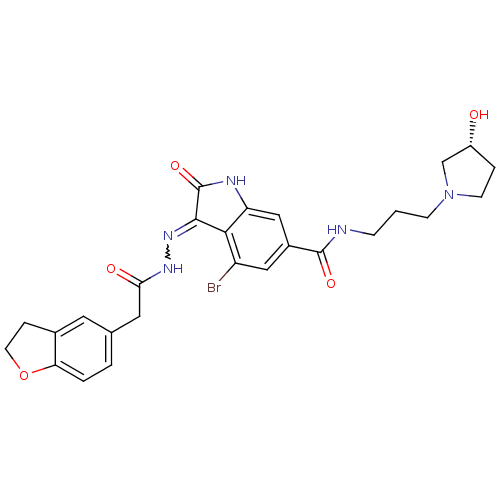 (CHEMBL1822368) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human c-MET | Eur J Med Chem 46: 3675-80 (2011) Article DOI: 10.1016/j.ejmech.2011.05.031 BindingDB Entry DOI: 10.7270/Q2891676 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50351662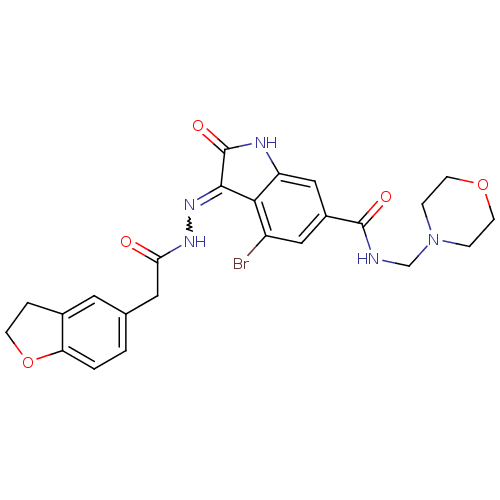 (CHEMBL1822366) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human c-MET | Eur J Med Chem 46: 3675-80 (2011) Article DOI: 10.1016/j.ejmech.2011.05.031 BindingDB Entry DOI: 10.7270/Q2891676 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50351633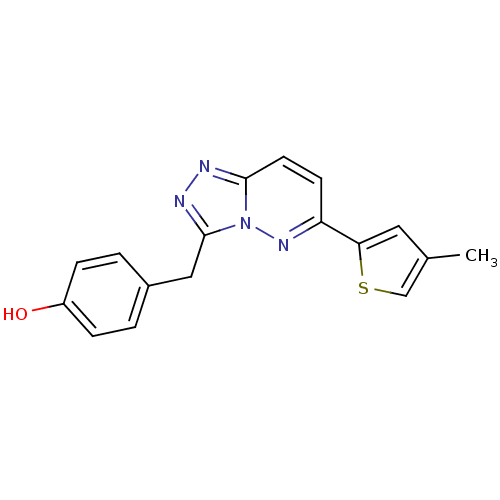 (CHEMBL497118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human c-MET | Eur J Med Chem 46: 3675-80 (2011) Article DOI: 10.1016/j.ejmech.2011.05.031 BindingDB Entry DOI: 10.7270/Q2891676 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50351579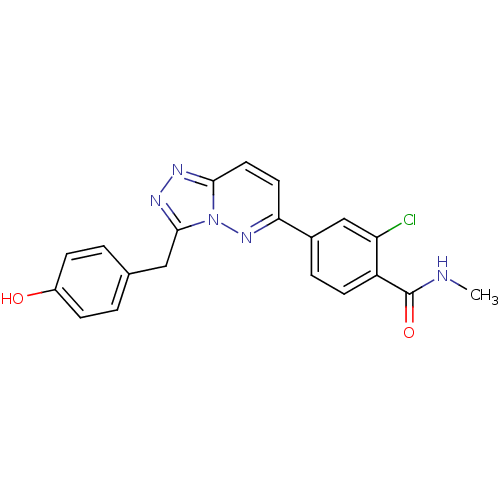 (CHEMBL494985) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human c-MET | Eur J Med Chem 46: 3675-80 (2011) Article DOI: 10.1016/j.ejmech.2011.05.031 BindingDB Entry DOI: 10.7270/Q2891676 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24460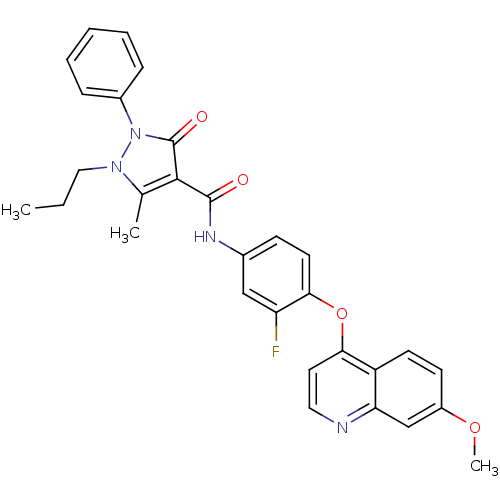 (N-{3-fluoro-4-[(7-methoxyquinolin-4-yl)oxy]phenyl}...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human c-MET | Eur J Med Chem 46: 3675-80 (2011) Article DOI: 10.1016/j.ejmech.2011.05.031 BindingDB Entry DOI: 10.7270/Q2891676 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM28028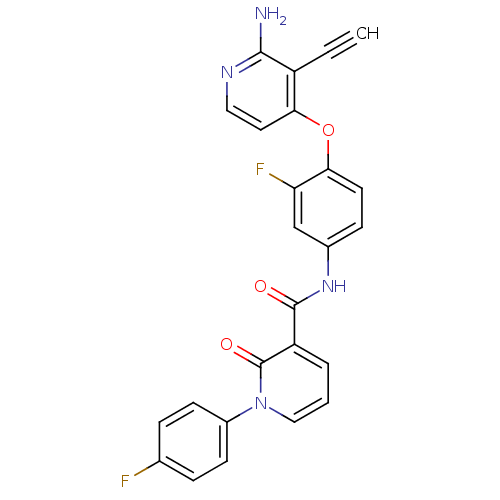 (2-aminopyridine analogue, 7 | N-{4-[(2-amino-3-eth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human c-MET | Eur J Med Chem 46: 3675-80 (2011) Article DOI: 10.1016/j.ejmech.2011.05.031 BindingDB Entry DOI: 10.7270/Q2891676 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50351641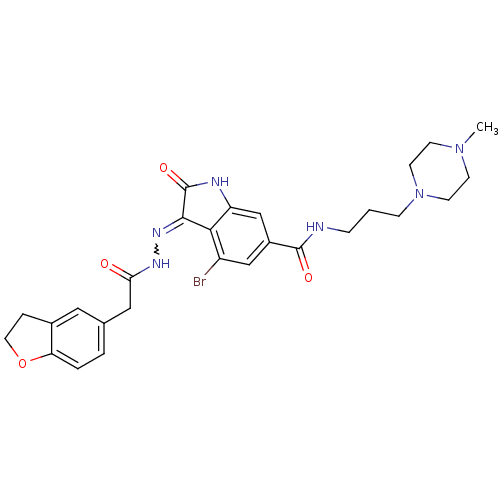 (CHEMBL1823128) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human c-MET | Eur J Med Chem 46: 3675-80 (2011) Article DOI: 10.1016/j.ejmech.2011.05.031 BindingDB Entry DOI: 10.7270/Q2891676 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM28030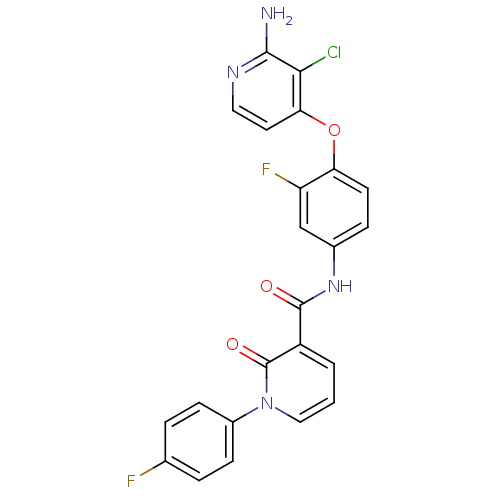 (2-aminopyridine analogue, 9 | N-{4-[(2-amino-3-chl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human c-MET | Eur J Med Chem 46: 3675-80 (2011) Article DOI: 10.1016/j.ejmech.2011.05.031 BindingDB Entry DOI: 10.7270/Q2891676 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24462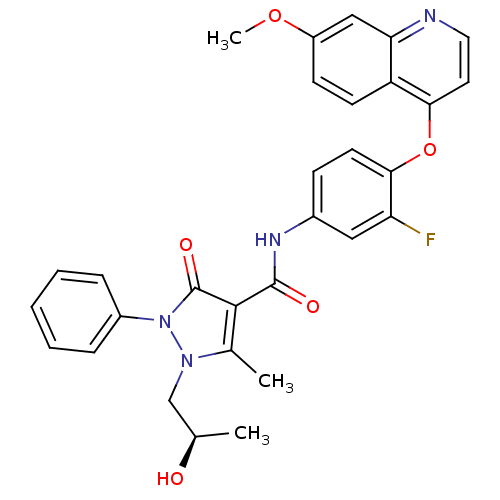 (N-{3-fluoro-4-[(7-methoxyquinolin-4-yl)oxy]phenyl}...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human c-MET | Eur J Med Chem 46: 3675-80 (2011) Article DOI: 10.1016/j.ejmech.2011.05.031 BindingDB Entry DOI: 10.7270/Q2891676 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24459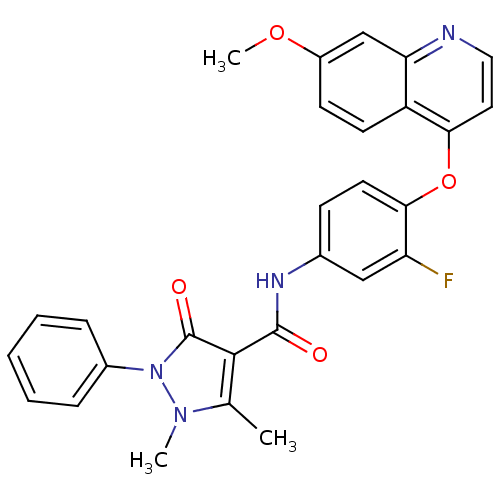 (N-{3-fluoro-4-[(7-methoxyquinolin-4-yl)oxy]phenyl}...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human c-MET | Eur J Med Chem 46: 3675-80 (2011) Article DOI: 10.1016/j.ejmech.2011.05.031 BindingDB Entry DOI: 10.7270/Q2891676 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50351650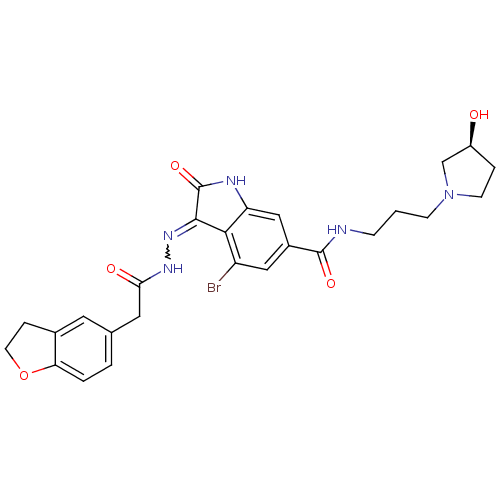 (CHEMBL1823252) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human c-MET | Eur J Med Chem 46: 3675-80 (2011) Article DOI: 10.1016/j.ejmech.2011.05.031 BindingDB Entry DOI: 10.7270/Q2891676 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24463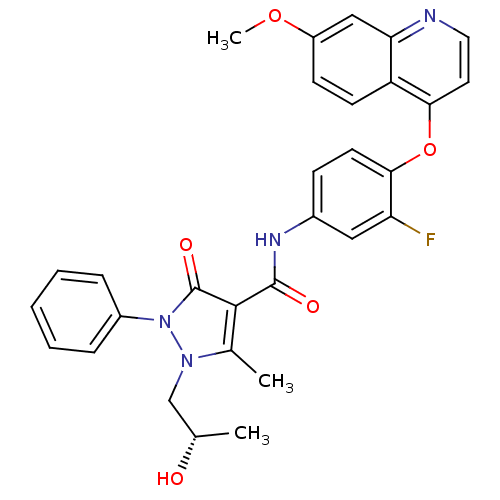 (N-{3-fluoro-4-[(7-methoxyquinolin-4-yl)oxy]phenyl}...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human c-MET | Eur J Med Chem 46: 3675-80 (2011) Article DOI: 10.1016/j.ejmech.2011.05.031 BindingDB Entry DOI: 10.7270/Q2891676 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24457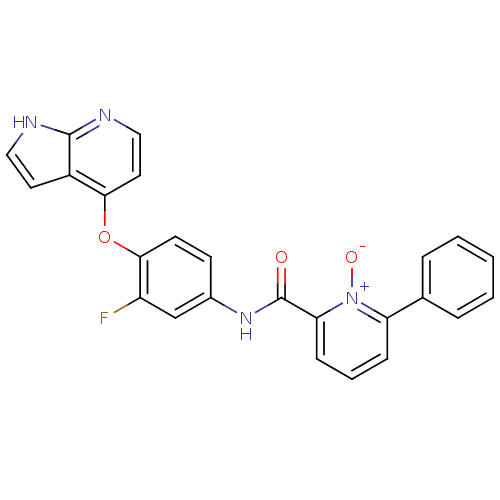 (2-[(3-fluoro-4-{1H-pyrrolo[2,3-b]pyridin-4-yloxy}p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human c-MET | Eur J Med Chem 46: 3675-80 (2011) Article DOI: 10.1016/j.ejmech.2011.05.031 BindingDB Entry DOI: 10.7270/Q2891676 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 703 total ) | Next | Last >> |