 Found 30257 hits with Last Name = 'wang' and Initial = 'z'
Found 30257 hits with Last Name = 'wang' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50430798
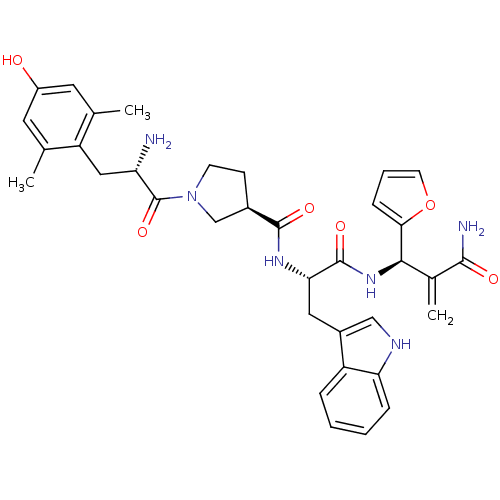
(CHEMBL2335120)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CC[C@H](C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccco1 |r| Show InChI InChI=1S/C35H40N6O6/c1-19-13-24(42)14-20(2)26(19)16-27(36)35(46)41-11-10-22(18-41)33(44)39-29(15-23-17-38-28-8-5-4-7-25(23)28)34(45)40-31(21(3)32(37)43)30-9-6-12-47-30/h4-9,12-14,17,22,27,29,31,38,42H,3,10-11,15-16,18,36H2,1-2H3,(H2,37,43)(H,39,44)(H,40,45)/t22-,27+,29+,31-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... |
J Med Chem 56: 3102-14 (2013)
Article DOI: 10.1021/jm400195y
BindingDB Entry DOI: 10.7270/Q2KS6SW8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50033531
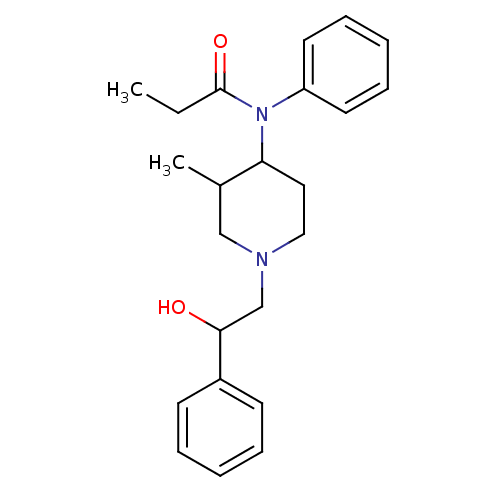
(CHEMBL333410 | N-[1-(2-Hydroxy-2-phenyl-ethyl)-3-m...)Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50430801
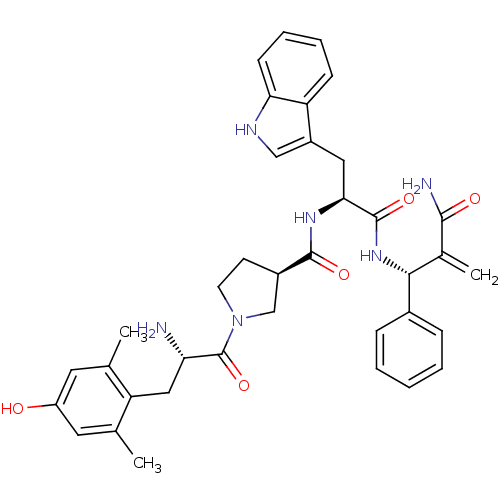
(CHEMBL2334776)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CC[C@H](C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccccc1 |r| Show InChI InChI=1S/C37H42N6O5/c1-21-15-27(44)16-22(2)29(21)18-30(38)37(48)43-14-13-25(20-43)35(46)41-32(17-26-19-40-31-12-8-7-11-28(26)31)36(47)42-33(23(3)34(39)45)24-9-5-4-6-10-24/h4-12,15-16,19,25,30,32-33,40,44H,3,13-14,17-18,20,38H2,1-2H3,(H2,39,45)(H,41,46)(H,42,47)/t25-,30+,32+,33-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00986 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... |
J Med Chem 56: 3102-14 (2013)
Article DOI: 10.1021/jm400195y
BindingDB Entry DOI: 10.7270/Q2KS6SW8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50430802
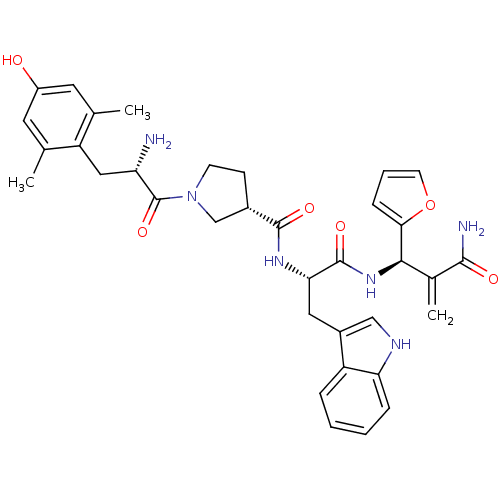
(CHEMBL2334775)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CC[C@@H](C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccco1 |r| Show InChI InChI=1S/C35H40N6O6/c1-19-13-24(42)14-20(2)26(19)16-27(36)35(46)41-11-10-22(18-41)33(44)39-29(15-23-17-38-28-8-5-4-7-25(23)28)34(45)40-31(21(3)32(37)43)30-9-6-12-47-30/h4-9,12-14,17,22,27,29,31,38,42H,3,10-11,15-16,18,36H2,1-2H3,(H2,37,43)(H,39,44)(H,40,45)/t22-,27-,29-,31+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... |
J Med Chem 56: 3102-14 (2013)
Article DOI: 10.1021/jm400195y
BindingDB Entry DOI: 10.7270/Q2KS6SW8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50430803
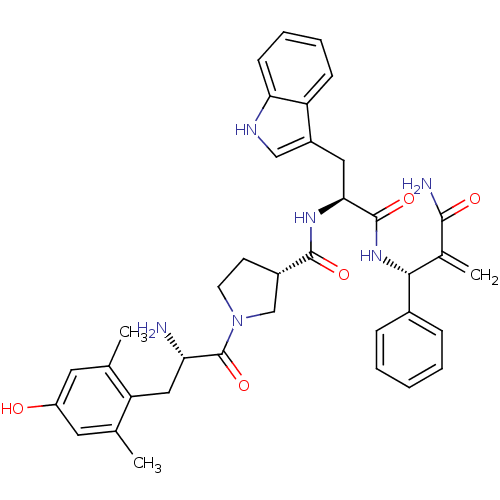
(CHEMBL2334774)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CC[C@@H](C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccccc1 |r| Show InChI InChI=1S/C37H42N6O5/c1-21-15-27(44)16-22(2)29(21)18-30(38)37(48)43-14-13-25(20-43)35(46)41-32(17-26-19-40-31-12-8-7-11-28(26)31)36(47)42-33(23(3)34(39)45)24-9-5-4-6-10-24/h4-12,15-16,19,25,30,32-33,40,44H,3,13-14,17-18,20,38H2,1-2H3,(H2,39,45)(H,41,46)(H,42,47)/t25-,30-,32-,33+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... |
J Med Chem 56: 3102-14 (2013)
Article DOI: 10.1021/jm400195y
BindingDB Entry DOI: 10.7270/Q2KS6SW8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340336
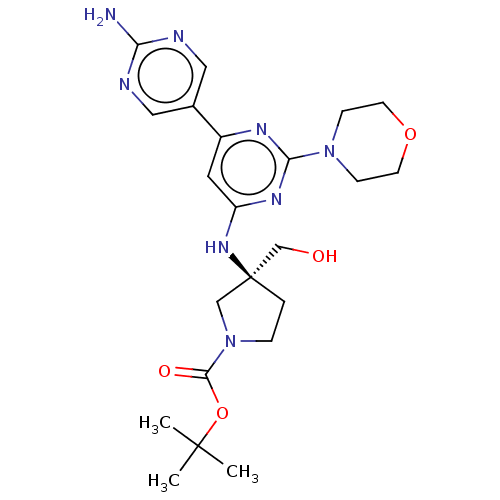
(US9758538, Example 24)Show SMILES CC(C)(C)OC(=O)N1CC[C@@](CO)(C1)Nc1cc(nc(n1)N1CCOCC1)-c1cnc(N)nc1 |r| Show InChI InChI=1S/C22H32N8O4/c1-21(2,3)34-20(32)30-5-4-22(13-30,14-31)28-17-10-16(15-11-24-18(23)25-12-15)26-19(27-17)29-6-8-33-9-7-29/h10-12,31H,4-9,13-14H2,1-3H3,(H2,23,24,25)(H,26,27,28)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207378
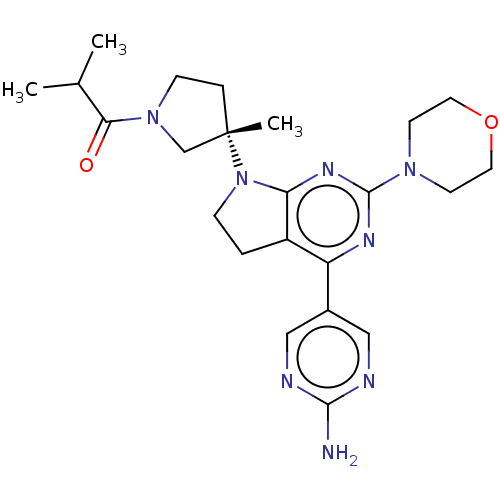
(US9260439, 262)Show SMILES CC(C)C(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C23H32N8O2/c1-15(2)20(32)30-7-5-23(3,14-30)31-6-4-17-18(16-12-25-21(24)26-13-16)27-22(28-19(17)31)29-8-10-33-11-9-29/h12-13,15H,4-11,14H2,1-3H3,(H2,24,25,26)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207217
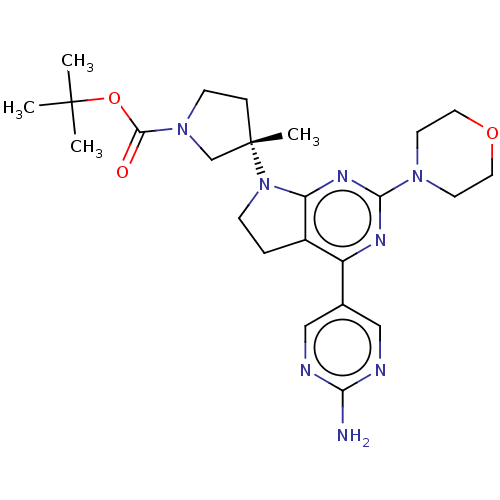
(US9260439, 194 | US9260439, 238 | US9260439, 239)Show SMILES CC(C)(C)OC(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C24H34N8O3/c1-23(2,3)35-22(33)31-8-6-24(4,15-31)32-7-5-17-18(16-13-26-20(25)27-14-16)28-21(29-19(17)32)30-9-11-34-12-10-30/h13-14H,5-12,15H2,1-4H3,(H2,25,26,27)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340384
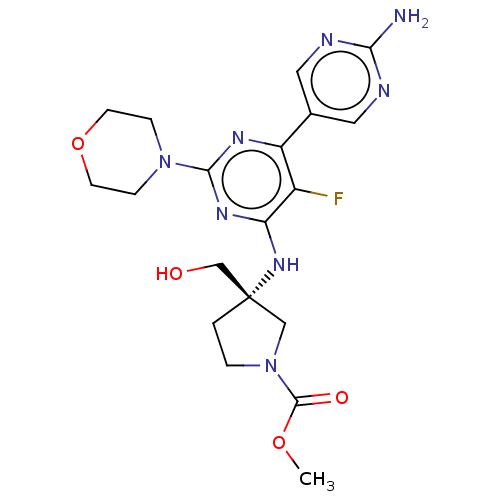
(US9758538, Example 72)Show SMILES COC(=O)N1CC[C@@](CO)(C1)Nc1nc(nc(c1F)-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C19H25FN8O4/c1-31-18(30)28-3-2-19(10-28,11-29)26-15-13(20)14(12-8-22-16(21)23-9-12)24-17(25-15)27-4-6-32-7-5-27/h8-9,29H,2-7,10-11H2,1H3,(H2,21,22,23)(H,24,25,26)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340314
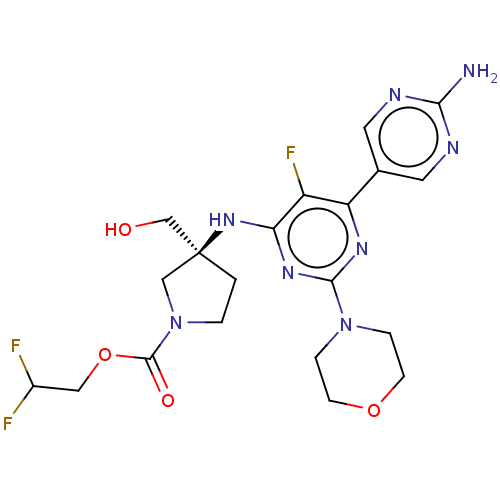
((Scheme A): Preparation of 2,2-difluoroethyl (3S)-...)Show SMILES Nc1ncc(cn1)-c1nc(nc(N[C@@]2(CO)CCN(C2)C(=O)OCC(F)F)c1F)N1CCOCC1 |r| Show InChI InChI=1S/C20H25F3N8O4/c21-13(22)9-35-19(33)31-2-1-20(10-31,11-32)29-16-14(23)15(12-7-25-17(24)26-8-12)27-18(28-16)30-3-5-34-6-4-30/h7-8,13,32H,1-6,9-11H2,(H2,24,25,26)(H,27,28,29)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207196
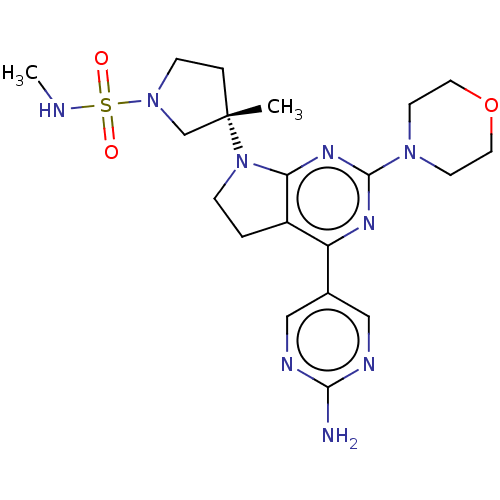
(US9260439, 173)Show SMILES CNS(=O)(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C20H29N9O3S/c1-20(4-6-28(13-20)33(30,31)22-2)29-5-3-15-16(14-11-23-18(21)24-12-14)25-19(26-17(15)29)27-7-9-32-10-8-27/h11-12,22H,3-10,13H2,1-2H3,(H2,21,23,24)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340346

(US9758538, Example 34)Show SMILES COC(=O)N1CC[C@@](CO)(C1)Nc1cc(nc(n1)N1CCOCC1)-c1cnc(N)nc1 |r| Show InChI InChI=1S/C19H26N8O4/c1-30-18(29)27-3-2-19(11-27,12-28)25-15-8-14(13-9-21-16(20)22-10-13)23-17(24-15)26-4-6-31-7-5-26/h8-10,28H,2-7,11-12H2,1H3,(H2,20,21,22)(H,23,24,25)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340391
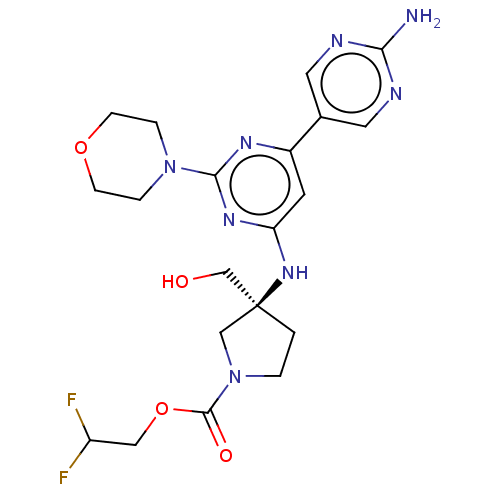
(US9758538, Example 79)Show SMILES Nc1ncc(cn1)-c1cc(N[C@@]2(CO)CCN(C2)C(=O)OCC(F)F)nc(n1)N1CCOCC1 |r| Show InChI InChI=1S/C20H26F2N8O4/c21-15(22)10-34-19(32)30-2-1-20(11-30,12-31)28-16-7-14(13-8-24-17(23)25-9-13)26-18(27-16)29-3-5-33-6-4-29/h7-9,15,31H,1-6,10-12H2,(H2,23,24,25)(H,26,27,28)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207236
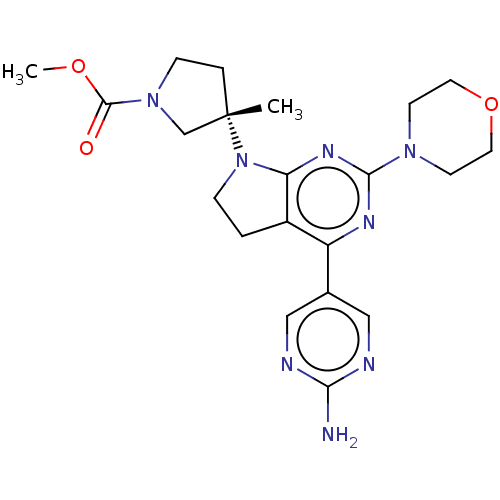
(US9260439, 213)Show SMILES COC(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C21H28N8O3/c1-21(4-6-28(13-21)20(30)31-2)29-5-3-15-16(14-11-23-18(22)24-12-14)25-19(26-17(15)29)27-7-9-32-10-8-27/h11-12H,3-10,13H2,1-2H3,(H2,22,23,24)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207028
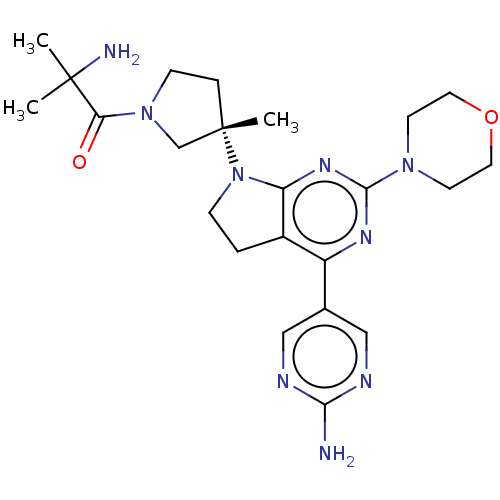
(US9260439, 10 | US9260439, 4)Show SMILES CC(C)(N)C(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C23H33N9O2/c1-22(2,25)19(33)31-7-5-23(3,14-31)32-6-4-16-17(15-12-26-20(24)27-13-15)28-21(29-18(16)32)30-8-10-34-11-9-30/h12-13H,4-11,14,25H2,1-3H3,(H2,24,26,27)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50148931
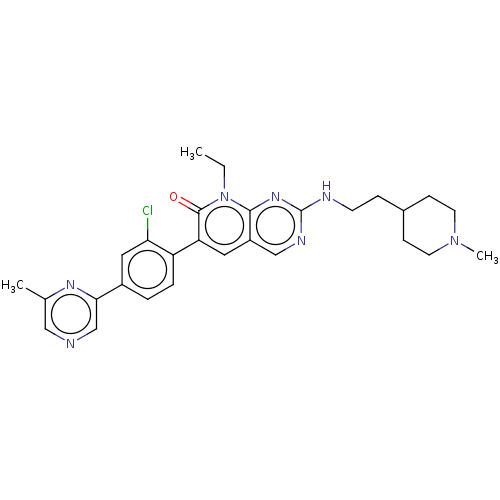
(CHEMBL3770186)Show SMILES CCn1c2nc(NCCC3CCN(C)CC3)ncc2cc(-c2ccc(cc2Cl)-c2cncc(C)n2)c1=O Show InChI InChI=1S/C28H32ClN7O/c1-4-36-26-21(16-32-28(34-26)31-10-7-19-8-11-35(3)12-9-19)13-23(27(36)37)22-6-5-20(14-24(22)29)25-17-30-15-18(2)33-25/h5-6,13-17,19H,4,7-12H2,1-3H3,(H,31,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length recombinant human N-terminal GST/His6-tagged PAK1 expressed in sf9 insect cells using tetra LRRWSLG as substrate preincubat... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112517
BindingDB Entry DOI: 10.7270/Q2Q243W7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207172
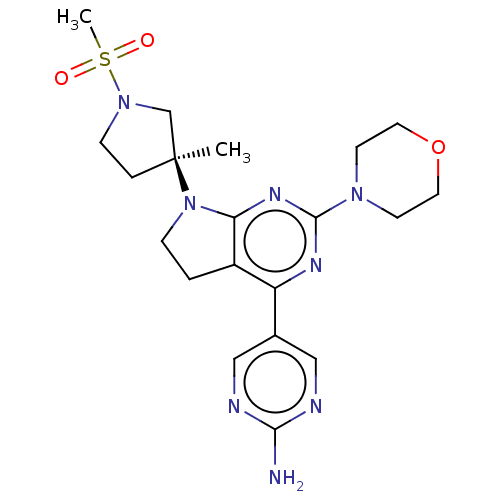
(US9260439, 149)Show SMILES C[C@@]1(CCN(C1)S(C)(=O)=O)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C20H28N8O3S/c1-20(4-6-27(13-20)32(2,29)30)28-5-3-15-16(14-11-22-18(21)23-12-14)24-19(25-17(15)28)26-7-9-31-10-8-26/h11-12H,3-10,13H2,1-2H3,(H2,21,22,23)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207391
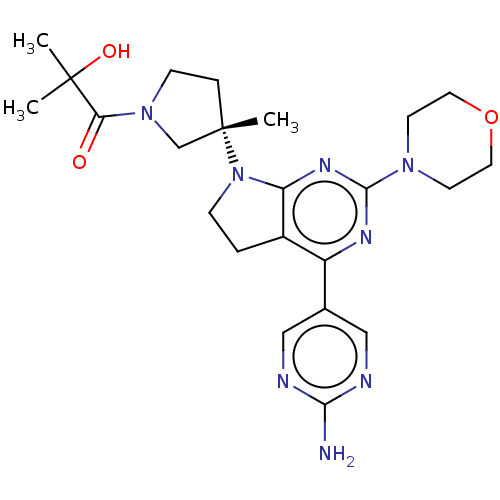
(US9260439, 275)Show SMILES CC(C)(O)C(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C23H32N8O3/c1-22(2,33)19(32)30-7-5-23(3,14-30)31-6-4-16-17(15-12-25-20(24)26-13-15)27-21(28-18(16)31)29-8-10-34-11-9-29/h12-13,33H,4-11,14H2,1-3H3,(H2,24,25,26)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50430799
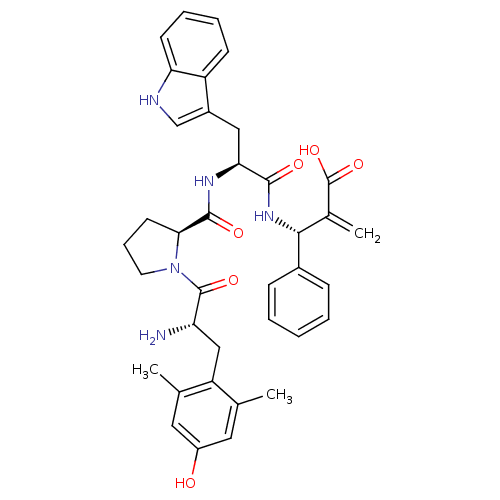
(CHEMBL2334772)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C37H41N5O6/c1-21-16-26(43)17-22(2)28(21)19-29(38)36(46)42-15-9-14-32(42)35(45)40-31(18-25-20-39-30-13-8-7-12-27(25)30)34(44)41-33(23(3)37(47)48)24-10-5-4-6-11-24/h4-8,10-13,16-17,20,29,31-33,39,43H,3,9,14-15,18-19,38H2,1-2H3,(H,40,45)(H,41,44)(H,47,48)/t29-,31-,32-,33+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0322 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... |
J Med Chem 56: 3102-14 (2013)
Article DOI: 10.1021/jm400195y
BindingDB Entry DOI: 10.7270/Q2KS6SW8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50245852
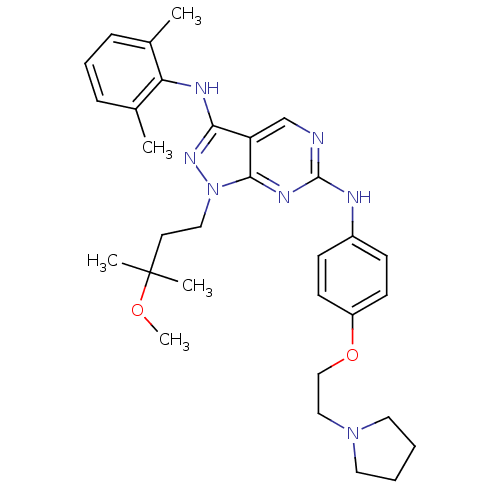
(CHEMBL458333 | N3-(2,6-dimethylphenyl)-1-(3-methox...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccc(OCCN4CCCC4)cc3)nc12 Show InChI InChI=1S/C31H41N7O2/c1-22-9-8-10-23(2)27(22)34-28-26-21-32-30(35-29(26)38(36-28)18-15-31(3,4)39-5)33-24-11-13-25(14-12-24)40-20-19-37-16-6-7-17-37/h8-14,21H,6-7,15-20H2,1-5H3,(H,34,36)(H,32,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LCK (unknown origin) |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50430800
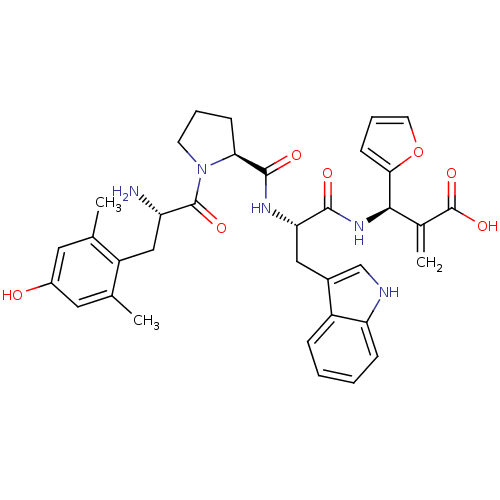
(CHEMBL2334773)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(O)=O)c1ccco1 |r| Show InChI InChI=1S/C35H39N5O7/c1-19-14-23(41)15-20(2)25(19)17-26(36)34(44)40-12-6-10-29(40)33(43)38-28(16-22-18-37-27-9-5-4-8-24(22)27)32(42)39-31(21(3)35(45)46)30-11-7-13-47-30/h4-5,7-9,11,13-15,18,26,28-29,31,37,41H,3,6,10,12,16-17,36H2,1-2H3,(H,38,43)(H,39,42)(H,45,46)/t26-,28-,29-,31+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... |
J Med Chem 56: 3102-14 (2013)
Article DOI: 10.1021/jm400195y
BindingDB Entry DOI: 10.7270/Q2KS6SW8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50033533

(CHEMBL121403 | N-[(3R,4R)-1-((S)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@@H]1CCN(C[C@@H](O)c2ccccc2)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207234
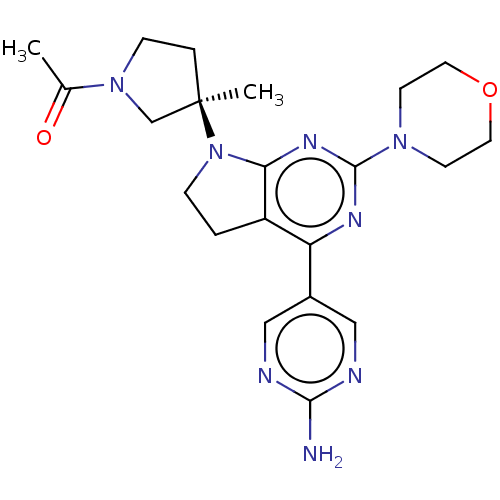
(US9260439, 211)Show SMILES CC(=O)N1CC[C@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C21H28N8O2/c1-14(30)28-6-4-21(2,13-28)29-5-3-16-17(15-11-23-19(22)24-12-15)25-20(26-18(16)29)27-7-9-31-10-8-27/h11-12H,3-10,13H2,1-2H3,(H2,22,23,24)/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50033534
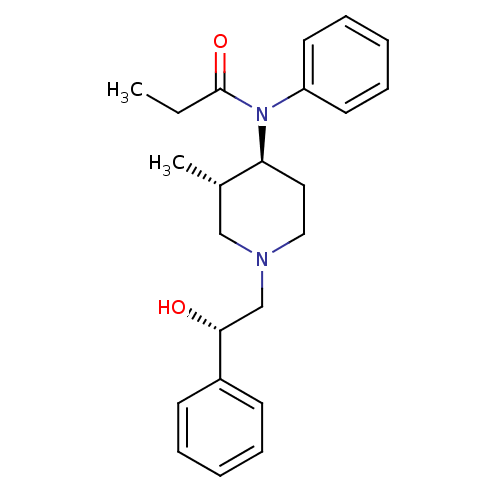
(CHEMBL338510 | N-[(3S,4S)-1-((S)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@H]1CCN(C[C@@H](O)c2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207239
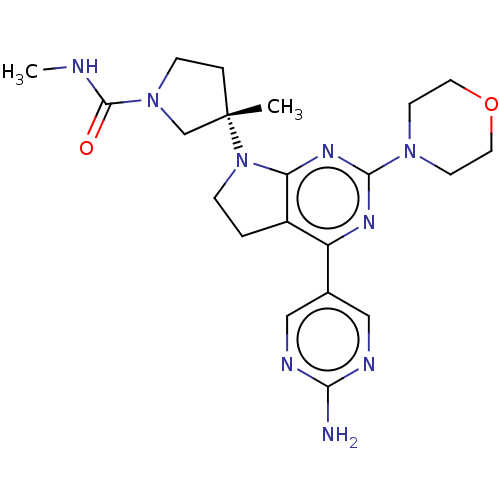
(US9260439, 216)Show SMILES CNC(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C21H29N9O2/c1-21(4-6-29(13-21)20(31)23-2)30-5-3-15-16(14-11-24-18(22)25-12-14)26-19(27-17(15)30)28-7-9-32-10-8-28/h11-12H,3-10,13H2,1-2H3,(H,23,31)(H2,22,24,25)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328391
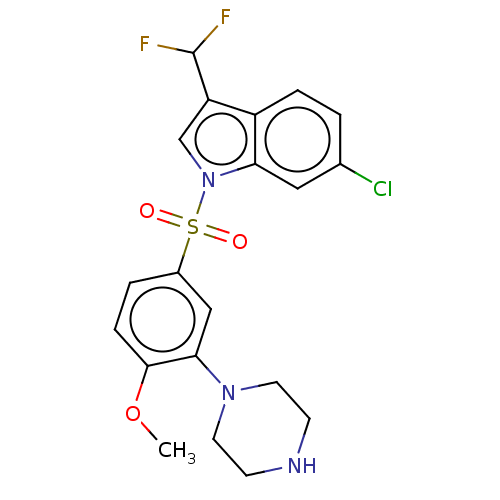
(6-chloro-3-(difluoromethyl)-1-((4-methoxy-3-(piper...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C(F)F)c2ccc(Cl)cc12 Show InChI InChI=1S/C20H20ClF2N3O3S/c1-29-19-5-3-14(11-18(19)25-8-6-24-7-9-25)30(27,28)26-12-16(20(22)23)15-4-2-13(21)10-17(15)26/h2-5,10-12,20,24H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340352
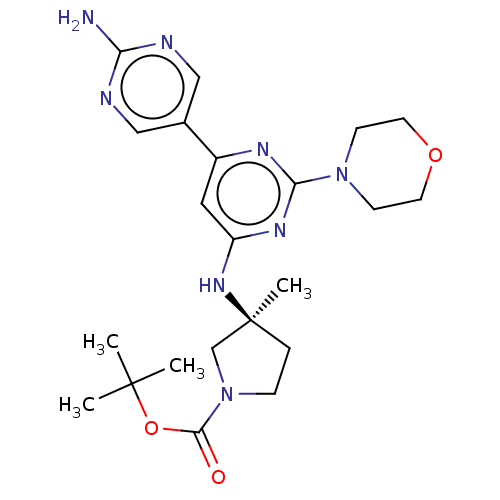
(US9758538, Example 40)Show SMILES CC(C)(C)OC(=O)N1CC[C@@](C)(C1)Nc1cc(nc(n1)N1CCOCC1)-c1cnc(N)nc1 |r| Show InChI InChI=1S/C22H32N8O3/c1-21(2,3)33-20(31)30-6-5-22(4,14-30)28-17-11-16(15-12-24-18(23)25-13-15)26-19(27-17)29-7-9-32-10-8-29/h11-13H,5-10,14H2,1-4H3,(H2,23,24,25)(H,26,27,28)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207061
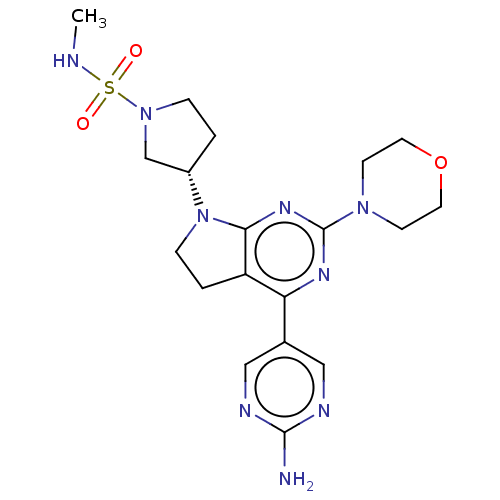
(US9260439, 38)Show SMILES CNS(=O)(=O)N1CC[C@@H](C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C19H27N9O3S/c1-21-32(29,30)27-4-2-14(12-27)28-5-3-15-16(13-10-22-18(20)23-11-13)24-19(25-17(15)28)26-6-8-31-9-7-26/h10-11,14,21H,2-9,12H2,1H3,(H2,20,22,23)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(MOUSE) | BDBM50033536

(CHEMBL121494 | N-[(3R,4R)-1-((R)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@@H]1CCN(C[C@H](O)c2ccccc2)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340347
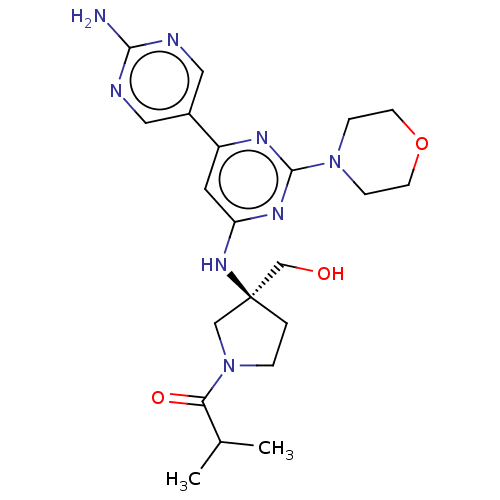
(US9758538, Example 35)Show SMILES CC(C)C(=O)N1CC[C@@](CO)(C1)Nc1cc(nc(n1)N1CCOCC1)-c1cnc(N)nc1 |r| Show InChI InChI=1S/C21H30N8O3/c1-14(2)18(31)29-4-3-21(12-29,13-30)27-17-9-16(15-10-23-19(22)24-11-15)25-20(26-17)28-5-7-32-8-6-28/h9-11,14,30H,3-8,12-13H2,1-2H3,(H2,22,23,24)(H,25,26,27)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50033530
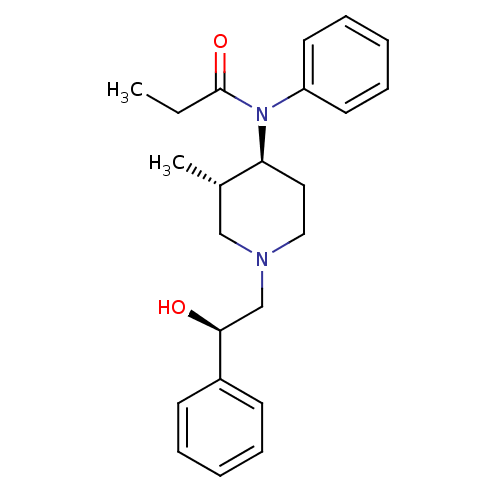
(CHEMBL121211 | N-[(3S,4S)-1-((R)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@H]1CCN(C[C@H](O)c2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207043
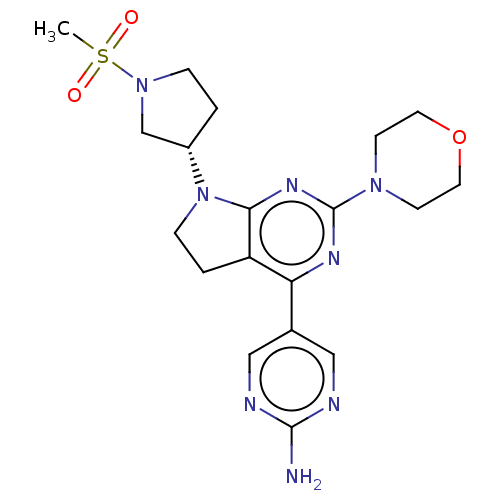
(US9260439, 20)Show SMILES CS(=O)(=O)N1CC[C@@H](C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C19H26N8O3S/c1-31(28,29)26-4-2-14(12-26)27-5-3-15-16(13-10-21-18(20)22-11-13)23-19(24-17(15)27)25-6-8-30-9-7-25/h10-11,14H,2-9,12H2,1H3,(H2,20,21,22)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50033530

(CHEMBL121211 | N-[(3S,4S)-1-((R)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@H]1CCN(C[C@H](O)c2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | CHEMBL5276586

| UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50430806
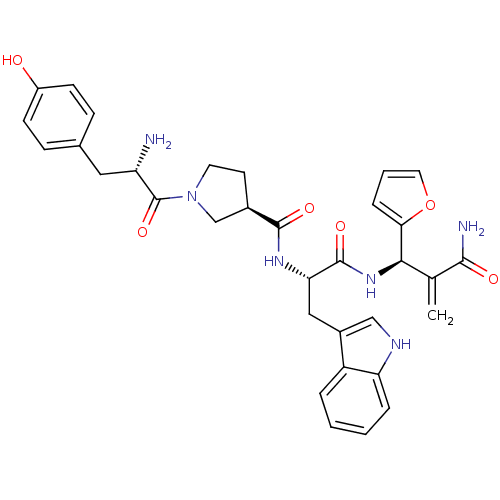
(CHEMBL2334771)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CC[C@H](C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccco1 |r| Show InChI InChI=1S/C33H36N6O6/c1-19(30(35)41)29(28-7-4-14-45-28)38-32(43)27(16-22-17-36-26-6-3-2-5-24(22)26)37-31(42)21-12-13-39(18-21)33(44)25(34)15-20-8-10-23(40)11-9-20/h2-11,14,17,21,25,27,29,36,40H,1,12-13,15-16,18,34H2,(H2,35,41)(H,37,42)(H,38,43)/t21-,25+,27+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... |
J Med Chem 56: 3102-14 (2013)
Article DOI: 10.1021/jm400195y
BindingDB Entry DOI: 10.7270/Q2KS6SW8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50592784
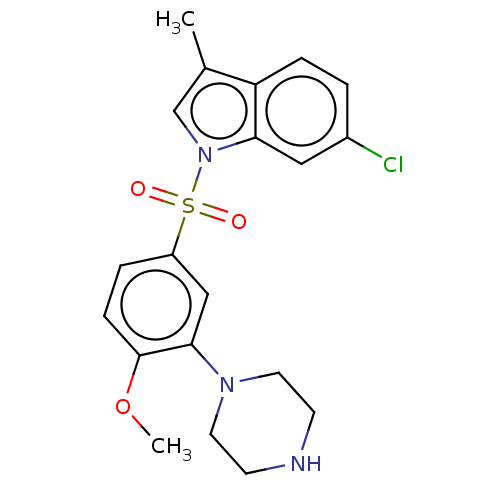
(CHEMBL5177311)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C)c2ccc(Cl)cc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | CHEMBL5269400
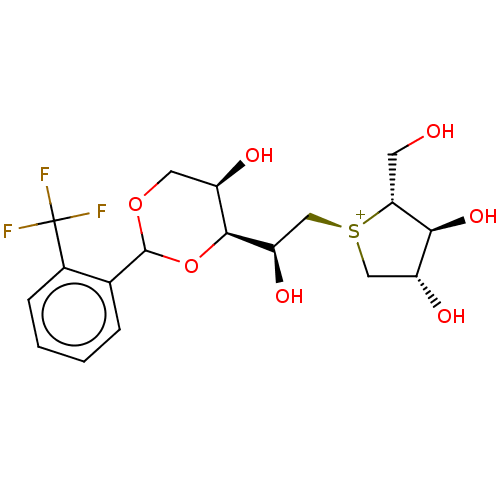
| UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12676

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H20F4N8O2/c1-34(2)12-21-30-7-8-35(21)13-3-5-17(16(25)10-13)31-23(37)18-11-20(24(26,27)28)32-36(18)14-4-6-19-15(9-14)22(29)33-38-19/h3-11H,12H2,1-2H3,(H2,29,33)(H,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50333737
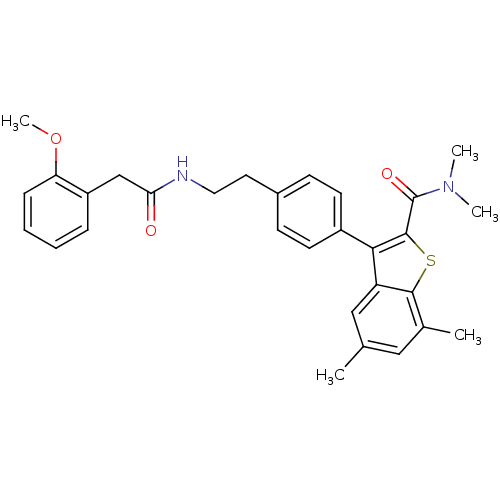
(3-(4-(2-(2-(2-methoxyphenyl)acetamido)ethyl)phenyl...)Show SMILES COc1ccccc1CC(=O)NCCc1ccc(cc1)-c1c(sc2c(C)cc(C)cc12)C(=O)N(C)C Show InChI InChI=1S/C30H32N2O3S/c1-19-16-20(2)28-24(17-19)27(29(36-28)30(34)32(3)4)22-12-10-21(11-13-22)14-15-31-26(33)18-23-8-6-7-9-25(23)35-5/h6-13,16-17H,14-15,18H2,1-5H3,(H,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP4 receptor |
Bioorg Med Chem Lett 21: 734-7 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.118
BindingDB Entry DOI: 10.7270/Q22N52JP |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383163
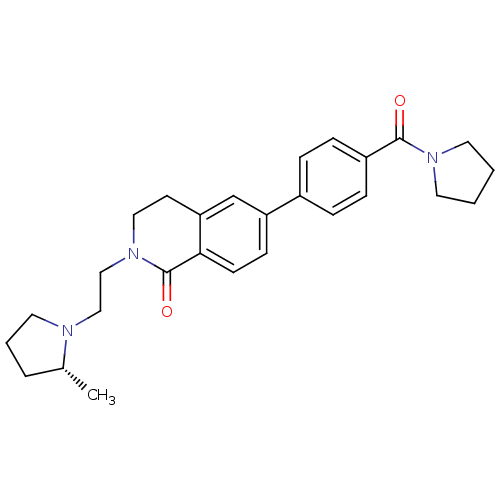
(CHEMBL2031885)Show SMILES C[C@@H]1CCCN1CCN1CCc2cc(ccc2C1=O)-c1ccc(cc1)C(=O)N1CCCC1 |r| Show InChI InChI=1S/C27H33N3O2/c1-20-5-4-15-28(20)17-18-30-16-12-24-19-23(10-11-25(24)27(30)32)21-6-8-22(9-7-21)26(31)29-13-2-3-14-29/h6-11,19-20H,2-5,12-18H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421256
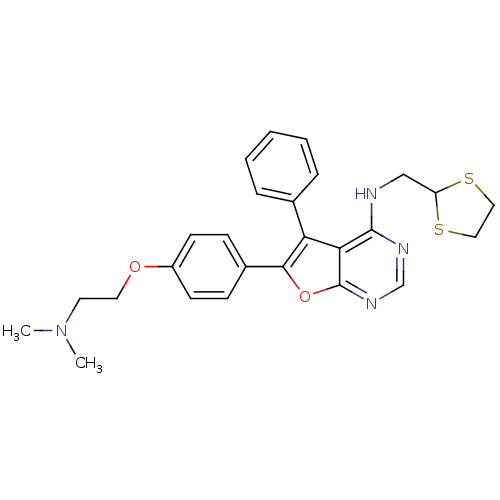
(CHEMBL2087874)Show SMILES CN(C)CCOc1ccc(cc1)-c1oc2ncnc(NCC3SCCS3)c2c1-c1ccccc1 Show InChI InChI=1S/C26H28N4O2S2/c1-30(2)12-13-31-20-10-8-19(9-11-20)24-22(18-6-4-3-5-7-18)23-25(28-17-29-26(23)32-24)27-16-21-33-14-15-34-21/h3-11,17,21H,12-16H2,1-2H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218305

(3-chloro-N-((1S,2R)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1[C@@H](Cc2ccccc12)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C30H23ClN4O3/c31-24-17-32-25-16-20(10-13-23(24)25)30(38)34-28-22-6-2-1-5-19(22)15-26(28)33-29(37)18-8-11-21(12-9-18)35-14-4-3-7-27(35)36/h1-14,16-17,26,28,32H,15H2,(H,33,37)(H,34,38)/t26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328379
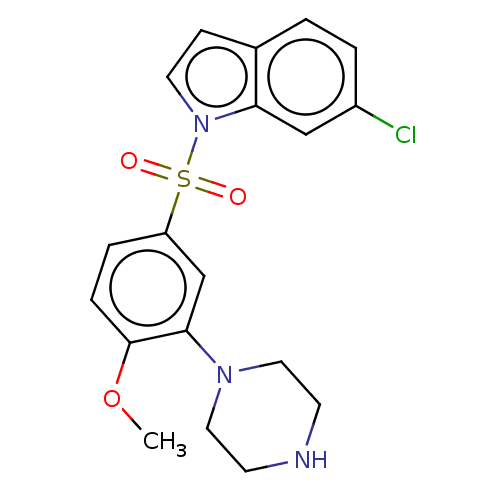
(6-chloro-1-((4-methoxy-3-(piperazin-1-yl)phenyl)su...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1ccc2ccc(Cl)cc12 Show InChI InChI=1S/C19H20ClN3O3S/c1-26-19-5-4-16(13-18(19)22-10-7-21-8-11-22)27(24,25)23-9-6-14-2-3-15(20)12-17(14)23/h2-6,9,12-13,21H,7-8,10-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | CHEMBL5268739

| UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50319837
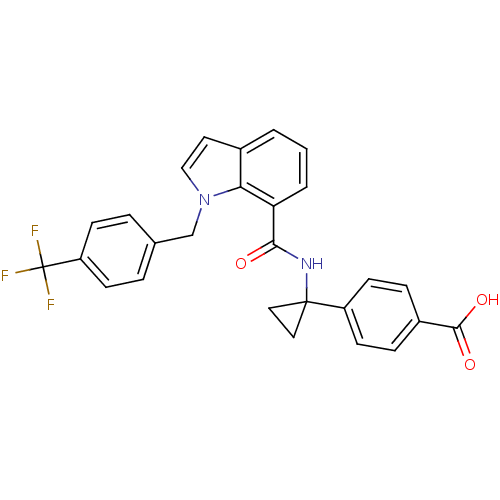
(4-(1-(1-(4-(trifluoromethyl)benzyl)-1H-indole-7-ca...)Show SMILES OC(=O)c1ccc(cc1)C1(CC1)NC(=O)c1cccc2ccn(Cc3ccc(cc3)C(F)(F)F)c12 Show InChI InChI=1S/C27H21F3N2O3/c28-27(29,30)21-8-4-17(5-9-21)16-32-15-12-18-2-1-3-22(23(18)32)24(33)31-26(13-14-26)20-10-6-19(7-11-20)25(34)35/h1-12,15H,13-14,16H2,(H,31,33)(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in HEK293-EBNA cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 3760-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.065
BindingDB Entry DOI: 10.7270/Q20P106K |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | CHEMBL5282029

| UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50245852

(CHEMBL458333 | N3-(2,6-dimethylphenyl)-1-(3-methox...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccc(OCCN4CCCC4)cc3)nc12 Show InChI InChI=1S/C31H41N7O2/c1-22-9-8-10-23(2)27(22)34-28-26-21-32-30(35-29(26)38(36-28)18-15-31(3,4)39-5)33-24-11-13-25(14-12-24)40-20-19-37-16-6-7-17-37/h8-14,21H,6-7,15-20H2,1-5H3,(H,34,36)(H,32,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50592785
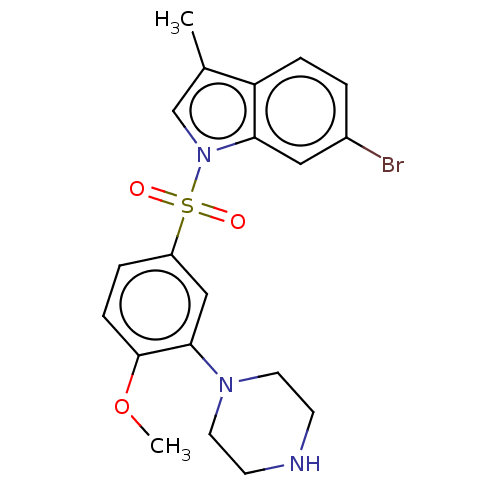
(CHEMBL5206617)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C)c2ccc(Br)cc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50130285
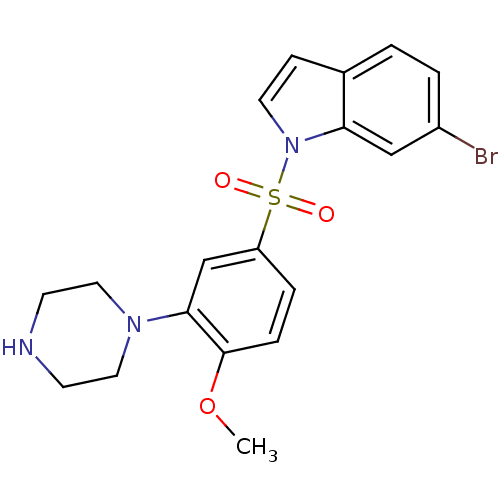
(6-Bromo-1-(4-methoxy-3-piperazin-1-yl-benzenesulfo...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1ccc2ccc(Br)cc12 Show InChI InChI=1S/C19H20BrN3O3S/c1-26-19-5-4-16(13-18(19)22-10-7-21-8-11-22)27(24,25)23-9-6-14-2-3-15(20)12-17(14)23/h2-6,9,12-13,21H,7-8,10-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data