

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50255365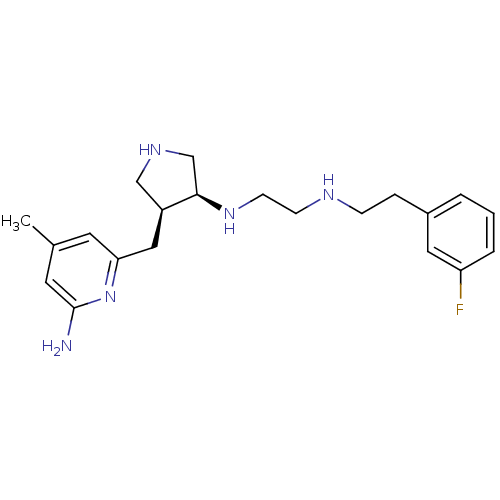 ((+/-)-cis-N1-((3S,4S)-4-((6-amino-4-methylpyridin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat recombinant nNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50278675 ((+/-)-cis-6-((4-(2-(3-Fluorophenethylamino)-ethoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat recombinant nNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM50537592 (CHEMBL4632881) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y181C reverse transcriptase. | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50278676 ((+/-)-cis-N-(4-((6-Amino-4-methylpyridin-2-yl)-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat recombinant nNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521018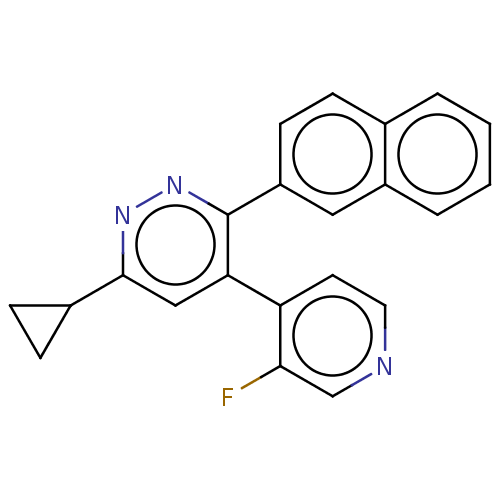 (US11149020, Compound 10 (MW-167)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521021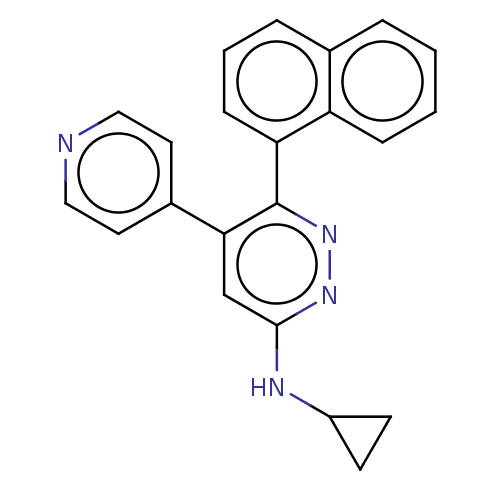 (US11149020, Compound 13 (MW-107)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521019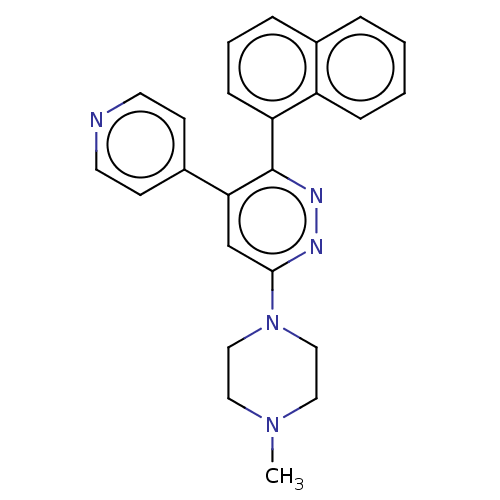 (US11149020, Compound 11 (MW-122)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521025 (US11149020, Compound 16 (MW-200)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537600 (CHEMBL4129018 | US11149020, Compound 27 (MW-150)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y188L reverse transcriptase. | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521045 (US11149020, Compound 36 (MW-164)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537600 (CHEMBL4129018 | US11149020, Compound 27 (MW-150)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537599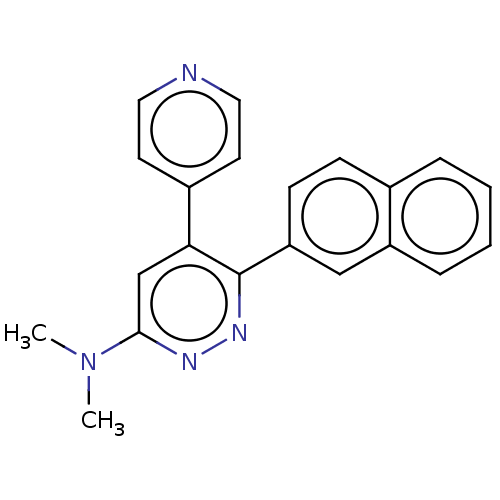 (CHEMBL4648060 | US11149020, Compound 2 (MW-108)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase G190A | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537599 (CHEMBL4648060 | US11149020, Compound 2 (MW-108)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521017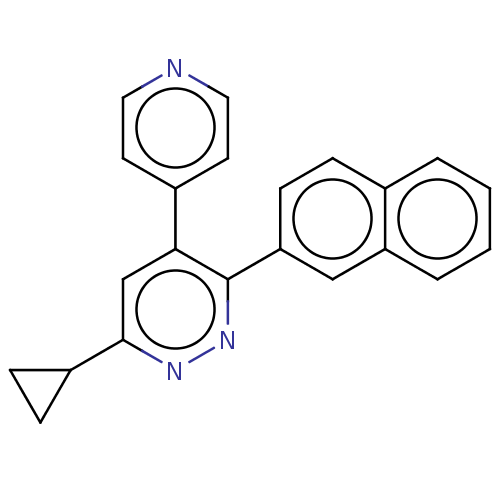 (US11149020, Compound 9 (MW-125)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537598 (CHEMBL4646628 | US11149020, Compound 1 (MW-181)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase P236L | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537598 (CHEMBL4646628 | US11149020, Compound 1 (MW-181)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521016 (US11149020, Compound 7 (MW-077)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521024 (US11149020, Compound 15 (MW-156)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 276 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11 (Homo sapiens (Human)) | BDBM50537598 (CHEMBL4646628 | US11149020, Compound 1 (MW-181)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y188L reverse transcriptase. | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521047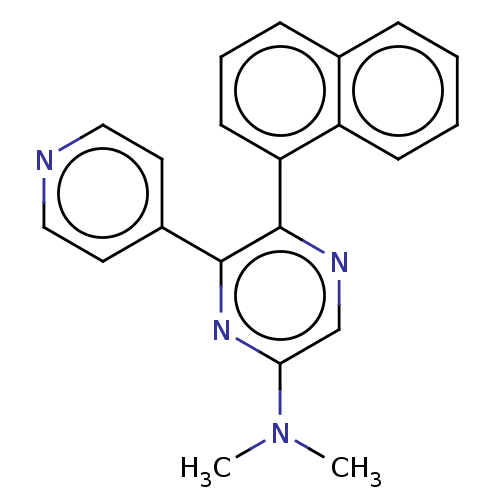 (N,N-dimethyl-5-(naphthalen-1-yl)-6-(pyridin-4-yl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 343 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50278744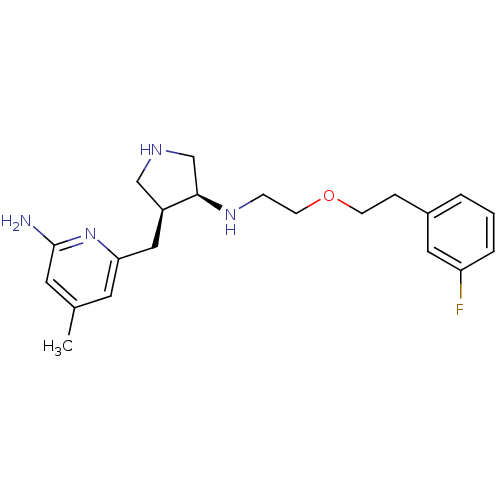 ((+/-)-cis-6-((4-(2-(3-Fluorophenethoxy)ethylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat recombinant nNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537592 (CHEMBL4632881) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase K103N | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537597 (CHEMBL4645737 | US11149020, Compound 6 (MW-105)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 657 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50278745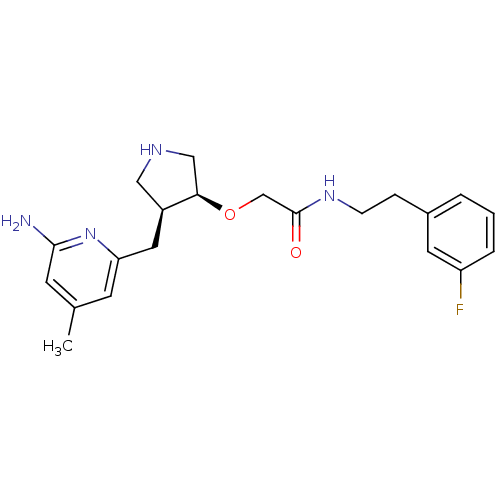 ((+/-)-cis-2-(4-((6-Amino-4-methylpyridin-2-yl)-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat recombinant nNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50255365 ((+/-)-cis-N1-((3S,4S)-4-((6-amino-4-methylpyridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant iNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50278744 ((+/-)-cis-6-((4-(2-(3-Fluorophenethoxy)ethylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant iNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50278746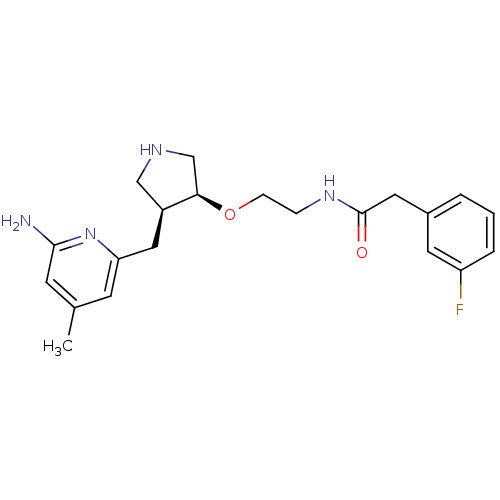 ((+/-)-cis-N-(2-(4-((6-Amino-4-methylpyridin-2-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat recombinant nNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50278676 ((+/-)-cis-N-(4-((6-Amino-4-methylpyridin-2-yl)-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant iNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50278675 ((+/-)-cis-6-((4-(2-(3-Fluorophenethylamino)-ethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant iNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50278676 ((+/-)-cis-N-(4-((6-Amino-4-methylpyridin-2-yl)-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of bovine recombinant eNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50255365 ((+/-)-cis-N1-((3S,4S)-4-((6-amino-4-methylpyridin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of bovine recombinant eNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50278675 ((+/-)-cis-6-((4-(2-(3-Fluorophenethylamino)-ethoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of bovine recombinant eNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50278744 ((+/-)-cis-6-((4-(2-(3-Fluorophenethoxy)ethylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of bovine recombinant eNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50278745 ((+/-)-cis-2-(4-((6-Amino-4-methylpyridin-2-yl)-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant iNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50278746 ((+/-)-cis-N-(2-(4-((6-Amino-4-methylpyridin-2-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant iNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50278746 ((+/-)-cis-N-(2-(4-((6-Amino-4-methylpyridin-2-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of bovine recombinant eNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50278745 ((+/-)-cis-2-(4-((6-Amino-4-methylpyridin-2-yl)-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of bovine recombinant eNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myosin light chain kinase, smooth muscle (Gallus gallus (chicken)) | BDBM50074653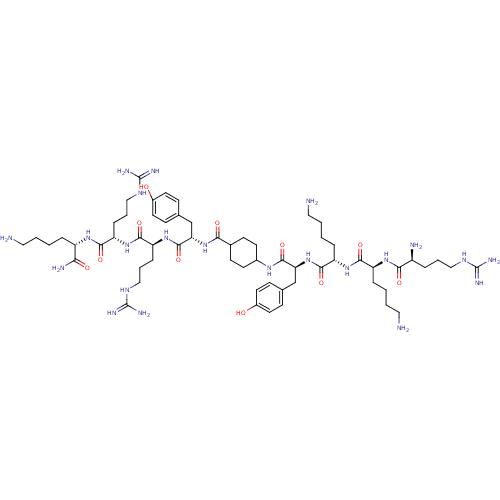 (CHEMBL385812 | R-K-K-Y-(Ach)-Y-R-R-K-NH2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against MLCK (smooth muscle myosin light chain kinase) | J Med Chem 42: 910-9 (1999) Article DOI: 10.1021/jm980573a BindingDB Entry DOI: 10.7270/Q2JS9R4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM50537594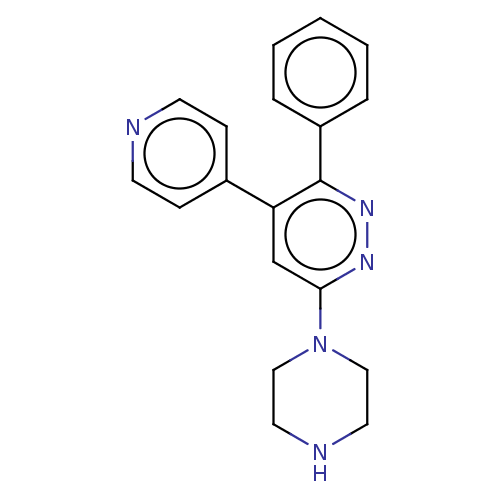 (CHEMBL4639028) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y181C reverse transcriptase. | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myosin light chain kinase, smooth muscle (Gallus gallus (chicken)) | BDBM50074638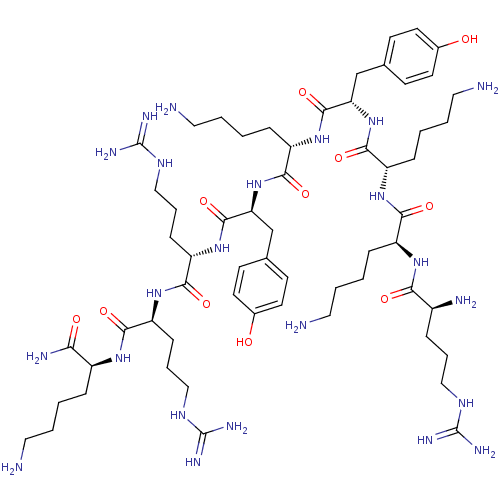 (CHEMBL443574 | R-K-K-Y-K-Y-R-R-K-NH2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against MLCK (smooth muscle myosin light chain kinase) | J Med Chem 42: 910-9 (1999) Article DOI: 10.1021/jm980573a BindingDB Entry DOI: 10.7270/Q2JS9R4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myosin light chain kinase, smooth muscle (Gallus gallus (chicken)) | BDBM50074648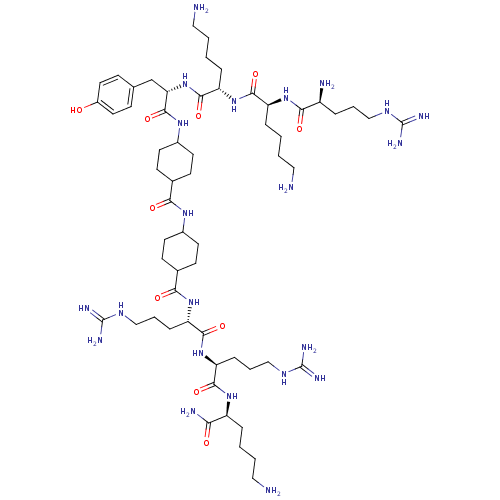 (CHEMBL268790 | R-K-K-Y-(Ach)-(Ach)-R-R-K-NH2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against MLCK (smooth muscle myosin light chain kinase) | J Med Chem 42: 910-9 (1999) Article DOI: 10.1021/jm980573a BindingDB Entry DOI: 10.7270/Q2JS9R4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM50537593 (CHEMBL4639555 | US11149020, Compound 3 (MW-066)) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 wild-type reverse transcriptase. | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537606 (CHEMBL4647072 | US11149020, Compound 34 (MW-154)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myosin light chain kinase, smooth muscle (Gallus gallus (chicken)) | BDBM50074635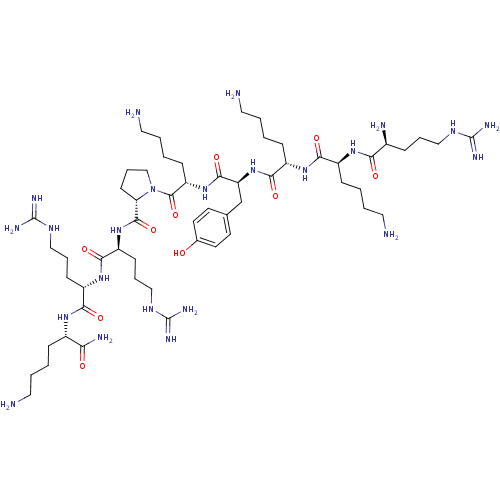 (CHEMBL425624 | R-K-K-Y-K-P-R-R-K-NH2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against MLCK (smooth muscle myosin light chain kinase) | J Med Chem 42: 910-9 (1999) Article DOI: 10.1021/jm980573a BindingDB Entry DOI: 10.7270/Q2JS9R4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM50537593 (CHEMBL4639555 | US11149020, Compound 3 (MW-066)) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521035 (US11149020, Compound 26 (MW-025)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521022 (US11149020, Compound 14 (MW-109)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537594 (CHEMBL4639028) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase G190A | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myosin light chain kinase, smooth muscle (Gallus gallus (chicken)) | BDBM50074657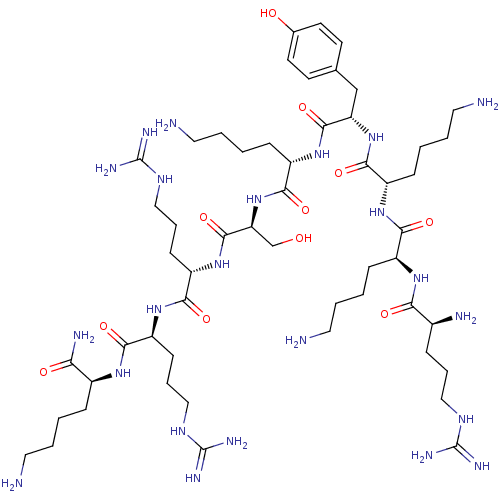 (CHEMBL267348 | R-K-K-Y-K-S-R-R-K-NH2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against MLCK (smooth muscle myosin light chain kinase) | J Med Chem 42: 910-9 (1999) Article DOI: 10.1021/jm980573a BindingDB Entry DOI: 10.7270/Q2JS9R4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myosin light chain kinase, smooth muscle (Gallus gallus (chicken)) | BDBM50074644 (CHEMBL409544 | R-K-K-Y-K-F-R-R-K-NH2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against MLCK (smooth muscle myosin light chain kinase) | J Med Chem 42: 910-9 (1999) Article DOI: 10.1021/jm980573a BindingDB Entry DOI: 10.7270/Q2JS9R4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 330 total ) | Next | Last >> |