

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50408442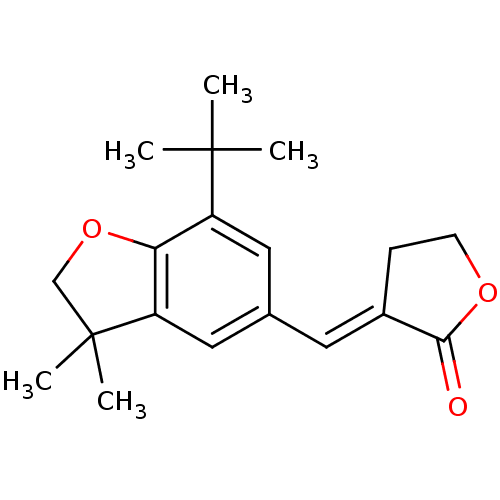 (CHEMBL2112567) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 2 (COX-2) | J Med Chem 41: 3515-29 (1998) Article DOI: 10.1021/jm9802416 BindingDB Entry DOI: 10.7270/Q2V98763 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28674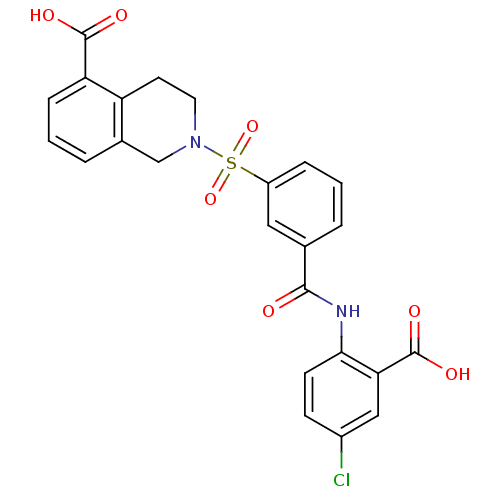 (2-({3-[(2-carboxy-4-chlorophenyl)carbamoyl]benzene...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | 1.26E+3 | n/a | n/a | 7.0 | 22 |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28691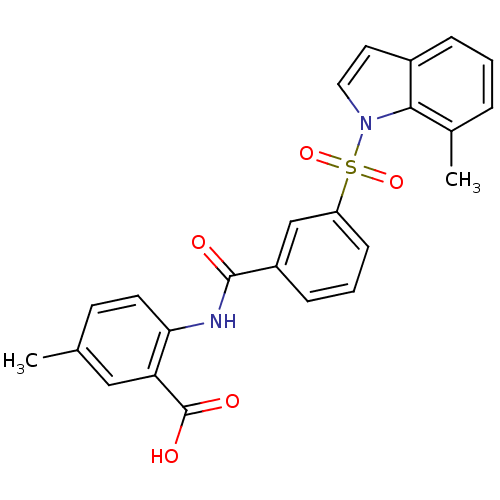 (5-methyl-2-({3-[(7-methyl-1H-indole-1-)sulfonyl]be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | 79 | n/a | n/a | n/a | n/a |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28661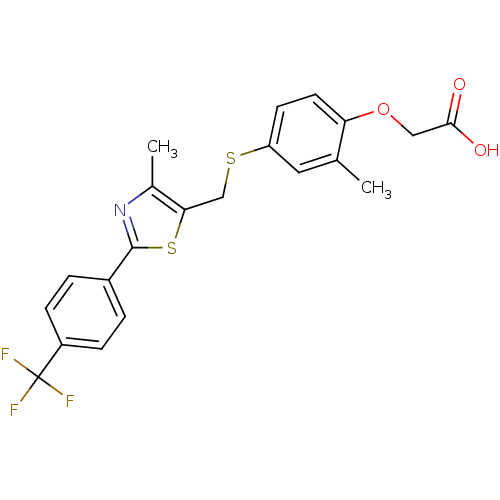 (2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]GW 2433 from human PPARdelta by scintillation proximity assay | Bioorg Med Chem Lett 21: 2345-50 (2011) Article DOI: 10.1016/j.bmcl.2011.02.077 BindingDB Entry DOI: 10.7270/Q2M61MHT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28661 (2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | 3 | n/a | n/a | 7.0 | 22 |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28670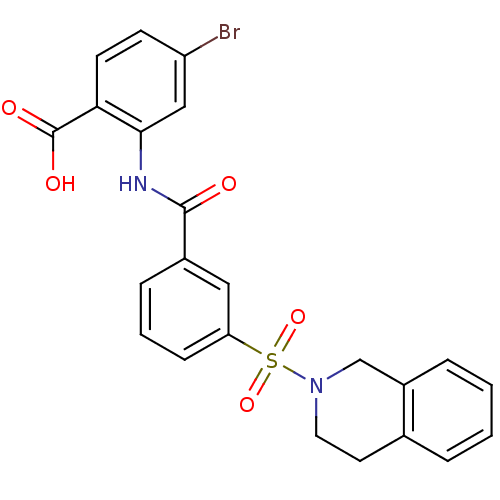 (4-bromo-2-{[3-(1,2,3,4-tetrahydroisoquinoline-2-su...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | 158 | n/a | n/a | 7.0 | 22 |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28669 (2-{[3-(1,2,3,4-tetrahydroisoquinoline-2-sulfonyl)b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | 158 | n/a | n/a | 7.0 | 22 |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28682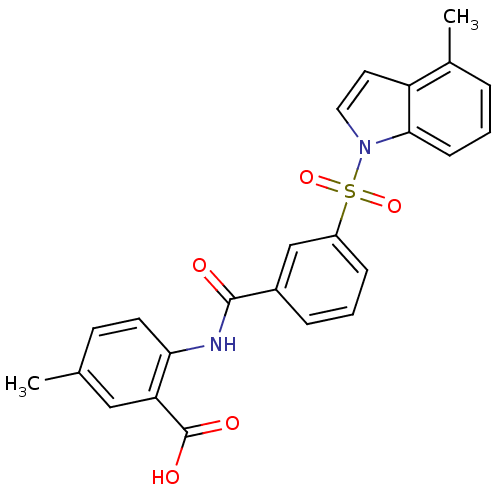 (5-methyl-2-({3-[(4-methyl-1H-indole-1-)sulfonyl]be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | 20 | n/a | n/a | n/a | n/a |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28695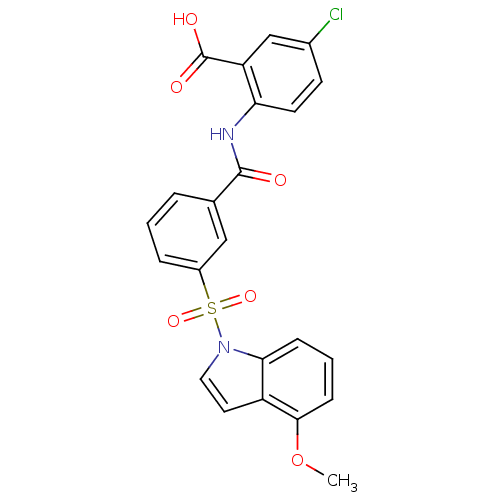 (5-chloro-2-({3-[(4-methoxy-1H-indole-1-)sulfonyl]b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | 158 | n/a | n/a | n/a | n/a |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50418124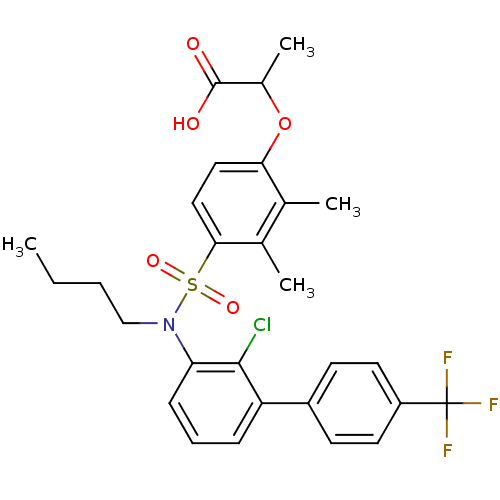 (CHEMBL1760428) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]GW 2433 from human PPARdelta by scintillation proximity assay | Bioorg Med Chem Lett 21: 2345-50 (2011) Article DOI: 10.1016/j.bmcl.2011.02.077 BindingDB Entry DOI: 10.7270/Q2M61MHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50063777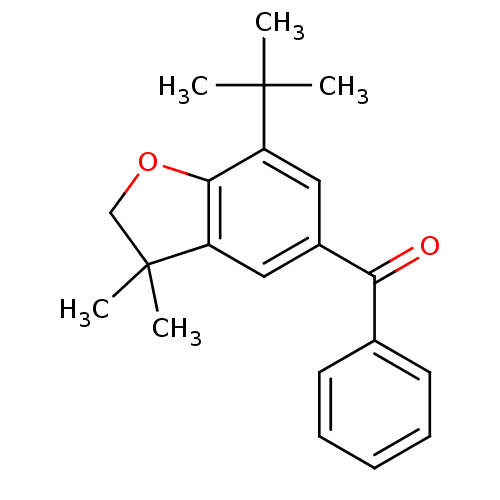 ((7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofuran-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 2 (COX-2) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 4 (Mus musculus) | BDBM50044874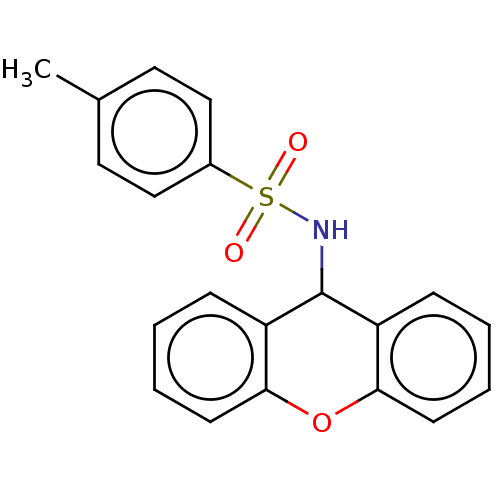 (CHEMBL3311302) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of mouse FFA4 receptor expressed in U2OS cells | Bioorg Med Chem Lett 24: 3100-3 (2014) Article DOI: 10.1016/j.bmcl.2014.05.012 BindingDB Entry DOI: 10.7270/Q2CC1292 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50418148 (CHEMBL1760411) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]GW 2433 from human PPARdelta by scintillation proximity assay | Bioorg Med Chem Lett 21: 2345-50 (2011) Article DOI: 10.1016/j.bmcl.2011.02.077 BindingDB Entry DOI: 10.7270/Q2M61MHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 4 (Rattus norvegicus) | BDBM50044874 (CHEMBL3311302) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of rat FFA4 receptor expressed in U2OS cells | Bioorg Med Chem Lett 24: 3100-3 (2014) Article DOI: 10.1016/j.bmcl.2014.05.012 BindingDB Entry DOI: 10.7270/Q2CC1292 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28666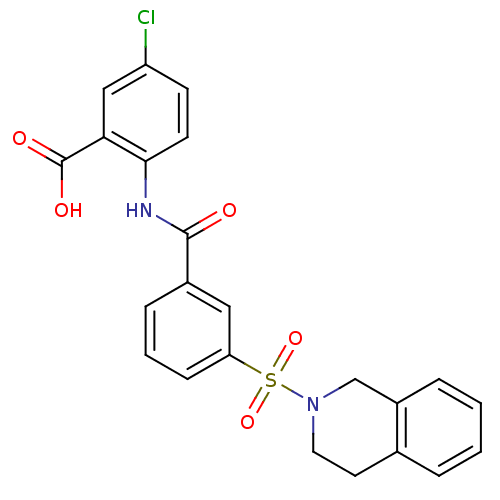 (5-chloro-2-{[3-(1,2,3,4-tetrahydroisoquinoline-2-s...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | 126 | n/a | n/a | 7.0 | 22 |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28667 (5-bromo-2-{[3-(1,2,3,4-tetrahydroisoquinoline-2-su...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | 200 | n/a | n/a | 7.0 | 22 |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28688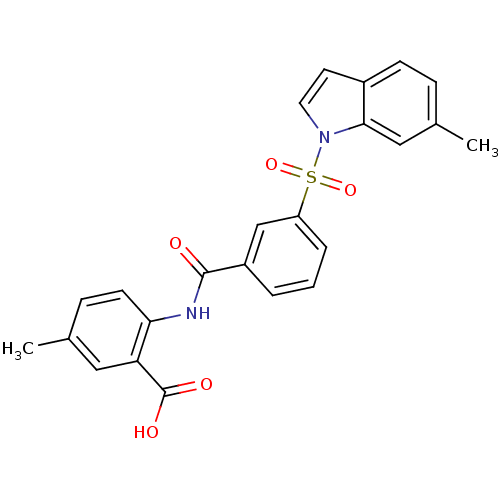 (5-methyl-2-({3-[(6-methyl-1H-indole-1-)sulfonyl]be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | 50 | n/a | n/a | n/a | n/a |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28685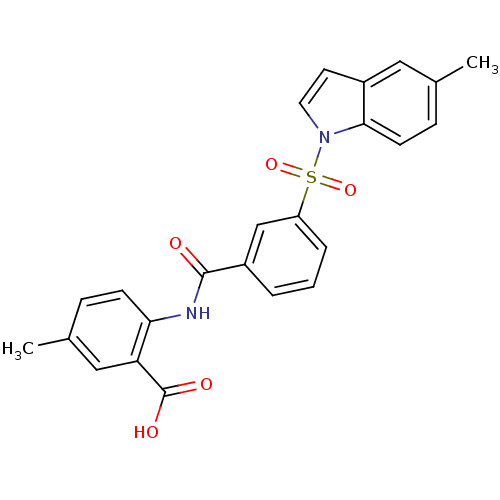 (5-methyl-2-({3-[(5-methyl-1H-indole-1-)sulfonyl]be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | 32 | n/a | n/a | n/a | n/a |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28684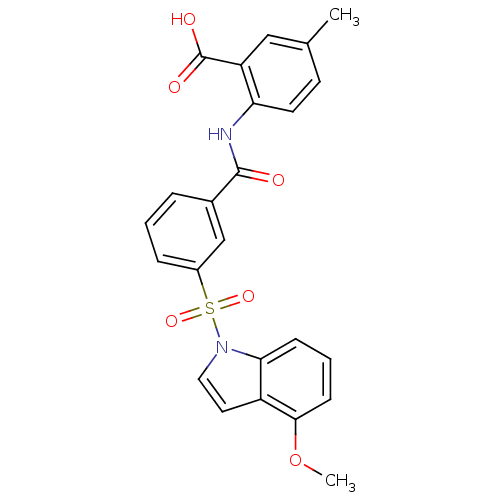 (2-({3-[(4-methoxy-1H-indole-1-)sulfonyl]benzene}am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | 40 | n/a | n/a | n/a | n/a |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28676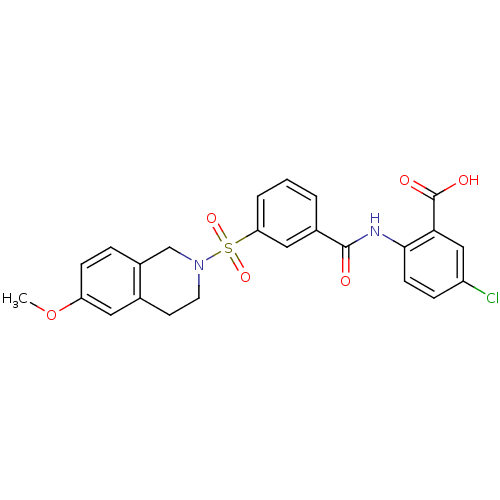 (5-chloro-2-({3-[(6-methoxy-1,2,3,4-tetrahydroisoqu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | 794 | n/a | n/a | 7.0 | 22 |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28675 (2-[(3-{[5-(carboxymethoxy)-1,2,3,4-tetrahydroisoqu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | >1.00E+4 | n/a | n/a | 7.0 | 22 |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28683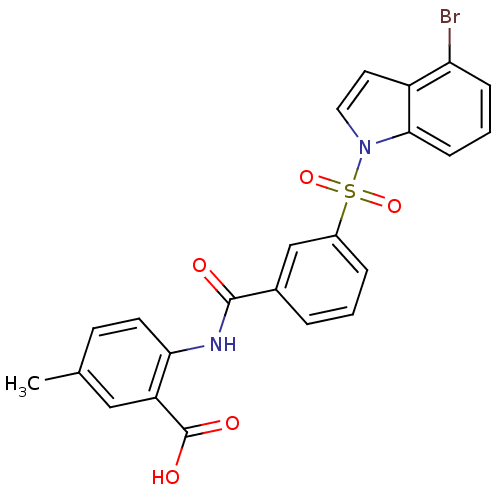 (2-({3-[(4-bromo-1H-indole-1-)sulfonyl]benzene}amid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | 13 | n/a | n/a | n/a | n/a |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28696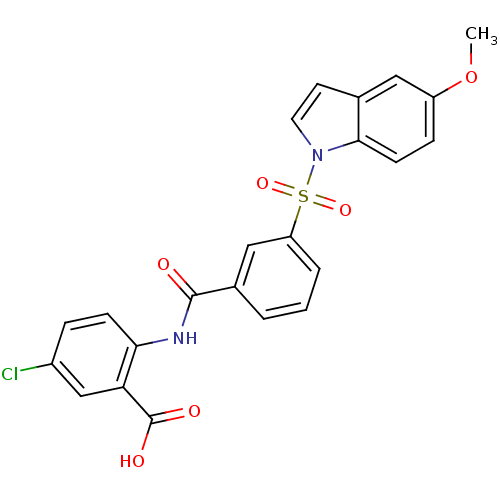 (5-chloro-2-({3-[(5-methoxy-1H-indole-1-)sulfonyl]b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | 158 | n/a | n/a | n/a | n/a |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28672 (5-chloro-2-({3-[(5-methoxy-1,2,3,4-tetrahydroisoqu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | 1.26E+3 | n/a | n/a | 7.0 | 22 |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28665 (5-methyl-2-{[3-(1,2,3,4-tetrahydroisoquinoline-2-s...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | 200 | n/a | n/a | 7.0 | 22 |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50418129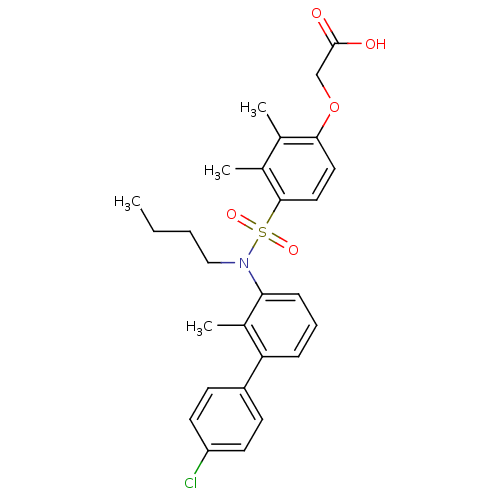 (CHEMBL1760268) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]GW 2433 from human PPARdelta by scintillation proximity assay | Bioorg Med Chem Lett 21: 2345-50 (2011) Article DOI: 10.1016/j.bmcl.2011.02.077 BindingDB Entry DOI: 10.7270/Q2M61MHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28697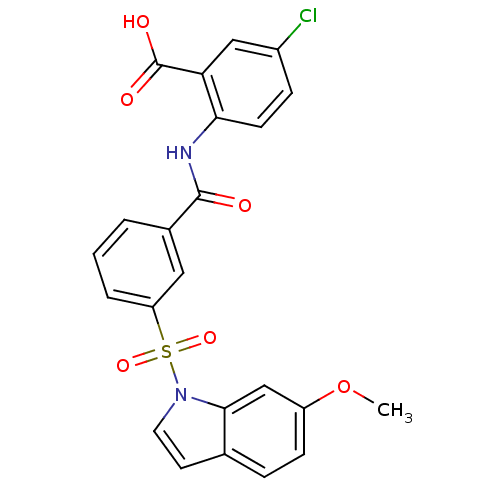 (5-chloro-2-({3-[(6-methoxy-1H-indole-1-)sulfonyl]b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | 100 | n/a | n/a | n/a | n/a |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50063780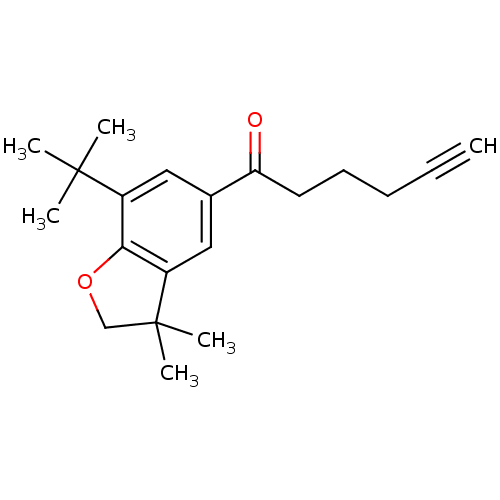 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 2 (COX-2) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50066572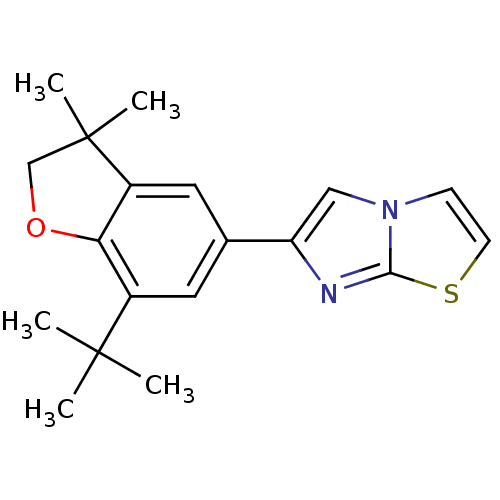 (6-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 2 (COX-2) | J Med Chem 41: 3515-29 (1998) Article DOI: 10.1021/jm9802416 BindingDB Entry DOI: 10.7270/Q2V98763 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50418123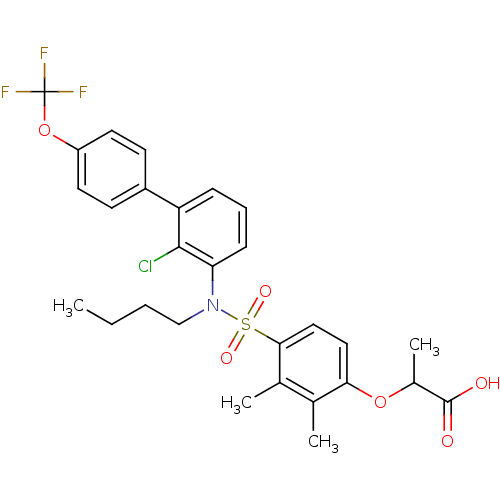 (CHEMBL1760005) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]GW 2433 from human PPARdelta by scintillation proximity assay | Bioorg Med Chem Lett 21: 2345-50 (2011) Article DOI: 10.1016/j.bmcl.2011.02.077 BindingDB Entry DOI: 10.7270/Q2M61MHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28677 (5-chloro-2-({3-[(7-fluoro-1,2,3,4-tetrahydroisoqui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | 1.00E+3 | n/a | n/a | 7.0 | 22 |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28698 (5-chloro-2-({3-[(5-methoxy-4-methyl-1H-indole-1-)s...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | 16 | n/a | n/a | n/a | n/a |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50418161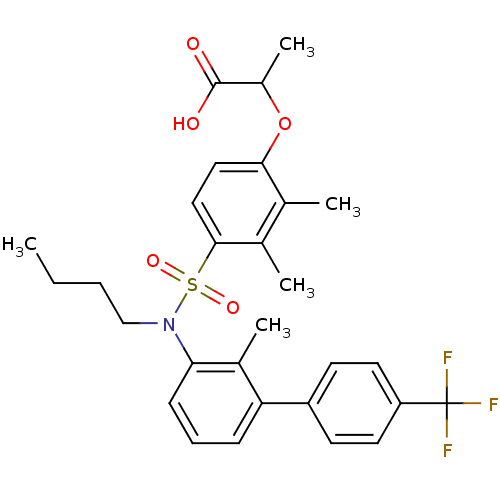 (CHEMBL1760424) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]GW 2433 from human PPARdelta by scintillation proximity assay | Bioorg Med Chem Lett 21: 2345-50 (2011) Article DOI: 10.1016/j.bmcl.2011.02.077 BindingDB Entry DOI: 10.7270/Q2M61MHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28679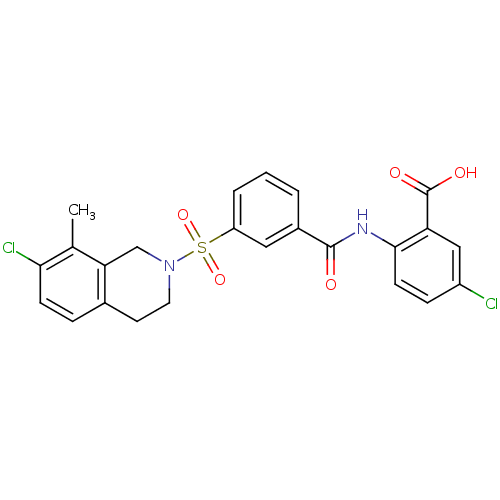 (5-chloro-2-({3-[(7-chloro-8-methyl-1,2,3,4-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | 316 | n/a | n/a | 7.0 | 22 |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28692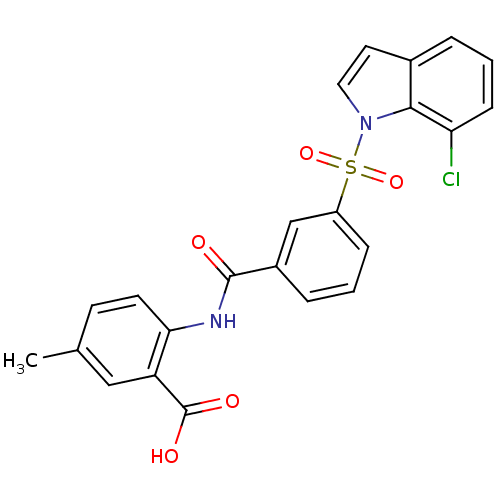 (2-({3-[(7-chloro-1H-indole-1-)sulfonyl]benzene}ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | 158 | n/a | n/a | n/a | n/a |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50418135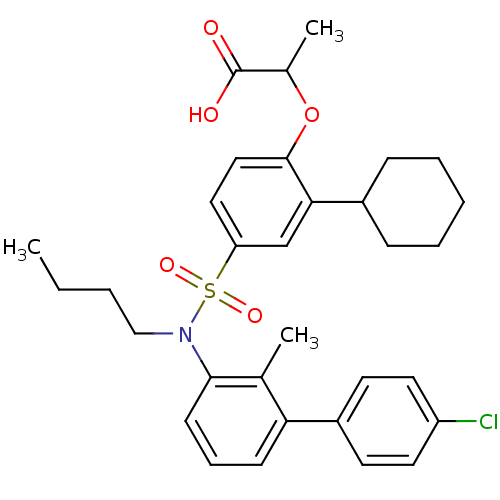 (CHEMBL1760274) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]GW 2433 from human PPARdelta by scintillation proximity assay | Bioorg Med Chem Lett 21: 2345-50 (2011) Article DOI: 10.1016/j.bmcl.2011.02.077 BindingDB Entry DOI: 10.7270/Q2M61MHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28673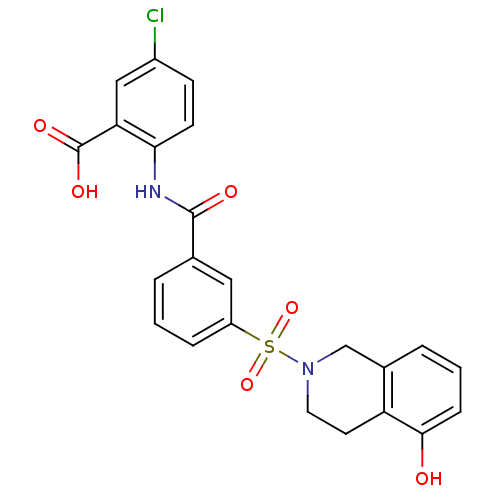 (5-chloro-2-({3-[(5-hydroxy-1,2,3,4-tetrahydroisoqu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | 2.00E+3 | n/a | n/a | 7.0 | 22 |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28694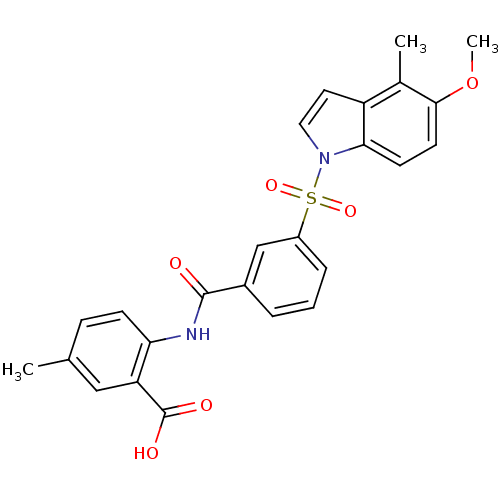 (2-({3-[(5-methoxy-4-methyl-1H-indole-1-)sulfonyl]b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | 13 | n/a | n/a | n/a | n/a |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50066561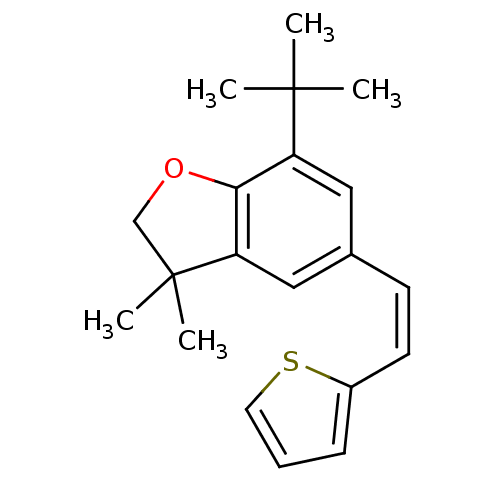 (7-tert-Butyl-3,3-dimethyl-5-((Z)-2-thiophen-2-yl-v...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 (COX-1) | J Med Chem 41: 3515-29 (1998) Article DOI: 10.1021/jm9802416 BindingDB Entry DOI: 10.7270/Q2V98763 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28686 (2-({3-[(5-chloro-1H-indole-1-)sulfonyl]benzene}ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | 50 | n/a | n/a | n/a | n/a |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28668 (5-fluoro-2-{[3-(1,2,3,4-tetrahydroisoquinoline-2-s...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | 501 | n/a | n/a | 7.0 | 22 |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50418149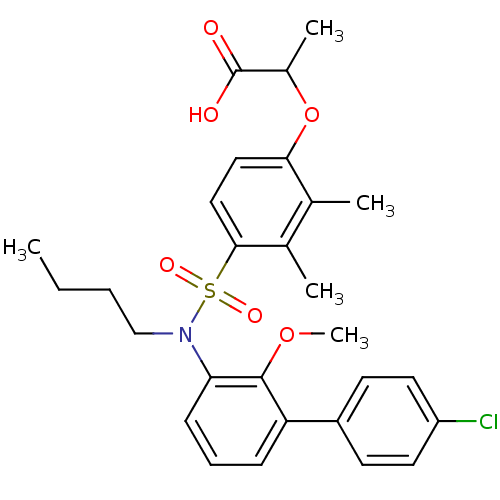 (CHEMBL1760412) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]GW 2433 from human PPARdelta by scintillation proximity assay | Bioorg Med Chem Lett 21: 2345-50 (2011) Article DOI: 10.1016/j.bmcl.2011.02.077 BindingDB Entry DOI: 10.7270/Q2M61MHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28678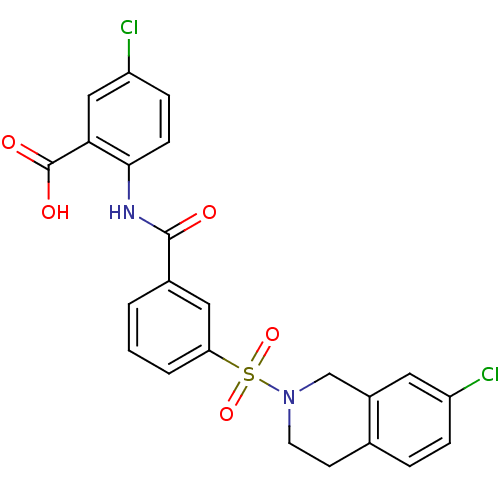 (5-chloro-2-({3-[(7-chloro-1,2,3,4-tetrahydroisoqui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | 794 | n/a | n/a | 7.0 | 22 |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50418134 (CHEMBL1760273) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]GW 2433 from human PPARdelta by scintillation proximity assay | Bioorg Med Chem Lett 21: 2345-50 (2011) Article DOI: 10.1016/j.bmcl.2011.02.077 BindingDB Entry DOI: 10.7270/Q2M61MHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50066564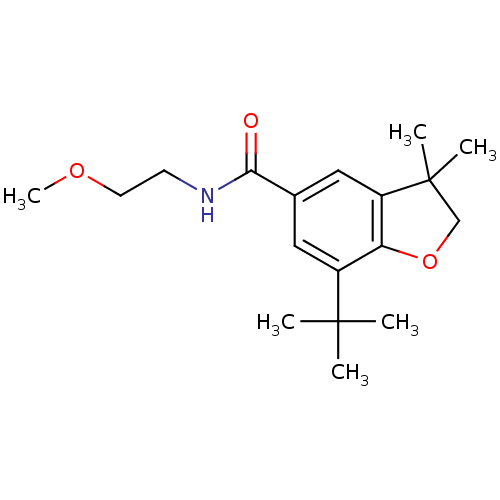 (7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofuran-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 2 (COX-2) | J Med Chem 41: 3515-29 (1998) Article DOI: 10.1021/jm9802416 BindingDB Entry DOI: 10.7270/Q2V98763 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28690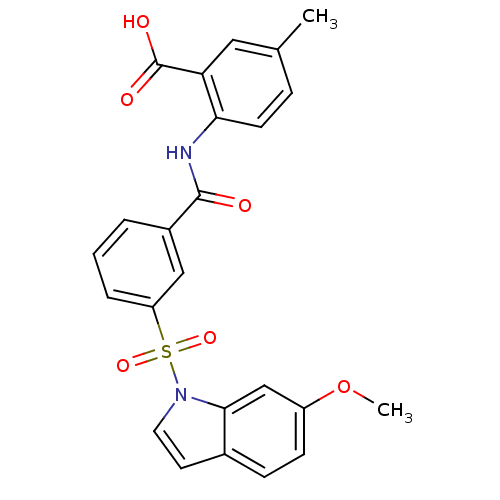 (2-({3-[(6-methoxy-1H-indole-1-)sulfonyl]benzene}am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | 100 | n/a | n/a | n/a | n/a |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50066561 (7-tert-Butyl-3,3-dimethyl-5-((Z)-2-thiophen-2-yl-v...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 2 (COX-2) | J Med Chem 41: 3515-29 (1998) Article DOI: 10.1021/jm9802416 BindingDB Entry DOI: 10.7270/Q2V98763 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50066569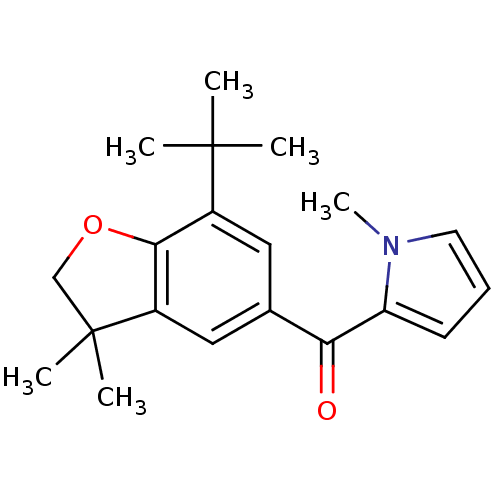 ((7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofuran-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 (COX-1) | J Med Chem 41: 3515-29 (1998) Article DOI: 10.1021/jm9802416 BindingDB Entry DOI: 10.7270/Q2V98763 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50066560 ((7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofuran-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 2 (COX-2) | J Med Chem 41: 3515-29 (1998) Article DOI: 10.1021/jm9802416 BindingDB Entry DOI: 10.7270/Q2V98763 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50063779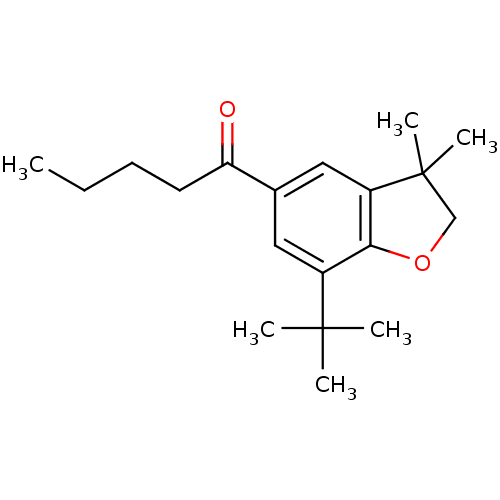 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 2 (COX-2) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 651 total ) | Next | Last >> |