 Found 648 hits with Last Name = 'weatherhead' and Initial = 'g'
Found 648 hits with Last Name = 'weatherhead' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aurora kinase A
(Mus musculus (mouse)) | BDBM50277545

(4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...)Show SMILES COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |c:11| Show InChI InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells in presence of ATP |
ACS Med Chem Lett 6: 630-4 (2015)
Article DOI: 10.1021/ml500409n
BindingDB Entry DOI: 10.7270/Q2WS8W1V |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM31093
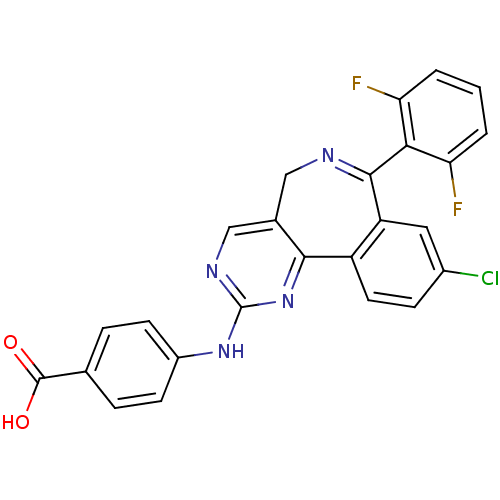
(4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...)Show SMILES OC(=O)c1ccc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2F)cc1 |c:13| Show InChI InChI=1S/C25H15ClF2N4O2/c26-15-6-9-17-18(10-15)23(21-19(27)2-1-3-20(21)28)29-11-14-12-30-25(32-22(14)17)31-16-7-4-13(5-8-16)24(33)34/h1-10,12H,11H2,(H,33,34)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells in presence of ATP |
ACS Med Chem Lett 6: 630-4 (2015)
Article DOI: 10.1021/ml500409n
BindingDB Entry DOI: 10.7270/Q2WS8W1V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Integrase
(Human immunodeficiency virus 1) | BDBM50484379
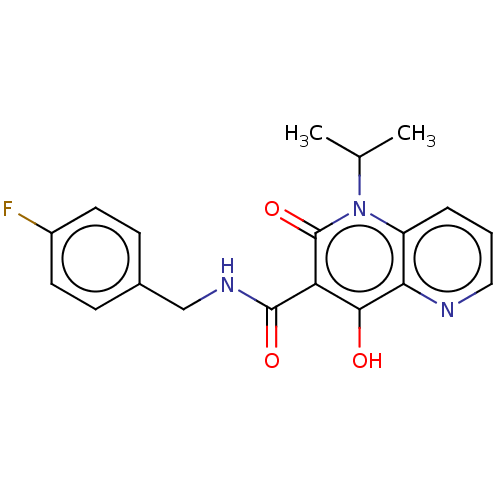
(CHEMBL1917873)Show SMILES CC(C)n1c2cccnc2c(O)c(C(=O)NCc2ccc(F)cc2)c1=O Show InChI InChI=1S/C19H18FN3O3/c1-11(2)23-14-4-3-9-21-16(14)17(24)15(19(23)26)18(25)22-10-12-5-7-13(20)8-6-12/h3-9,11,24H,10H2,1-2H3,(H,22,25) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer by biochemical assay |
Bioorg Med Chem Lett 21: 6461-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.082
BindingDB Entry DOI: 10.7270/Q22Z18CH |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM50277545

(4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...)Show SMILES COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |c:11| Show InChI InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells using biotin-GLRRASLG as substrate in presence of [gamma-33P]ATP |
ACS Med Chem Lett 6: 630-4 (2015)
Article DOI: 10.1021/ml500409n
BindingDB Entry DOI: 10.7270/Q2WS8W1V |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50362985

(CHEMBL1945502)Show SMILES COc1ccc(Nc2ncc3CC(=S)Nc4cc(Cl)ccc4-c3n2)cc1CCCN(C)C Show InChI InChI=1S/C24H26ClN5OS/c1-30(2)10-4-5-15-11-18(7-9-21(15)31-3)27-24-26-14-16-12-22(32)28-20-13-17(25)6-8-19(20)23(16)29-24/h6-9,11,13-14H,4-5,10,12H2,1-3H3,(H,28,32)(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
J Med Chem 55: 197-208 (2012)
Article DOI: 10.1021/jm2011172
BindingDB Entry DOI: 10.7270/Q21C1XBR |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50489299
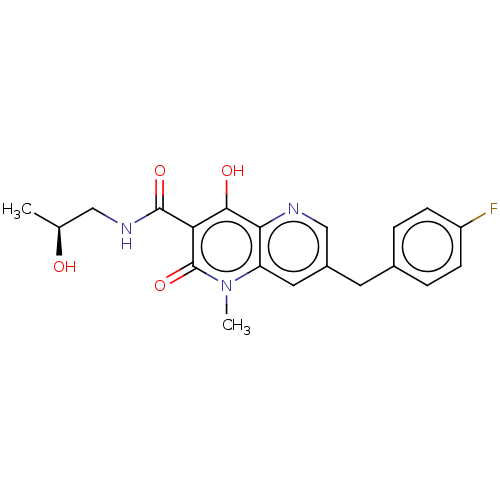
(CHEMBL2316125)Show SMILES C[C@H](O)CNC(=O)c1c(O)c2ncc(Cc3ccc(F)cc3)cc2n(C)c1=O |r| Show InChI InChI=1S/C20H20FN3O4/c1-11(25)9-23-19(27)16-18(26)17-15(24(2)20(16)28)8-13(10-22-17)7-12-3-5-14(21)6-4-12/h3-6,8,10-11,25-26H,7,9H2,1-2H3,(H,23,27)/t11-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity |
Bioorg Med Chem Lett 23: 422-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.071
BindingDB Entry DOI: 10.7270/Q2XK8JFB |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294683
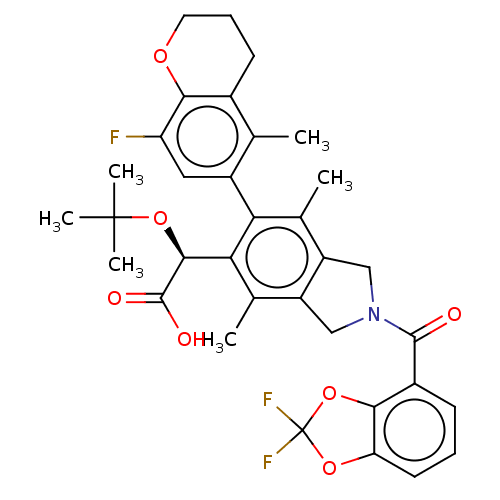
(US10112899, Example 162)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cccc2OC(F)(F)Oc12 |r,wD:23.27,(2.08,-1.5,;.75,-.73,;-.59,-1.5,;-.91,-3.01,;-2.44,-3.17,;-3.06,-1.76,;-1.92,-.73,;-1.92,.81,;-3.25,1.58,;-.59,1.58,;-.59,3.12,;.75,3.89,;.75,5.43,;2.08,6.2,;-.59,6.2,;-.59,7.74,;-1.92,8.51,;-3.25,7.74,;-3.25,6.2,;-1.92,5.43,;-1.92,3.89,;-3.25,3.12,;.75,.81,;2.08,1.58,;2.08,3.12,;3.41,3.89,;3.41,5.43,;4.75,3.12,;4.75,4.66,;3.41,.81,;4.75,1.58,;3.41,-.73,;-3.21,-4.51,;-4.75,-4.51,;-2.44,-5.84,;-3.21,-7.17,;-2.44,-8.51,;-.9,-8.51,;-.13,-7.17,;1.38,-6.85,;1.54,-5.32,;3.08,-5.32,;1.94,-3.83,;.13,-4.69,;-.9,-5.84,)| Show InChI InChI=1S/C34H34F3NO7/c1-16-19-10-8-12-42-28(19)24(35)13-21(16)26-17(2)22-14-38(31(39)20-9-7-11-25-29(20)45-34(36,37)43-25)15-23(22)18(3)27(26)30(32(40)41)44-33(4,5)6/h7,9,11,13,30H,8,10,12,14-15H2,1-6H3,(H,40,41)/t30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294834

(US10112899, Example 305)Show SMILES Cc1c2CN(Cc2c(C)c(-c2ccc3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cccc(F)c1 |r,wD:22.26,(2.08,-1.5,;.75,-.73,;-.59,-1.5,;-.91,-3.01,;-2.44,-3.17,;-3.06,-1.76,;-1.92,-.73,;-1.92,.81,;-3.25,1.58,;-.59,1.58,;-.59,3.12,;.75,3.89,;.75,5.43,;-.59,6.2,;-.59,7.74,;-1.92,8.51,;-3.25,7.74,;-3.25,6.2,;-1.92,5.43,;-1.92,3.89,;-3.25,3.12,;.75,.81,;2.08,1.58,;2.08,3.12,;3.41,3.89,;3.41,5.43,;4.75,3.12,;4.75,4.66,;3.41,.81,;4.75,1.58,;3.41,-.73,;-3.21,-4.51,;-4.75,-4.51,;-2.44,-5.84,;-3.21,-7.17,;-2.44,-8.51,;-.9,-8.51,;-.13,-7.17,;1.41,-7.17,;-.9,-5.84,)| Show InChI InChI=1S/C33H36FNO5/c1-18-23-11-8-14-39-27(23)13-12-24(18)28-19(2)25-16-35(31(36)21-9-7-10-22(34)15-21)17-26(25)20(3)29(28)30(32(37)38)40-33(4,5)6/h7,9-10,12-13,15,30H,8,11,14,16-17H2,1-6H3,(H,37,38)/t30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294851
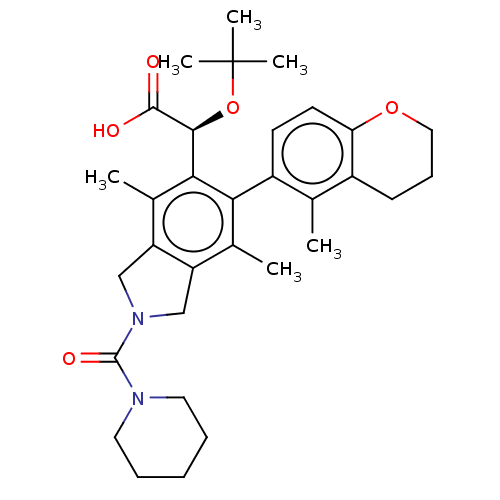
(US10112899, Example 321)Show SMILES Cc1c2CN(Cc2c(C)c(-c2ccc3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)N1CCCCC1 |r,wD:22.26,(2.08,-1.5,;.75,-.73,;-.59,-1.5,;-.91,-3.01,;-2.44,-3.17,;-3.06,-1.76,;-1.92,-.73,;-1.92,.81,;-3.25,1.58,;-.59,1.58,;-.59,3.12,;.75,3.89,;.75,5.43,;-.59,6.2,;-.59,7.74,;-1.92,8.51,;-3.25,7.74,;-3.25,6.2,;-1.92,5.43,;-1.92,3.89,;-3.25,3.12,;.75,.81,;2.08,1.58,;2.08,3.12,;3.41,3.89,;3.41,5.43,;4.75,3.12,;4.75,4.66,;3.41,.81,;4.75,1.58,;3.41,-.73,;-3.21,-4.51,;-4.75,-4.51,;-2.44,-5.84,;-3.21,-7.17,;-2.44,-8.51,;-.9,-8.51,;-.13,-7.17,;-.9,-5.84,)| Show InChI InChI=1S/C32H42N2O5/c1-19-22-11-10-16-38-26(22)13-12-23(19)27-20(2)24-17-34(31(37)33-14-8-7-9-15-33)18-25(24)21(3)28(27)29(30(35)36)39-32(4,5)6/h12-13,29H,7-11,14-18H2,1-6H3,(H,35,36)/t29-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294738
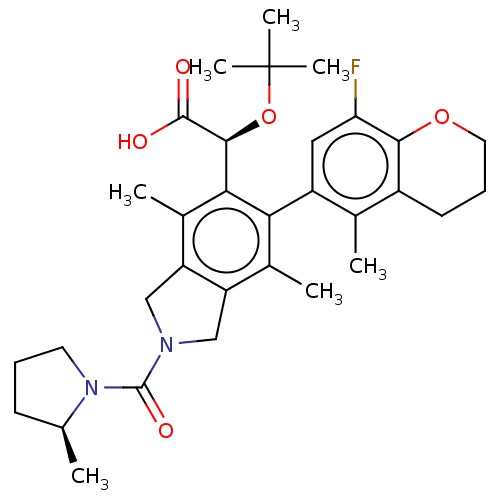
((S)-2-(tert-butoxy)-2-((M)-6-(8-fluoro-5-methylchr...)Show SMILES C[C@H]1CCCN1C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wU:1.0,wD:29.33,(-4.16,-7.82,;-4.56,-6.33,;-5.97,-5.71,;-5.81,-4.18,;-4.3,-3.86,;-3.53,-5.19,;-1.99,-5.19,;-1.22,-6.52,;-1.22,-3.86,;.31,-3.69,;.63,-2.19,;-.7,-1.42,;-1.85,-2.45,;-.7,.12,;-2.04,.89,;.63,.89,;.63,2.43,;1.97,3.2,;1.97,4.74,;3.3,5.51,;.63,5.51,;.63,7.05,;-.7,7.82,;-2.04,7.05,;-2.04,5.51,;-.7,4.74,;-.7,3.2,;-2.04,2.43,;1.97,.12,;3.3,.89,;3.3,2.43,;4.63,3.2,;5.97,3.97,;4.63,4.74,;5.97,2.43,;4.63,.12,;5.97,.89,;4.63,-1.42,;1.97,-1.42,;3.3,-2.19,)| Show InChI InChI=1S/C32H41FN2O5/c1-17-10-8-12-35(17)31(38)34-15-23-19(3)26(22-14-25(33)28-21(18(22)2)11-9-13-39-28)27(20(4)24(23)16-34)29(30(36)37)40-32(5,6)7/h14,17,29H,8-13,15-16H2,1-7H3,(H,36,37)/t17-,29-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294814
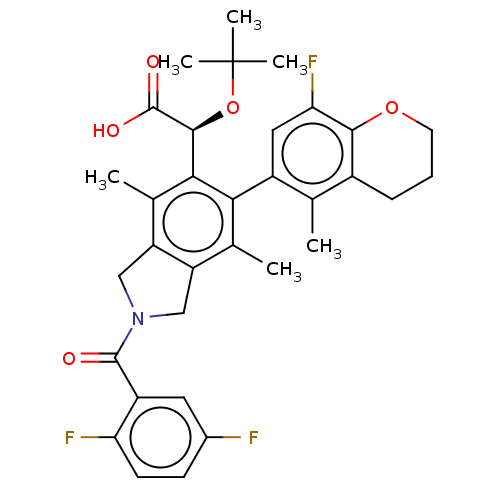
(US10112899, Example 285)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cc(F)ccc1F |r,wD:23.27,(3.62,-2.17,;2.29,-1.4,;.95,-2.17,;.63,-3.68,;-.9,-3.84,;-1.52,-2.43,;-.38,-1.4,;-.38,.14,;-1.71,.91,;.95,.91,;.95,2.45,;2.29,3.22,;2.29,4.76,;3.62,5.53,;.95,5.53,;.95,7.07,;-.38,7.84,;-1.71,7.07,;-1.71,5.53,;-.38,4.76,;-.38,3.22,;-1.71,2.45,;2.29,.14,;3.62,.91,;3.62,2.45,;4.95,3.22,;6.29,2.45,;4.95,4.76,;6.29,3.99,;4.95,.14,;6.29,.91,;4.95,-1.4,;-1.67,-5.17,;-.9,-6.51,;-3.21,-5.17,;-3.98,-3.84,;-5.52,-3.84,;-6.29,-2.5,;-6.29,-5.17,;-5.52,-6.51,;-3.98,-6.51,;-3.21,-7.84,)| Show InChI InChI=1S/C33H34F3NO5/c1-16-20-8-7-11-41-29(20)26(36)13-21(16)27-17(2)23-14-37(31(38)22-12-19(34)9-10-25(22)35)15-24(23)18(3)28(27)30(32(39)40)42-33(4,5)6/h9-10,12-13,30H,7-8,11,14-15H2,1-6H3,(H,39,40)/t30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294657

((S)-2-(tert-butoxy)-2-((R)-2-(5-fluoro-2-methylben...)Show SMILES Cc1ccc(F)cc1C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:31.35,(-3.21,-7.84,;-3.98,-6.51,;-5.52,-6.51,;-6.29,-5.17,;-5.52,-3.84,;-6.29,-2.5,;-3.98,-3.84,;-3.21,-5.17,;-1.67,-5.17,;-.9,-6.51,;-.9,-3.84,;.63,-3.68,;.95,-2.17,;-.38,-1.4,;-1.52,-2.43,;-.38,.14,;-1.71,.91,;.95,.91,;.95,2.45,;2.29,3.22,;2.29,4.76,;3.62,5.53,;.95,5.53,;.95,7.07,;-.38,7.84,;-1.71,7.07,;-1.71,5.53,;-.38,4.76,;-.38,3.22,;-1.71,2.45,;2.29,.14,;3.62,.91,;3.62,2.45,;4.95,3.22,;4.95,4.76,;6.29,2.45,;6.29,3.99,;4.95,.14,;6.29,.91,;4.95,-1.4,;2.29,-1.4,;3.62,-2.17,)| Show InChI InChI=1S/C34H37F2NO5/c1-17-10-11-21(35)13-23(17)32(38)37-15-25-19(3)28(24-14-27(36)30-22(18(24)2)9-8-12-41-30)29(20(4)26(25)16-37)31(33(39)40)42-34(5,6)7/h10-11,13-14,31H,8-9,12,15-16H2,1-7H3,(H,39,40)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294658
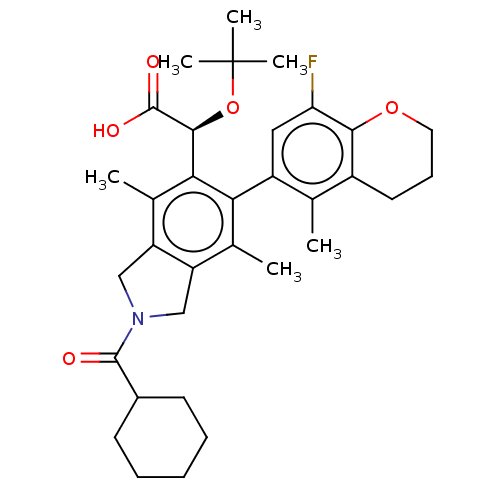
(US10112899, Example 137)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)C1CCCCC1 |r,wD:23.27,(3.62,-2.84,;2.29,-2.07,;.95,-2.84,;.63,-4.34,;-.9,-4.51,;-1.52,-3.1,;-.38,-2.07,;-.38,-.53,;-1.71,.24,;.95,.24,;.95,1.78,;2.29,2.55,;2.29,4.09,;3.62,4.86,;.95,4.86,;.95,6.4,;-.38,7.17,;-1.71,6.4,;-1.71,4.86,;-.38,4.09,;-.38,2.55,;-1.71,1.78,;2.29,-.53,;3.62,.24,;3.62,1.78,;4.95,2.55,;4.95,4.09,;6.29,1.78,;6.29,3.32,;4.95,-.53,;6.29,.24,;4.95,-2.07,;-1.67,-5.84,;-.9,-7.17,;-3.21,-5.84,;-3.98,-4.51,;-5.52,-4.51,;-6.29,-5.84,;-5.52,-7.17,;-3.98,-7.17,)| Show InChI InChI=1S/C33H42FNO5/c1-18-22-13-10-14-39-29(22)26(34)15-23(18)27-19(2)24-16-35(31(36)21-11-8-7-9-12-21)17-25(24)20(3)28(27)30(32(37)38)40-33(4,5)6/h15,21,30H,7-14,16-17H2,1-6H3,(H,37,38)/t30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294659

(US10112899, Example 138)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cccc2OCOc12 |r,wD:23.27,(3.62,-1.65,;2.29,-.88,;.95,-1.65,;.63,-3.16,;-.9,-3.32,;-1.52,-1.91,;-.38,-.88,;-.38,.66,;-1.71,1.43,;.95,1.43,;.95,2.97,;2.29,3.74,;2.29,5.28,;3.62,6.05,;.95,6.05,;.95,7.59,;-.38,8.36,;-1.71,7.59,;-1.71,6.05,;-.38,5.28,;-.38,3.74,;-1.71,2.97,;2.29,.66,;3.62,1.43,;3.62,2.97,;4.95,3.74,;4.95,5.28,;6.29,2.97,;6.29,4.51,;4.95,.66,;6.29,1.43,;4.95,-.88,;-1.67,-4.65,;-.9,-5.99,;-3.21,-4.65,;-3.98,-3.32,;-5.52,-3.32,;-6.29,-4.65,;-5.52,-5.99,;-5.99,-7.45,;-4.75,-8.36,;-3.5,-7.45,;-3.98,-5.99,)| Show InChI InChI=1S/C34H36FNO7/c1-17-20-10-8-12-40-29(20)25(35)13-22(17)27-18(2)23-14-36(32(37)21-9-7-11-26-30(21)42-16-41-26)15-24(23)19(3)28(27)31(33(38)39)43-34(4,5)6/h7,9,11,13,31H,8,10,12,14-16H2,1-6H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294661
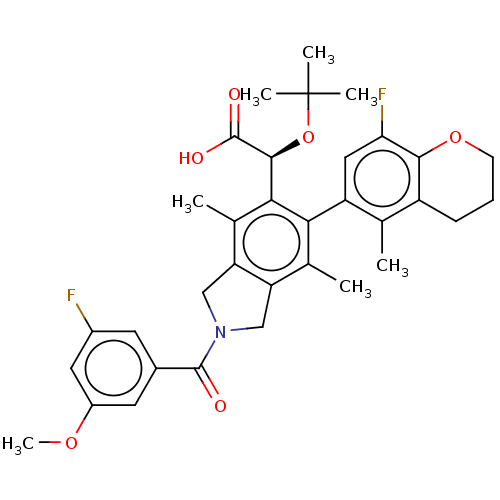
(US10112899, Example 140)Show SMILES COc1cc(F)cc(c1)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:32.36,(2.24,-5.17,;1.47,-6.51,;-.07,-6.51,;-.9,-7.84,;-2.44,-7.84,;-3.21,-9.17,;-3.21,-6.51,;-2.44,-5.17,;-.9,-5.17,;-3.21,-3.84,;-4.75,-3.84,;-2.44,-2.5,;-.91,-2.34,;-.59,-.84,;-1.92,-.07,;-3.06,-1.1,;-1.92,1.47,;-3.25,2.24,;-.59,2.24,;-.59,3.78,;.75,4.55,;.75,6.09,;2.08,6.86,;-.59,6.86,;-.59,8.4,;-1.92,9.17,;-3.25,8.4,;-3.25,6.86,;-1.92,6.09,;-1.92,4.55,;-3.25,3.78,;.75,1.47,;2.08,2.24,;2.08,3.78,;3.41,4.55,;3.41,6.09,;4.75,3.78,;4.75,5.32,;3.41,1.47,;4.75,2.24,;3.41,-.07,;.75,-.07,;2.08,-.84,)| Show InChI InChI=1S/C34H37F2NO6/c1-17-23-9-8-10-42-30(23)27(36)14-24(17)28-18(2)25-15-37(32(38)20-11-21(35)13-22(12-20)41-7)16-26(25)19(3)29(28)31(33(39)40)43-34(4,5)6/h11-14,31H,8-10,15-16H2,1-7H3,(H,39,40)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294666
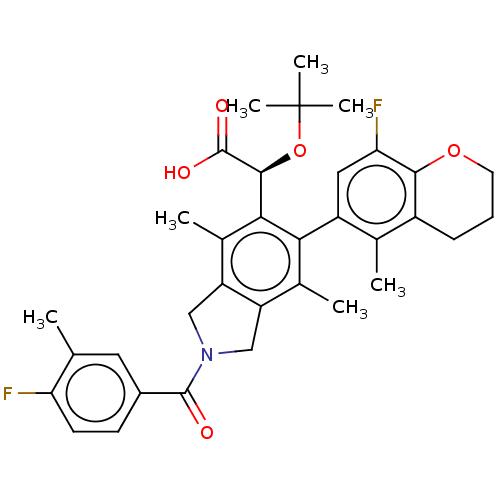
(US10112899, Example 145)Show SMILES Cc1cc(ccc1F)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:31.35,(-3.21,-9.17,;-2.44,-7.84,;-3.21,-6.51,;-2.44,-5.17,;-.9,-5.17,;-.13,-6.51,;-.9,-7.84,;-.13,-9.17,;-3.21,-3.84,;-4.75,-3.84,;-2.44,-2.5,;-.91,-2.34,;-.59,-.84,;-1.92,-.07,;-3.06,-1.1,;-1.92,1.47,;-3.25,2.24,;-.59,2.24,;-.59,3.78,;.75,4.55,;.75,6.09,;2.08,6.86,;-.59,6.86,;-.59,8.4,;-1.92,9.17,;-3.25,8.4,;-3.25,6.86,;-1.92,6.09,;-1.92,4.55,;-3.25,3.78,;.75,1.47,;2.08,2.24,;2.08,3.78,;3.41,4.55,;3.41,6.09,;4.75,3.78,;4.75,5.32,;3.41,1.47,;4.75,2.24,;3.41,-.07,;.75,-.07,;2.08,-.84,)| Show InChI InChI=1S/C34H37F2NO5/c1-17-13-21(10-11-26(17)35)32(38)37-15-24-19(3)28(23-14-27(36)30-22(18(23)2)9-8-12-41-30)29(20(4)25(24)16-37)31(33(39)40)42-34(5,6)7/h10-11,13-14,31H,8-9,12,15-16H2,1-7H3,(H,39,40)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294670

(US10112899, Example 149)Show SMILES Cc1cccc(c1)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:30.34,(-3.21,-9.17,;-2.44,-7.84,;-.9,-7.84,;-.13,-6.51,;-.9,-5.17,;-2.44,-5.17,;-3.21,-6.51,;-3.21,-3.84,;-4.75,-3.84,;-2.44,-2.5,;-.91,-2.34,;-.59,-.84,;-1.92,-.07,;-3.06,-1.1,;-1.92,1.47,;-3.25,2.24,;-.59,2.24,;-.59,3.78,;.75,4.55,;.75,6.09,;2.08,6.86,;-.59,6.86,;-.59,8.4,;-1.92,9.17,;-3.25,8.4,;-3.25,6.86,;-1.92,6.09,;-1.92,4.55,;-3.25,3.78,;.75,1.47,;2.08,2.24,;2.08,3.78,;3.41,4.55,;3.41,6.09,;4.75,3.78,;4.75,5.32,;3.41,1.47,;4.75,2.24,;3.41,-.07,;.75,-.07,;2.08,-.84,)| Show InChI InChI=1S/C34H38FNO5/c1-18-10-8-11-22(14-18)32(37)36-16-25-20(3)28(24-15-27(35)30-23(19(24)2)12-9-13-40-30)29(21(4)26(25)17-36)31(33(38)39)41-34(5,6)7/h8,10-11,14-15,31H,9,12-13,16-17H2,1-7H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294673

(US10112899, Example 152)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cccc2CCOc12 |r,wD:23.27,(2.08,-1.5,;.75,-.73,;-.59,-1.5,;-.91,-3.01,;-2.44,-3.17,;-3.06,-1.76,;-1.92,-.73,;-1.92,.81,;-3.25,1.58,;-.59,1.58,;-.59,3.12,;.75,3.89,;.75,5.43,;2.08,6.2,;-.59,6.2,;-.59,7.74,;-1.92,8.51,;-3.25,7.74,;-3.25,6.2,;-1.92,5.43,;-1.92,3.89,;-3.25,3.12,;.75,.81,;2.08,1.58,;2.08,3.12,;3.41,3.89,;3.41,5.43,;4.75,3.12,;4.75,4.66,;3.41,.81,;4.75,1.58,;3.41,-.73,;-3.21,-4.51,;-4.75,-4.51,;-2.44,-5.84,;-3.21,-7.17,;-2.44,-8.51,;-.9,-8.51,;-.13,-7.17,;1.38,-6.85,;1.54,-5.32,;.13,-4.69,;-.9,-5.84,)| Show InChI InChI=1S/C35H38FNO6/c1-18-22-11-8-13-41-31(22)27(36)15-24(18)28-19(2)25-16-37(33(38)23-10-7-9-21-12-14-42-30(21)23)17-26(25)20(3)29(28)32(34(39)40)43-35(4,5)6/h7,9-10,15,32H,8,11-14,16-17H2,1-6H3,(H,39,40)/t32-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294679

(US10112899, Example 158)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1ccccc1 |r,wD:23.27,(3.62,-2.84,;2.29,-2.07,;.95,-2.84,;.63,-4.34,;-.9,-4.51,;-1.52,-3.1,;-.38,-2.07,;-.38,-.53,;-1.71,.24,;.95,.24,;.95,1.78,;2.29,2.55,;2.29,4.09,;3.62,4.86,;.95,4.86,;.95,6.4,;-.38,7.17,;-1.71,6.4,;-1.71,4.86,;-.38,4.09,;-.38,2.55,;-1.71,1.78,;2.29,-.53,;3.62,.24,;3.62,1.78,;4.95,2.55,;4.95,4.09,;6.29,1.78,;6.29,3.32,;4.95,-.53,;6.29,.24,;4.95,-2.07,;-1.67,-5.84,;-.9,-7.17,;-3.21,-5.84,;-3.98,-4.51,;-5.52,-4.51,;-6.29,-5.84,;-5.52,-7.17,;-3.98,-7.17,)| Show InChI InChI=1S/C33H36FNO5/c1-18-22-13-10-14-39-29(22)26(34)15-23(18)27-19(2)24-16-35(31(36)21-11-8-7-9-12-21)17-25(24)20(3)28(27)30(32(37)38)40-33(4,5)6/h7-9,11-12,15,30H,10,13-14,16-17H2,1-6H3,(H,37,38)/t30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294680
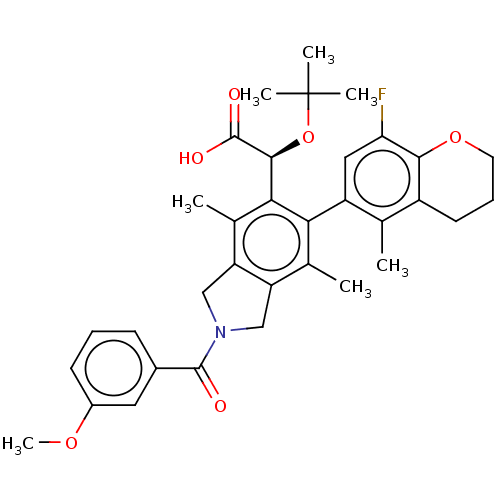
(US10112899, Example 159)Show SMILES COc1cccc(c1)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:31.35,(-7.06,-3.17,;-5.52,-3.17,;-4.75,-4.51,;-5.52,-5.84,;-4.75,-7.17,;-3.21,-7.17,;-2.44,-5.84,;-3.21,-4.51,;-.9,-5.84,;-.13,-7.17,;-.13,-4.51,;1.4,-4.34,;1.72,-2.84,;.39,-2.07,;-.75,-3.1,;.39,-.53,;-.94,.24,;1.72,.24,;1.72,1.78,;3.06,2.55,;3.06,4.09,;4.39,4.86,;1.72,4.86,;1.72,6.4,;.39,7.17,;-.94,6.4,;-.94,4.86,;.39,4.09,;.39,2.55,;-.94,1.78,;3.06,-.53,;4.39,.24,;4.39,1.78,;5.72,2.55,;5.72,4.09,;7.06,1.78,;7.06,3.32,;5.72,-.53,;7.06,.24,;5.72,-2.07,;3.06,-2.07,;4.39,-2.84,)| Show InChI InChI=1S/C34H38FNO6/c1-18-23-12-9-13-41-30(23)27(35)15-24(18)28-19(2)25-16-36(32(37)21-10-8-11-22(14-21)40-7)17-26(25)20(3)29(28)31(33(38)39)42-34(4,5)6/h8,10-11,14-15,31H,9,12-13,16-17H2,1-7H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294691
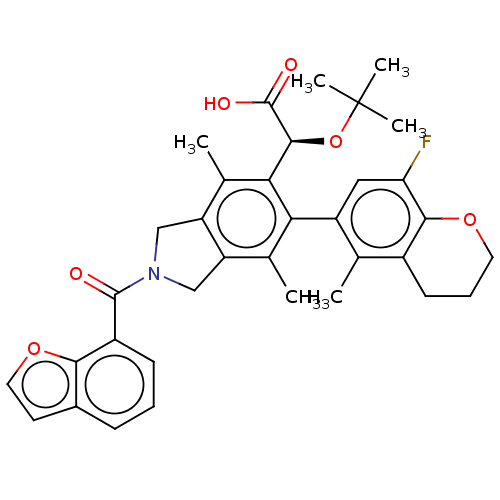
(US10112899, Example 169)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cccc2ccoc12 |r,wD:23.27,(3.62,-2.84,;2.29,-2.07,;.95,-2.84,;.63,-4.34,;-.9,-4.51,;-1.52,-3.1,;-.38,-2.07,;-.38,-.53,;-1.71,.24,;.95,.24,;.95,1.78,;2.29,2.55,;2.29,4.09,;3.62,4.86,;.95,4.86,;.95,6.4,;-.38,7.17,;-1.71,6.4,;-1.71,4.86,;-.38,4.09,;-.38,2.55,;-1.71,1.78,;2.29,-.53,;3.62,.24,;3.62,1.78,;4.95,2.55,;4.95,4.09,;6.29,1.78,;6.29,3.32,;4.95,-.53,;6.29,.24,;4.95,-2.07,;-1.67,-5.84,;-.9,-7.17,;-3.21,-5.84,;-3.98,-7.17,;-5.52,-7.17,;-6.29,-5.84,;-5.52,-4.51,;-5.99,-3.04,;-4.75,-2.14,;-3.5,-3.04,;-3.98,-4.51,)| Show InChI InChI=1S/C35H36FNO6/c1-18-22-11-8-13-41-31(22)27(36)15-24(18)28-19(2)25-16-37(33(38)23-10-7-9-21-12-14-42-30(21)23)17-26(25)20(3)29(28)32(34(39)40)43-35(4,5)6/h7,9-10,12,14-15,32H,8,11,13,16-17H2,1-6H3,(H,39,40)/t32-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294692
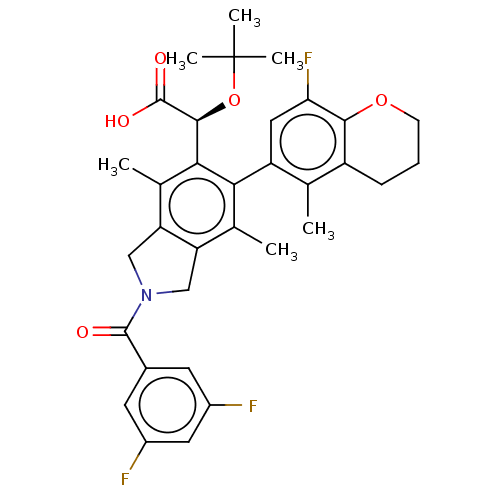
(US10112899, Example 170)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cc(F)cc(F)c1 |r,wD:23.27,(3.62,-2.17,;2.29,-1.4,;.95,-2.17,;.63,-3.68,;-.9,-3.84,;-1.52,-2.43,;-.38,-1.4,;-.38,.14,;-1.71,.91,;.95,.91,;.95,2.45,;2.29,3.22,;2.29,4.76,;3.62,5.53,;.95,5.53,;.95,7.07,;-.38,7.84,;-1.71,7.07,;-1.71,5.53,;-.38,4.76,;-.38,3.22,;-1.71,2.45,;2.29,.14,;3.62,.91,;3.62,2.45,;4.95,3.22,;4.95,4.76,;6.29,2.45,;6.29,3.99,;4.95,.14,;6.29,.91,;4.95,-1.4,;-1.67,-5.17,;-.9,-6.51,;-3.21,-5.17,;-3.98,-3.84,;-5.52,-3.84,;-6.29,-2.5,;-6.29,-5.17,;-5.52,-6.51,;-6.29,-7.84,;-3.98,-6.51,)| Show InChI InChI=1S/C33H34F3NO5/c1-16-22-8-7-9-41-29(22)26(36)13-23(16)27-17(2)24-14-37(31(38)19-10-20(34)12-21(35)11-19)15-25(24)18(3)28(27)30(32(39)40)42-33(4,5)6/h10-13,30H,7-9,14-15H2,1-6H3,(H,39,40)/t30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294693

(US10112899, Example 171)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1ccc(F)c(Cl)c1 |r,wD:23.27,(4.39,-2.84,;3.06,-2.07,;1.72,-2.84,;1.4,-4.34,;-.13,-4.51,;-.75,-3.1,;.39,-2.07,;.39,-.53,;-.94,.24,;1.72,.24,;1.72,1.78,;3.06,2.55,;3.06,4.09,;4.39,4.86,;1.72,4.86,;1.72,6.4,;.39,7.17,;-.94,6.4,;-.94,4.86,;.39,4.09,;.39,2.55,;-.94,1.78,;3.06,-.53,;4.39,.24,;4.39,1.78,;5.72,2.55,;5.72,4.09,;7.06,1.78,;7.06,3.32,;5.72,-.53,;7.06,.24,;5.72,-2.07,;-.9,-5.84,;-.13,-7.17,;-2.44,-5.84,;-3.21,-7.17,;-4.75,-7.17,;-5.52,-5.84,;-7.06,-5.84,;-4.75,-4.51,;-5.52,-3.17,;-3.21,-4.51,)| Show InChI InChI=1S/C33H34ClF2NO5/c1-16-20-8-7-11-41-29(20)26(36)13-21(16)27-17(2)22-14-37(31(38)19-9-10-25(35)24(34)12-19)15-23(22)18(3)28(27)30(32(39)40)42-33(4,5)6/h9-10,12-13,30H,7-8,11,14-15H2,1-6H3,(H,39,40)/t30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294657

((S)-2-(tert-butoxy)-2-((R)-2-(5-fluoro-2-methylben...)Show SMILES Cc1ccc(F)cc1C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:31.35,(-3.21,-7.84,;-3.98,-6.51,;-5.52,-6.51,;-6.29,-5.17,;-5.52,-3.84,;-6.29,-2.5,;-3.98,-3.84,;-3.21,-5.17,;-1.67,-5.17,;-.9,-6.51,;-.9,-3.84,;.63,-3.68,;.95,-2.17,;-.38,-1.4,;-1.52,-2.43,;-.38,.14,;-1.71,.91,;.95,.91,;.95,2.45,;2.29,3.22,;2.29,4.76,;3.62,5.53,;.95,5.53,;.95,7.07,;-.38,7.84,;-1.71,7.07,;-1.71,5.53,;-.38,4.76,;-.38,3.22,;-1.71,2.45,;2.29,.14,;3.62,.91,;3.62,2.45,;4.95,3.22,;4.95,4.76,;6.29,2.45,;6.29,3.99,;4.95,.14,;6.29,.91,;4.95,-1.4,;2.29,-1.4,;3.62,-2.17,)| Show InChI InChI=1S/C34H37F2NO5/c1-17-10-11-21(35)13-23(17)32(38)37-15-25-19(3)28(24-14-27(36)30-22(18(24)2)9-8-12-41-30)29(20(4)26(25)16-37)31(33(39)40)42-34(5,6)7/h10-11,13-14,31H,8-9,12,15-16H2,1-7H3,(H,39,40)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294700
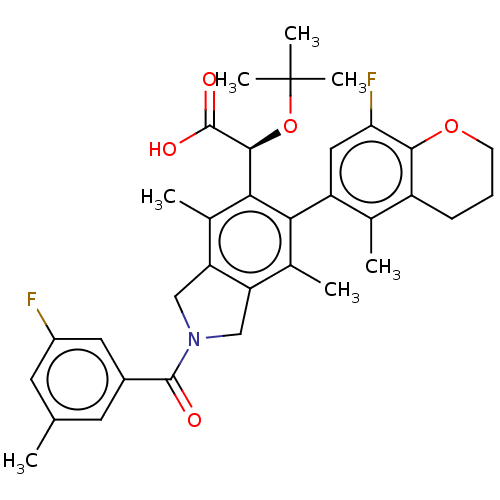
(US10112899, Example 178)Show SMILES Cc1cc(F)cc(c1)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:31.35,(-6.29,-7.84,;-5.52,-6.51,;-6.29,-5.17,;-5.52,-3.84,;-6.29,-2.5,;-3.98,-3.84,;-3.21,-5.17,;-3.98,-6.51,;-1.67,-5.17,;-.9,-6.51,;-.9,-3.84,;.63,-3.68,;.95,-2.17,;-.38,-1.4,;-1.52,-2.43,;-.38,.14,;-1.71,.91,;.95,.91,;.95,2.45,;2.29,3.22,;2.29,4.76,;3.62,5.53,;.95,5.53,;.95,7.07,;-.38,7.84,;-1.71,7.07,;-1.71,5.53,;-.38,4.76,;-.38,3.22,;-1.71,2.45,;2.29,.14,;3.62,.91,;3.62,2.45,;4.95,3.22,;4.95,4.76,;6.29,2.45,;6.29,3.99,;4.95,.14,;6.29,.91,;4.95,-1.4,;2.29,-1.4,;3.62,-2.17,)| Show InChI InChI=1S/C34H37F2NO5/c1-17-11-21(13-22(35)12-17)32(38)37-15-25-19(3)28(24-14-27(36)30-23(18(24)2)9-8-10-41-30)29(20(4)26(25)16-37)31(33(39)40)42-34(5,6)7/h11-14,31H,8-10,15-16H2,1-7H3,(H,39,40)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294706

(US10112899, Example 184)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1ccccn1 |r,wD:23.27,(3.62,-2.84,;2.29,-2.07,;.95,-2.84,;.63,-4.34,;-.9,-4.51,;-1.52,-3.1,;-.38,-2.07,;-.38,-.53,;-1.71,.24,;.95,.24,;.95,1.78,;2.29,2.55,;2.29,4.09,;3.62,4.86,;.95,4.86,;.95,6.4,;-.38,7.17,;-1.71,6.4,;-1.71,4.86,;-.38,4.09,;-.38,2.55,;-1.71,1.78,;2.29,-.53,;3.62,.24,;3.62,1.78,;4.95,2.55,;4.95,4.09,;6.29,1.78,;6.29,3.32,;4.95,-.53,;6.29,.24,;4.95,-2.07,;-1.67,-5.84,;-.9,-7.17,;-3.21,-5.84,;-3.98,-4.51,;-5.52,-4.51,;-6.29,-5.84,;-5.52,-7.17,;-3.98,-7.17,)| Show InChI InChI=1S/C32H35FN2O5/c1-17-20-10-9-13-39-28(20)24(33)14-21(17)26-18(2)22-15-35(30(36)25-11-7-8-12-34-25)16-23(22)19(3)27(26)29(31(37)38)40-32(4,5)6/h7-8,11-12,14,29H,9-10,13,15-16H2,1-6H3,(H,37,38)/t29-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294708
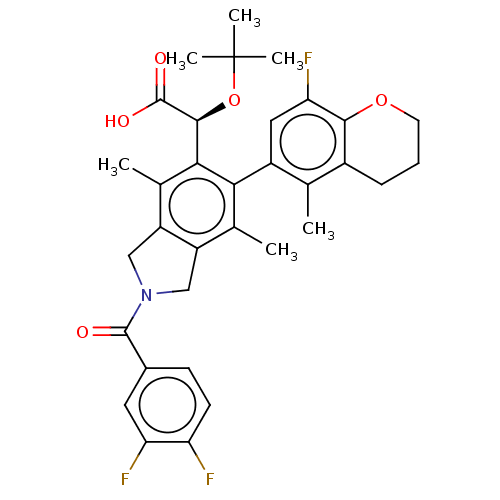
(US10112899, Example 186)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1ccc(F)c(F)c1 |r,wD:23.27,(4.39,-2.84,;3.06,-2.07,;1.72,-2.84,;1.4,-4.34,;-.13,-4.51,;-.75,-3.1,;.39,-2.07,;.39,-.53,;-.94,.24,;1.72,.24,;1.72,1.78,;3.06,2.55,;3.06,4.09,;4.39,4.86,;1.72,4.86,;1.72,6.4,;.39,7.17,;-.94,6.4,;-.94,4.86,;.39,4.09,;.39,2.55,;-.94,1.78,;3.06,-.53,;4.39,.24,;4.39,1.78,;5.72,2.55,;5.72,4.09,;7.06,1.78,;7.06,3.32,;5.72,-.53,;7.06,.24,;5.72,-2.07,;-.9,-5.84,;-.13,-7.17,;-2.44,-5.84,;-3.21,-7.17,;-4.75,-7.17,;-5.52,-5.84,;-7.06,-5.84,;-4.75,-4.51,;-5.52,-3.17,;-3.21,-4.51,)| Show InChI InChI=1S/C33H34F3NO5/c1-16-20-8-7-11-41-29(20)26(36)13-21(16)27-17(2)22-14-37(31(38)19-9-10-24(34)25(35)12-19)15-23(22)18(3)28(27)30(32(39)40)42-33(4,5)6/h9-10,12-13,30H,7-8,11,14-15H2,1-6H3,(H,39,40)/t30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294714
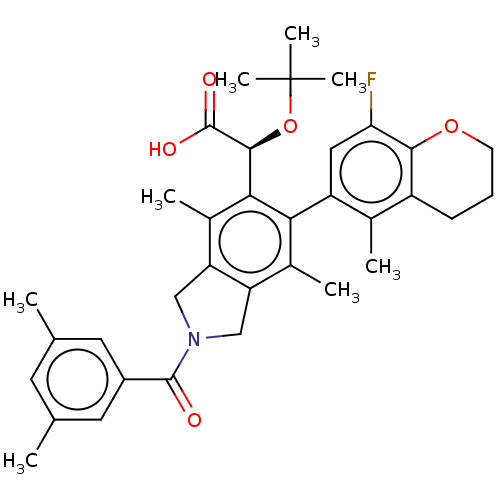
(US10112899, Example 191)Show SMILES Cc1cc(C)cc(c1)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:31.35,(-6.29,-2.5,;-5.52,-3.84,;-6.29,-5.17,;-5.52,-6.51,;-6.29,-7.84,;-3.98,-6.51,;-3.21,-5.17,;-3.98,-3.84,;-1.67,-5.17,;-.9,-6.51,;-.9,-3.84,;.63,-3.68,;.95,-2.17,;-.38,-1.4,;-1.52,-2.43,;-.38,.14,;-1.71,.91,;.95,.91,;.95,2.45,;2.29,3.22,;2.29,4.76,;3.62,5.53,;.95,5.53,;.95,7.07,;-.38,7.84,;-1.71,7.07,;-1.71,5.53,;-.38,4.76,;-.38,3.22,;-1.71,2.45,;2.29,.14,;3.62,.91,;3.62,2.45,;4.95,3.22,;4.95,4.76,;6.29,2.45,;6.29,3.99,;4.95,.14,;6.29,.91,;4.95,-1.4,;2.29,-1.4,;3.62,-2.17,)| Show InChI InChI=1S/C35H40FNO5/c1-18-12-19(2)14-23(13-18)33(38)37-16-26-21(4)29(25-15-28(36)31-24(20(25)3)10-9-11-41-31)30(22(5)27(26)17-37)32(34(39)40)42-35(6,7)8/h12-15,32H,9-11,16-17H2,1-8H3,(H,39,40)/t32-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294719
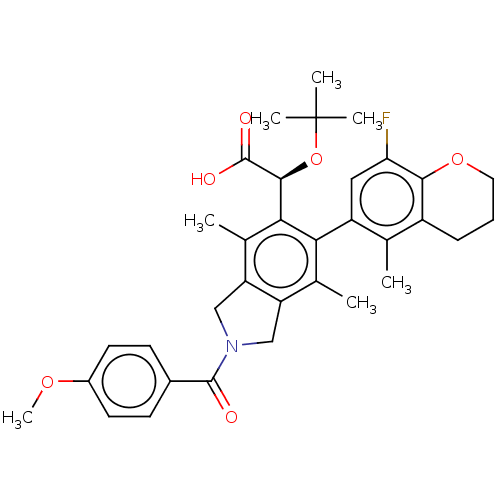
(US10112899, Example 196)Show SMILES COc1ccc(cc1)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:31.35,(-7.44,-4.51,;-6.67,-5.84,;-5.13,-5.84,;-4.36,-4.51,;-2.82,-4.51,;-2.05,-5.84,;-2.82,-7.17,;-4.36,-7.17,;-.51,-5.84,;.26,-7.17,;.26,-4.51,;1.79,-4.34,;2.11,-2.84,;.77,-2.07,;-.37,-3.1,;.77,-.53,;-.56,.24,;2.11,.24,;2.11,1.78,;3.44,2.55,;3.44,4.09,;4.78,4.86,;2.11,4.86,;2.11,6.4,;.77,7.17,;-.56,6.4,;-.56,4.86,;.77,4.09,;.77,2.55,;-.56,1.78,;3.44,-.53,;4.78,.24,;4.78,1.78,;6.11,2.55,;6.11,4.09,;7.44,1.78,;7.44,3.32,;6.11,-.53,;7.44,.24,;6.11,-2.07,;3.44,-2.07,;4.78,-2.84,)| Show InChI InChI=1S/C34H38FNO6/c1-18-23-9-8-14-41-30(23)27(35)15-24(18)28-19(2)25-16-36(32(37)21-10-12-22(40-7)13-11-21)17-26(25)20(3)29(28)31(33(38)39)42-34(4,5)6/h10-13,15,31H,8-9,14,16-17H2,1-7H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294622

((S)-2-(tert-butoxy)-2-((R)-2-(3,3-difluoropiperidi...)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)N1CCCC(F)(F)C1 |r,wD:23.27,(3.9,-2.84,;2.57,-2.07,;1.24,-2.84,;.92,-4.34,;-.62,-4.51,;-1.24,-3.1,;-.1,-2.07,;-.1,-.53,;-1.43,.24,;1.24,.24,;1.24,1.78,;2.57,2.55,;2.57,4.09,;3.9,4.86,;1.24,4.86,;1.24,6.4,;-.1,7.17,;-1.43,6.4,;-1.43,4.86,;-.1,4.09,;-.1,2.55,;-1.43,1.78,;2.57,-.53,;3.9,.24,;3.9,1.78,;5.24,2.55,;6.57,1.78,;5.24,4.09,;6.57,3.32,;5.24,-.53,;6.57,.24,;5.24,-2.07,;-1.39,-5.84,;-.62,-7.17,;-2.93,-5.84,;-3.7,-7.17,;-5.24,-7.17,;-6.01,-5.84,;-5.24,-4.51,;-5.24,-2.97,;-6.57,-3.74,;-3.7,-4.51,)| Show InChI InChI=1S/C32H39F3N2O5/c1-17-20-9-7-12-41-27(20)24(33)13-21(17)25-18(2)22-14-37(30(40)36-11-8-10-32(34,35)16-36)15-23(22)19(3)26(25)28(29(38)39)42-31(4,5)6/h13,28H,7-12,14-16H2,1-6H3,(H,38,39)/t28-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294623

(US10112899, Example 103)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cccc(F)c1 |r,wD:23.27,(3.62,-2.17,;2.29,-1.4,;.95,-2.17,;.63,-3.68,;-.9,-3.84,;-1.52,-2.43,;-.38,-1.4,;-.38,.14,;-1.71,.91,;.95,.91,;.95,2.45,;2.29,3.22,;2.29,4.76,;3.62,5.53,;.95,5.53,;.95,7.07,;-.38,7.84,;-1.71,7.07,;-1.71,5.53,;-.38,4.76,;-.38,3.22,;-1.71,2.45,;2.29,.14,;3.62,.91,;3.62,2.45,;4.95,3.22,;6.29,2.45,;4.95,4.76,;6.29,3.99,;4.95,.14,;6.29,.91,;4.95,-1.4,;-1.67,-5.17,;-.9,-6.51,;-3.21,-5.17,;-3.98,-3.84,;-5.52,-3.84,;-6.29,-5.17,;-5.52,-6.51,;-6.29,-7.84,;-3.98,-6.51,)| Show InChI InChI=1S/C33H35F2NO5/c1-17-22-11-8-12-40-29(22)26(35)14-23(17)27-18(2)24-15-36(31(37)20-9-7-10-21(34)13-20)16-25(24)19(3)28(27)30(32(38)39)41-33(4,5)6/h7,9-10,13-14,30H,8,11-12,15-16H2,1-6H3,(H,38,39)/t30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294624
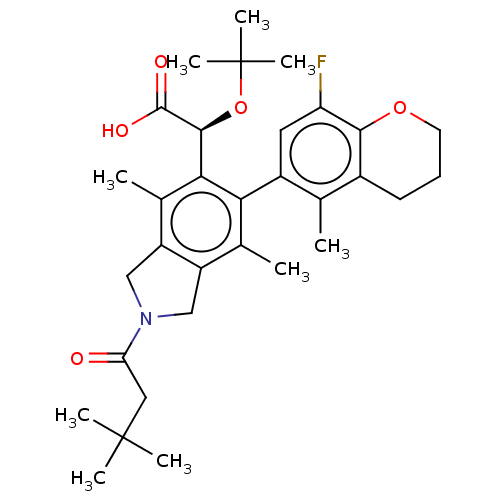
((S)-2-(tert-butoxy)-2-((M)-2-(3,3-dimethylbutanoyl...)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)CC(C)(C)C |r,wD:23.27,(3.24,-2.17,;1.9,-1.4,;.57,-2.17,;.25,-3.68,;-1.28,-3.84,;-1.91,-2.43,;-.77,-1.4,;-.77,.14,;-2.1,.91,;.57,.91,;.57,2.45,;1.9,3.22,;1.9,4.76,;3.24,5.53,;.57,5.53,;.57,7.07,;-.77,7.84,;-2.1,7.07,;-2.1,5.53,;-.77,4.76,;-.77,3.22,;-2.1,2.45,;1.9,.14,;3.24,.91,;3.24,2.45,;4.57,3.22,;5.9,2.45,;4.57,4.76,;5.9,3.99,;4.57,.14,;5.9,.91,;4.57,-1.4,;-2.05,-5.17,;-1.28,-6.51,;-3.59,-5.17,;-4.36,-6.51,;-5.9,-6.51,;-3.59,-7.84,;-5.13,-7.84,)| Show InChI InChI=1S/C32H42FNO5/c1-17-20-11-10-12-38-28(20)24(33)13-21(17)26-18(2)22-15-34(25(35)14-31(4,5)6)16-23(22)19(3)27(26)29(30(36)37)39-32(7,8)9/h13,29H,10-12,14-16H2,1-9H3,(H,36,37)/t29-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [1148-1435]
(Human immunodeficiency virus type 1) | BDBM28239

(2-(7-{5-[(4-fluorophenyl)methyl]-1,3,4-oxadiazol-2...)Show SMILES Oc1c(nc(N2CCCCS2(=O)=O)c2cccnc12)-c1nnc(Cc2ccc(F)cc2)o1 Show InChI InChI=1S/C21H18FN5O4S/c22-14-7-5-13(6-8-14)12-16-25-26-21(31-16)18-19(28)17-15(4-3-9-23-17)20(24-18)27-10-1-2-11-32(27,29)30/h3-9,28H,1-2,10-12H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 37 |
GSK
| Assay Description
Purified recombinant integrase was first combined in a complex with biotinylated donor DNA- streptavidin-coated SPA beads. The complex was preincubat... |
Bioorg Med Chem Lett 19: 1802-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.090
BindingDB Entry DOI: 10.7270/Q2Z899QM |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [1148-1435]
(Human immunodeficiency virus type 1) | BDBM28269

(1,3,4-oxadiazole substituted naphthyridine, 37 | 3...)Show SMILES OC(=O)c1cccc(c1)-c1nc(-c2nnc(Cc3ccc(F)cc3)o2)c(O)c2ncccc12 Show InChI InChI=1S/C24H15FN4O4/c25-16-8-6-13(7-9-16)11-18-28-29-23(33-18)21-22(30)20-17(5-2-10-26-20)19(27-21)14-3-1-4-15(12-14)24(31)32/h1-10,12,30H,11H2,(H,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
Purified recombinant integrase was first combined in a complex with biotinylated donor DNA- streptavidin-coated SPA beads. The complex was preincubat... |
Bioorg Med Chem Lett 19: 1807-10 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.089
BindingDB Entry DOI: 10.7270/Q2TH8K1R |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [1148-1435]
(Human immunodeficiency virus type 1) | BDBM28274

(1,3,4-oxadiazole substituted naphthyridine, 42 | 4...)Show SMILES OC(=O)c1ccc(cc1)-c1nc(-c2nnc(Cc3ccc(F)cc3)o2)c(O)c2ncccc12 Show InChI InChI=1S/C24H15FN4O4/c25-16-9-3-13(4-10-16)12-18-28-29-23(33-18)21-22(30)20-17(2-1-11-26-20)19(27-21)14-5-7-15(8-6-14)24(31)32/h1-11,30H,12H2,(H,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
Purified recombinant integrase was first combined in a complex with biotinylated donor DNA- streptavidin-coated SPA beads. The complex was preincubat... |
Bioorg Med Chem Lett 19: 1807-10 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.089
BindingDB Entry DOI: 10.7270/Q2TH8K1R |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [1148-1435]
(Human immunodeficiency virus type 1) | BDBM28239

(2-(7-{5-[(4-fluorophenyl)methyl]-1,3,4-oxadiazol-2...)Show SMILES Oc1c(nc(N2CCCCS2(=O)=O)c2cccnc12)-c1nnc(Cc2ccc(F)cc2)o1 Show InChI InChI=1S/C21H18FN5O4S/c22-14-7-5-13(6-8-14)12-16-25-26-21(31-16)18-19(28)17-15(4-3-9-23-17)20(24-18)27-10-1-2-11-32(27,29)30/h3-9,28H,1-2,10-12H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
Purified recombinant integrase was first combined in a complex with biotinylated donor DNA- streptavidin-coated SPA beads. The complex was preincubat... |
Bioorg Med Chem Lett 19: 1807-10 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.089
BindingDB Entry DOI: 10.7270/Q2TH8K1R |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50062551

(CHEBI:76010 | Dolutegravir | GSK1349572 | S-349572)Show SMILES [H][C@]12Cn3cc(C(=O)NCc4ccc(F)cc4F)c(=O)c(O)c3C(=O)N1[C@H](C)CCO2 |r| Show InChI InChI=1S/C20H19F2N3O5/c1-10-4-5-30-15-9-24-8-13(17(26)18(27)16(24)20(29)25(10)15)19(28)23-7-11-2-3-12(21)6-14(11)22/h2-3,6,8,10,15,27H,4-5,7,9H2,1H3,(H,23,28)/t10-,15+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 integrase strand transfer activity using [3H]-DNA as substrate preincubated for 60 mins prior to substrate addition measured afte... |
J Med Chem 56: 5901-16 (2013)
Article DOI: 10.1021/jm400645w
BindingDB Entry DOI: 10.7270/Q21G0Q64 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Integrase
(Human immunodeficiency virus 1) | BDBM50489280

(CHEMBL2311585)Show SMILES C[C@H](O)CNC(=O)c1c(O)c2ncc(Cc3ccc(F)cc3)cc2[nH]c1=O |r| Show InChI InChI=1S/C19H18FN3O4/c1-10(24)8-22-18(26)15-17(25)16-14(23-19(15)27)7-12(9-21-16)6-11-2-4-13(20)5-3-11/h2-5,7,9-10,24H,6,8H2,1H3,(H,22,26)(H2,23,25,27)/t10-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity |
Bioorg Med Chem Lett 23: 422-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.071
BindingDB Entry DOI: 10.7270/Q2XK8JFB |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50489287

(CHEMBL2316116)Show SMILES CC(C)(CO)NC(=O)c1c(O)c2ncc(Cc3ccc(F)cc3)cc2[nH]c1=O Show InChI InChI=1S/C20H20FN3O4/c1-20(2,10-25)24-19(28)15-17(26)16-14(23-18(15)27)8-12(9-22-16)7-11-3-5-13(21)6-4-11/h3-6,8-9,25H,7,10H2,1-2H3,(H,24,28)(H2,23,26,27) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity |
Bioorg Med Chem Lett 23: 422-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.071
BindingDB Entry DOI: 10.7270/Q2XK8JFB |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294847

(US10112899, Example 317)Show SMILES Cc1c2CN(Cc2c(C)c(-c2ccc3OCCCc3c2)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)N1CCCCC1 |r| Show InChI InChI=1S/C31H40N2O5/c1-19-23-17-33(30(36)32-13-7-6-8-14-32)18-24(23)20(2)27(28(29(34)35)38-31(3,4)5)26(19)22-11-12-25-21(16-22)10-9-15-37-25/h11-12,16,28H,6-10,13-15,17-18H2,1-5H3,(H,34,35)/t28-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294850
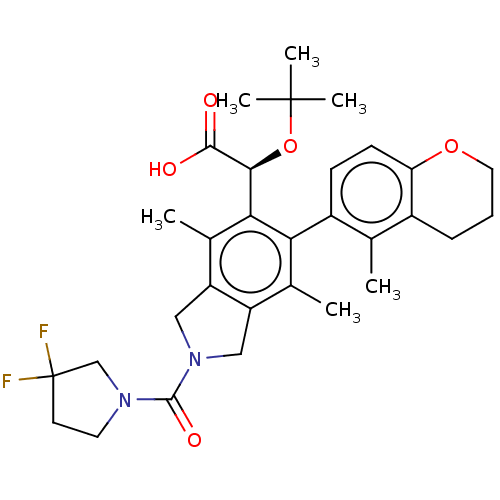
(US10112899, Example 320)Show SMILES Cc1c2CN(Cc2c(C)c(-c2ccc3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)N1CCC(F)(F)C1 |r,wD:22.26,(2.08,-1.44,;.75,-.67,;-.59,-1.44,;-.91,-2.95,;-2.44,-3.11,;-3.06,-1.71,;-1.92,-.67,;-1.92,.87,;-3.25,1.64,;-.59,1.64,;-.59,3.18,;.75,3.95,;.75,5.49,;-.59,6.26,;-.59,7.8,;-1.92,8.57,;-3.25,7.8,;-3.25,6.26,;-1.92,5.49,;-1.92,3.95,;-3.25,3.18,;.75,.87,;2.08,1.64,;2.08,3.18,;3.41,3.95,;3.41,5.49,;4.75,3.18,;4.75,4.72,;3.41,.87,;4.75,1.64,;3.41,-.67,;-3.21,-4.45,;-4.75,-4.45,;-2.44,-5.78,;-3.34,-7.03,;-2.44,-8.27,;-.97,-7.8,;.36,-7.03,;.36,-8.57,;-.97,-6.26,)| Show InChI InChI=1S/C31H38F2N2O5/c1-17-20-8-7-13-39-24(20)10-9-21(17)25-18(2)22-14-35(29(38)34-12-11-31(32,33)16-34)15-23(22)19(3)26(25)27(28(36)37)40-30(4,5)6/h9-10,27H,7-8,11-16H2,1-6H3,(H,36,37)/t27-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50492496
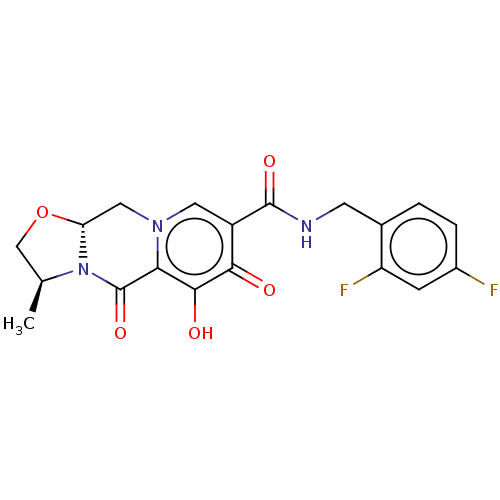
(Cabotegravir | GSK-1265744A | GSK1265744 | GSK1265...)Show SMILES [H][C@@]12Cn3cc(C(=O)NCc4ccc(F)cc4F)c(=O)c(O)c3C(=O)N1[C@@H](C)CO2 |r| Show InChI InChI=1S/C19H17F2N3O5/c1-9-8-29-14-7-23-6-12(16(25)17(26)15(23)19(28)24(9)14)18(27)22-5-10-2-3-11(20)4-13(10)21/h2-4,6,9,14,26H,5,7-8H2,1H3,(H,22,27)/t9-,14+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 integrase strand transfer activity using [3H]-DNA as substrate preincubated for 60 mins prior to substrate addition measured afte... |
J Med Chem 56: 5901-16 (2013)
Article DOI: 10.1021/jm400645w
BindingDB Entry DOI: 10.7270/Q21G0Q64 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50489263
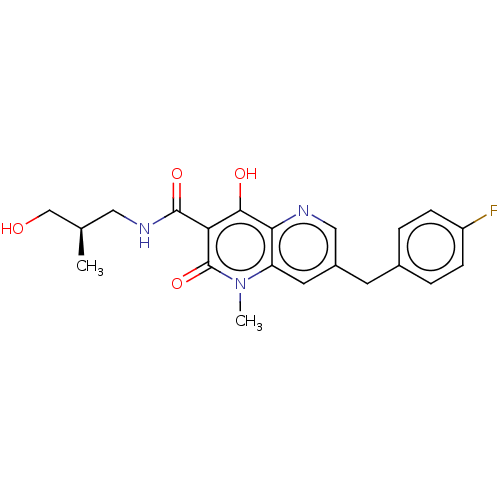
(CHEMBL2316126)Show SMILES C[C@@H](CO)CNC(=O)c1c(O)c2ncc(Cc3ccc(F)cc3)cc2n(C)c1=O |r| Show InChI InChI=1S/C21H22FN3O4/c1-12(11-26)9-24-20(28)17-19(27)18-16(25(2)21(17)29)8-14(10-23-18)7-13-3-5-15(22)6-4-13/h3-6,8,10,12,26-27H,7,9,11H2,1-2H3,(H,24,28)/t12-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity |
Bioorg Med Chem Lett 23: 422-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.071
BindingDB Entry DOI: 10.7270/Q2XK8JFB |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294739

((S)-2-(tert-butoxy)-2-((M)-2-(4,4-difluoropiperidi...)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)N1CCC(F)(F)CC1 |r,wD:23.27,(4.29,-2.84,;2.95,-2.07,;1.62,-2.84,;1.3,-4.34,;-.23,-4.51,;-.86,-3.1,;.29,-2.07,;.29,-.53,;-1.05,.24,;1.62,.24,;1.62,1.78,;2.95,2.55,;2.95,4.09,;4.29,4.86,;1.62,4.86,;1.62,6.4,;.29,7.17,;-1.05,6.4,;-1.05,4.86,;.29,4.09,;.29,2.55,;-1.05,1.78,;2.95,-.53,;4.29,.24,;4.29,1.78,;5.62,2.55,;6.96,3.32,;5.62,4.09,;6.96,1.78,;5.62,-.53,;6.96,.24,;5.62,-2.07,;-1,-5.84,;-.23,-7.17,;-2.54,-5.84,;-3.31,-4.51,;-4.85,-4.51,;-5.62,-5.84,;-6.96,-6.61,;-6.96,-5.07,;-4.85,-7.17,;-3.31,-7.17,)| Show InChI InChI=1S/C32H39F3N2O5/c1-17-20-8-7-13-41-27(20)24(33)14-21(17)25-18(2)22-15-37(30(40)36-11-9-32(34,35)10-12-36)16-23(22)19(3)26(25)28(29(38)39)42-31(4,5)6/h14,28H,7-13,15-16H2,1-6H3,(H,38,39)/t28-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294740

((S)-2-(tert-butoxy)-2-((M)-2-(3,3-dimethylpyrrolid...)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)N1CCC(C)(C)C1 |r,wD:23.27,(4.07,-2.58,;2.74,-1.81,;1.4,-2.58,;1.08,-4.09,;-.45,-4.25,;-1.08,-2.84,;.07,-1.81,;.07,-.27,;-1.27,.5,;1.4,.5,;1.4,2.04,;2.74,2.81,;2.74,4.35,;4.07,5.12,;1.4,5.12,;1.4,6.66,;.07,7.43,;-1.27,6.66,;-1.27,5.12,;.07,4.35,;.07,2.81,;-1.27,2.04,;2.74,-.27,;4.07,.5,;4.07,2.04,;5.4,2.81,;6.74,3.58,;5.4,4.35,;6.74,2.04,;5.4,-.27,;6.74,.5,;5.4,-1.81,;-1.22,-5.58,;-.45,-6.91,;-2.76,-5.58,;-3.53,-4.25,;-5.04,-4.57,;-5.2,-6.1,;-6.74,-6.1,;-5.97,-7.43,;-3.79,-6.72,)| Show InChI InChI=1S/C33H43FN2O5/c1-18-21-10-9-13-40-28(21)25(34)14-22(18)26-19(2)23-15-36(31(39)35-12-11-33(7,8)17-35)16-24(23)20(3)27(26)29(30(37)38)41-32(4,5)6/h14,29H,9-13,15-17H2,1-8H3,(H,37,38)/t29-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294779
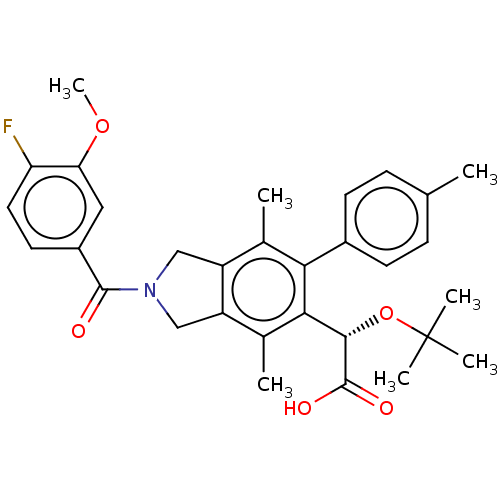
(US10112899, Example 251)Show SMILES COc1cc(ccc1F)C(=O)N1Cc2c(C1)c(C)c(-c1ccc(C)cc1)c([C@H](OC(C)(C)C)C(O)=O)c2C |r| Show InChI InChI=1S/C31H34FNO5/c1-17-8-10-20(11-9-17)26-18(2)22-15-33(29(34)21-12-13-24(32)25(14-21)37-7)16-23(22)19(3)27(26)28(30(35)36)38-31(4,5)6/h8-14,28H,15-16H2,1-7H3,(H,35,36)/t28-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294667
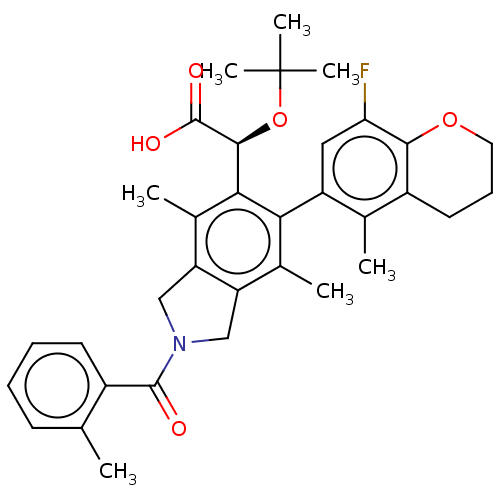
(US10112899, Example 146)Show SMILES Cc1ccccc1C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:30.34,(-4.75,-7.17,;-3.21,-7.17,;-2.44,-8.51,;-.9,-8.51,;-.13,-7.17,;-.9,-5.84,;-2.44,-5.84,;-3.21,-4.51,;-4.75,-4.51,;-2.44,-3.17,;-.91,-3.01,;-.59,-1.5,;-1.92,-.73,;-3.06,-1.76,;-1.92,.81,;-3.25,1.58,;-.59,1.58,;-.59,3.12,;.75,3.89,;.75,5.43,;2.08,6.2,;-.59,6.2,;-.59,7.74,;-1.92,8.51,;-3.25,7.74,;-3.25,6.2,;-1.92,5.43,;-1.92,3.89,;-3.25,3.12,;.75,.81,;2.08,1.58,;2.08,3.12,;3.41,3.89,;3.41,5.43,;4.75,3.12,;4.75,4.66,;3.41,.81,;4.75,1.58,;3.41,-.73,;.75,-.73,;2.08,-1.5,)| Show InChI InChI=1S/C34H38FNO5/c1-18-11-8-9-12-22(18)32(37)36-16-25-20(3)28(24-15-27(35)30-23(19(24)2)13-10-14-40-30)29(21(4)26(25)17-36)31(33(38)39)41-34(5,6)7/h8-9,11-12,15,31H,10,13-14,16-17H2,1-7H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294677

(US10112899, Example 156)Show SMILES COc1cc(ccc1C)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:32.36,(-7.06,-3.17,;-5.52,-3.17,;-4.75,-4.51,;-3.21,-4.51,;-2.44,-5.84,;-3.21,-7.17,;-4.75,-7.17,;-5.52,-5.84,;-7.06,-5.84,;-.9,-5.84,;-.13,-7.17,;-.13,-4.51,;1.4,-4.34,;1.72,-2.84,;.39,-2.07,;-.75,-3.1,;.39,-.53,;-.94,.24,;1.72,.24,;1.72,1.78,;3.06,2.55,;3.06,4.09,;4.39,4.86,;1.72,4.86,;1.72,6.4,;.39,7.17,;-.94,6.4,;-.94,4.86,;.39,4.09,;.39,2.55,;-.94,1.78,;3.06,-.53,;4.39,.24,;4.39,1.78,;5.72,2.55,;5.72,4.09,;7.06,1.78,;7.06,3.32,;5.72,-.53,;7.06,.24,;5.72,-2.07,;3.06,-2.07,;4.39,-2.84,)| Show InChI InChI=1S/C35H40FNO6/c1-18-11-12-22(14-28(18)41-8)33(38)37-16-25-20(3)29(24-15-27(36)31-23(19(24)2)10-9-13-42-31)30(21(4)26(25)17-37)32(34(39)40)43-35(5,6)7/h11-12,14-15,32H,9-10,13,16-17H2,1-8H3,(H,39,40)/t32-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294678
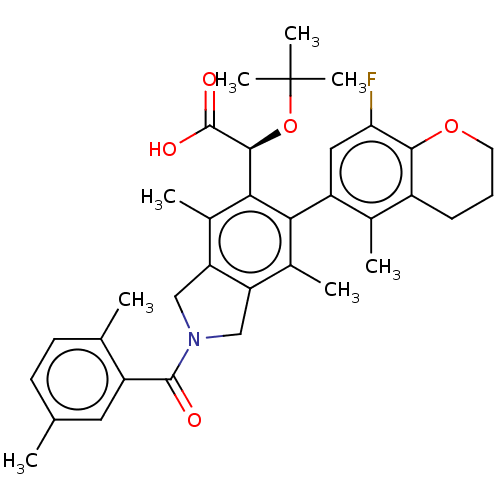
(US10112899, Example 157)Show SMILES Cc1ccc(C)c(c1)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:31.35,(-6.29,-2.5,;-5.52,-3.84,;-6.29,-5.17,;-5.52,-6.51,;-3.98,-6.51,;-3.21,-7.84,;-3.21,-5.17,;-3.98,-3.84,;-1.67,-5.17,;-.9,-6.51,;-.9,-3.84,;.63,-3.68,;.95,-2.17,;-.38,-1.4,;-1.52,-2.43,;-.38,.14,;-1.71,.91,;.95,.91,;.95,2.45,;2.29,3.22,;2.29,4.76,;3.62,5.53,;.95,5.53,;.95,7.07,;-.38,7.84,;-1.71,7.07,;-1.71,5.53,;-.38,4.76,;-.38,3.22,;-1.71,2.45,;2.29,.14,;3.62,.91,;3.62,2.45,;4.95,3.22,;4.95,4.76,;6.29,2.45,;6.29,3.99,;4.95,.14,;6.29,.91,;4.95,-1.4,;2.29,-1.4,;3.62,-2.17,)| Show InChI InChI=1S/C35H40FNO5/c1-18-11-12-19(2)24(14-18)33(38)37-16-26-21(4)29(25-15-28(36)31-23(20(25)3)10-9-13-41-31)30(22(5)27(26)17-37)32(34(39)40)42-35(6,7)8/h11-12,14-15,32H,9-10,13,16-17H2,1-8H3,(H,39,40)/t32-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294682

(US10112899, Example 161)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cccc2CCCc12 |r,wD:23.27,(3.62,-2.84,;2.29,-2.07,;.95,-2.84,;.63,-4.34,;-.9,-4.51,;-1.52,-3.1,;-.38,-2.07,;-.38,-.53,;-1.71,.24,;.95,.24,;.95,1.78,;2.29,2.55,;2.29,4.09,;3.62,4.86,;.95,4.86,;.95,6.4,;-.38,7.17,;-1.71,6.4,;-1.71,4.86,;-.38,4.09,;-.38,2.55,;-1.71,1.78,;2.29,-.53,;3.62,.24,;3.62,1.78,;4.95,2.55,;4.95,4.09,;6.29,1.78,;6.29,3.32,;4.95,-.53,;6.29,.24,;4.95,-2.07,;-1.67,-5.84,;-.9,-7.17,;-3.21,-5.84,;-3.98,-7.17,;-5.52,-7.17,;-6.29,-5.84,;-5.52,-4.51,;-5.99,-3.04,;-4.75,-2.14,;-3.5,-3.04,;-3.98,-4.51,)| Show InChI InChI=1S/C36H40FNO5/c1-19-23-14-9-15-42-32(23)29(37)16-26(19)30-20(2)27-17-38(34(39)25-13-8-11-22-10-7-12-24(22)25)18-28(27)21(3)31(30)33(35(40)41)43-36(4,5)6/h8,11,13,16,33H,7,9-10,12,14-15,17-18H2,1-6H3,(H,40,41)/t33-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data



















































