 Found 24 hits with Last Name = 'wei' and Initial = 'gf'
Found 24 hits with Last Name = 'wei' and Initial = 'gf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248709
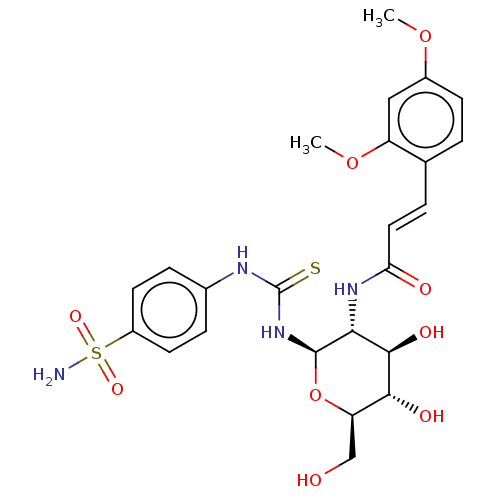
(CHEMBL4103517)Show SMILES COc1ccc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)c(OC)c1 |r| Show InChI InChI=1S/C24H30N4O9S2/c1-35-15-7-3-13(17(11-15)36-2)4-10-19(30)27-20-22(32)21(31)18(12-29)37-23(20)28-24(38)26-14-5-8-16(9-6-14)39(25,33)34/h3-11,18,20-23,29,31-32H,12H2,1-2H3,(H,27,30)(H2,25,33,34)(H2,26,28,38)/b10-4+/t18-,20-,21-,22-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50248709

(CHEMBL4103517)Show SMILES COc1ccc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)c(OC)c1 |r| Show InChI InChI=1S/C24H30N4O9S2/c1-35-15-7-3-13(17(11-15)36-2)4-10-19(30)27-20-22(32)21(31)18(12-29)37-23(20)28-24(38)26-14-5-8-16(9-6-14)39(25,33)34/h3-11,18,20-23,29,31-32H,12H2,1-2H3,(H,27,30)(H2,25,33,34)(H2,26,28,38)/b10-4+/t18-,20-,21-,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248718

(CHEMBL4075252)Show SMILES COc1cccc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)c1OC |r| Show InChI InChI=1S/C24H30N4O9S2/c1-35-16-5-3-4-13(22(16)36-2)6-11-18(30)27-19-21(32)20(31)17(12-29)37-23(19)28-24(38)26-14-7-9-15(10-8-14)39(25,33)34/h3-11,17,19-21,23,29,31-32H,12H2,1-2H3,(H,27,30)(H2,25,33,34)(H2,26,28,38)/b11-6+/t17-,19-,20-,21-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50248718

(CHEMBL4075252)Show SMILES COc1cccc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)c1OC |r| Show InChI InChI=1S/C24H30N4O9S2/c1-35-16-5-3-4-13(22(16)36-2)6-11-18(30)27-19-21(32)20(31)17(12-29)37-23(19)28-24(38)26-14-7-9-15(10-8-14)39(25,33)34/h3-11,17,19-21,23,29,31-32H,12H2,1-2H3,(H,27,30)(H2,25,33,34)(H2,26,28,38)/b11-6+/t17-,19-,20-,21-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248710
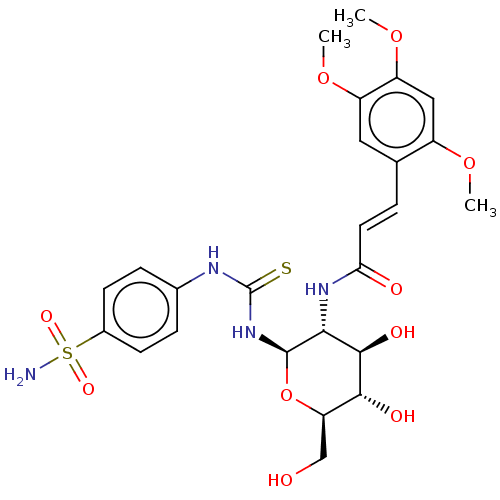
(CHEMBL4070358)Show SMILES COc1cc(OC)c(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)cc1OC |r| Show InChI InChI=1S/C25H32N4O10S2/c1-36-16-11-18(38-3)17(37-2)10-13(16)4-9-20(31)28-21-23(33)22(32)19(12-30)39-24(21)29-25(40)27-14-5-7-15(8-6-14)41(26,34)35/h4-11,19,21-24,30,32-33H,12H2,1-3H3,(H,28,31)(H2,26,34,35)(H2,27,29,40)/b9-4+/t19-,21-,22-,23-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50248707
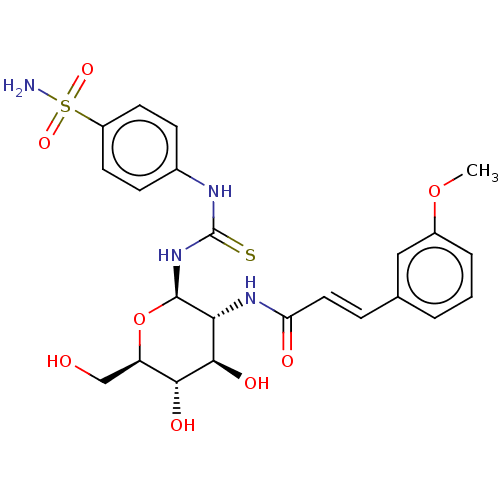
(CHEMBL4075581)Show SMILES COc1cccc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)c1 |r| Show InChI InChI=1S/C23H28N4O8S2/c1-34-15-4-2-3-13(11-15)5-10-18(29)26-19-21(31)20(30)17(12-28)35-22(19)27-23(36)25-14-6-8-16(9-7-14)37(24,32)33/h2-11,17,19-22,28,30-31H,12H2,1H3,(H,26,29)(H2,24,32,33)(H2,25,27,36)/b10-5+/t17-,19-,20-,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248719
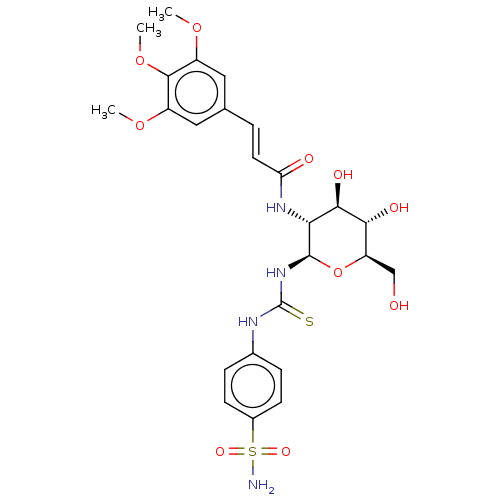
(CHEMBL4096770)Show SMILES COc1cc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)cc(OC)c1OC |r| Show InChI InChI=1S/C25H32N4O10S2/c1-36-16-10-13(11-17(37-2)23(16)38-3)4-9-19(31)28-20-22(33)21(32)18(12-30)39-24(20)29-25(40)27-14-5-7-15(8-6-14)41(26,34)35/h4-11,18,20-22,24,30,32-33H,12H2,1-3H3,(H,28,31)(H2,26,34,35)(H2,27,29,40)/b9-4+/t18-,20-,21-,22-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50248708
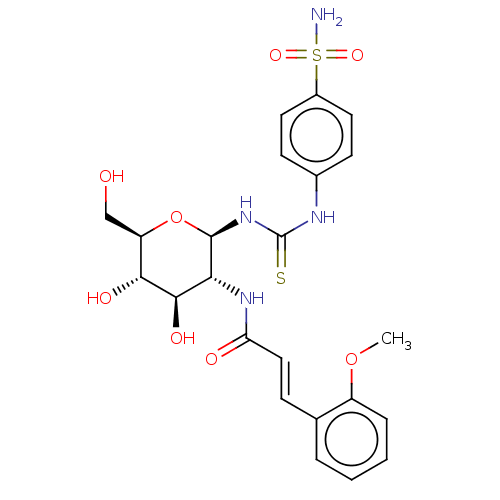
(CHEMBL4090618)Show SMILES COc1ccccc1\C=C\C(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1NC(=S)Nc1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C23H28N4O8S2/c1-34-16-5-3-2-4-13(16)6-11-18(29)26-19-21(31)20(30)17(12-28)35-22(19)27-23(36)25-14-7-9-15(10-8-14)37(24,32)33/h2-11,17,19-22,28,30-31H,12H2,1H3,(H,26,29)(H2,24,32,33)(H2,25,27,36)/b11-6+/t17-,19-,20-,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50248719

(CHEMBL4096770)Show SMILES COc1cc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)cc(OC)c1OC |r| Show InChI InChI=1S/C25H32N4O10S2/c1-36-16-10-13(11-17(37-2)23(16)38-3)4-9-19(31)28-20-22(33)21(32)18(12-30)39-24(20)29-25(40)27-14-5-7-15(8-6-14)41(26,34)35/h4-11,18,20-22,24,30,32-33H,12H2,1-3H3,(H,28,31)(H2,26,34,35)(H2,27,29,40)/b9-4+/t18-,20-,21-,22-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248708

(CHEMBL4090618)Show SMILES COc1ccccc1\C=C\C(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1NC(=S)Nc1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C23H28N4O8S2/c1-34-16-5-3-2-4-13(16)6-11-18(29)26-19-21(31)20(30)17(12-28)35-22(19)27-23(36)25-14-7-9-15(10-8-14)37(24,32)33/h2-11,17,19-22,28,30-31H,12H2,1H3,(H,26,29)(H2,24,32,33)(H2,25,27,36)/b11-6+/t17-,19-,20-,21-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50248710

(CHEMBL4070358)Show SMILES COc1cc(OC)c(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)cc1OC |r| Show InChI InChI=1S/C25H32N4O10S2/c1-36-16-11-18(38-3)17(37-2)10-13(16)4-9-20(31)28-21-23(33)22(32)19(12-30)39-24(21)29-25(40)27-14-5-7-15(8-6-14)41(26,34)35/h4-11,19,21-24,30,32-33H,12H2,1-3H3,(H,28,31)(H2,26,34,35)(H2,27,29,40)/b9-4+/t19-,21-,22-,23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50248706
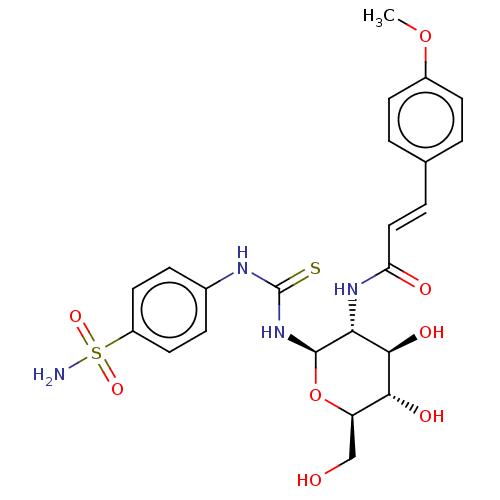
(CHEMBL4063719)Show SMILES COc1ccc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)cc1 |r| Show InChI InChI=1S/C23H28N4O8S2/c1-34-15-7-2-13(3-8-15)4-11-18(29)26-19-21(31)20(30)17(12-28)35-22(19)27-23(36)25-14-5-9-16(10-6-14)37(24,32)33/h2-11,17,19-22,28,30-31H,12H2,1H3,(H,26,29)(H2,24,32,33)(H2,25,27,36)/b11-4+/t17-,19-,20-,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248707

(CHEMBL4075581)Show SMILES COc1cccc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)c1 |r| Show InChI InChI=1S/C23H28N4O8S2/c1-34-15-4-2-3-13(11-15)5-10-18(29)26-19-21(31)20(30)17(12-28)35-22(19)27-23(36)25-14-6-8-16(9-7-14)37(24,32)33/h2-11,17,19-22,28,30-31H,12H2,1H3,(H,26,29)(H2,24,32,33)(H2,25,27,36)/b10-5+/t17-,19-,20-,21-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248706

(CHEMBL4063719)Show SMILES COc1ccc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)cc1 |r| Show InChI InChI=1S/C23H28N4O8S2/c1-34-15-7-2-13(3-8-15)4-11-18(29)26-19-21(31)20(30)17(12-28)35-22(19)27-23(36)25-14-5-9-16(10-6-14)37(24,32)33/h2-11,17,19-22,28,30-31H,12H2,1H3,(H,26,29)(H2,24,32,33)(H2,25,27,36)/b11-4+/t17-,19-,20-,21-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50248717
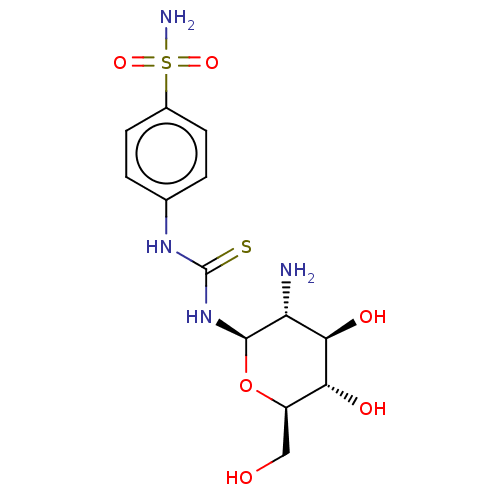
(CHEMBL4085325)Show SMILES N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1NC(=S)Nc1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C13H20N4O6S2/c14-9-11(20)10(19)8(5-18)23-12(9)17-13(24)16-6-1-3-7(4-2-6)25(15,21)22/h1-4,8-12,18-20H,5,14H2,(H2,15,21,22)(H2,16,17,24)/t8-,9-,10-,11-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 203 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248717

(CHEMBL4085325)Show SMILES N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1NC(=S)Nc1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C13H20N4O6S2/c14-9-11(20)10(19)8(5-18)23-12(9)17-13(24)16-6-1-3-7(4-2-6)25(15,21)22/h1-4,8-12,18-20H,5,14H2,(H2,15,21,22)(H2,16,17,24)/t8-,9-,10-,11-,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 667 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10857
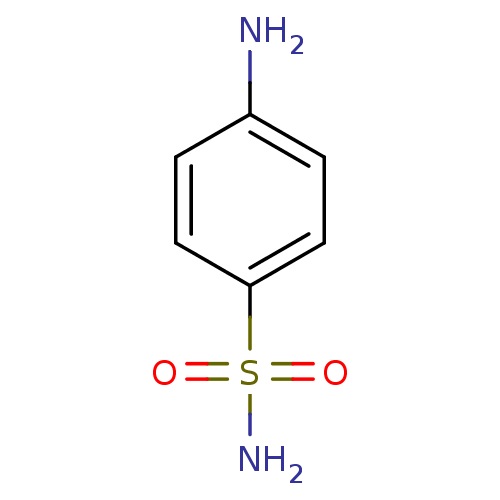
(4-aminobenzene-1-sulfonamide | CHEMBL21 | Sulfanil...)Show InChI InChI=1S/C6H8N2O2S/c7-5-1-3-6(4-2-5)11(8,9)10/h1-4H,7H2,(H2,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10857

(4-aminobenzene-1-sulfonamide | CHEMBL21 | Sulfanil...)Show InChI InChI=1S/C6H8N2O2S/c7-5-1-3-6(4-2-5)11(8,9)10/h1-4H,7H2,(H2,8,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50248711
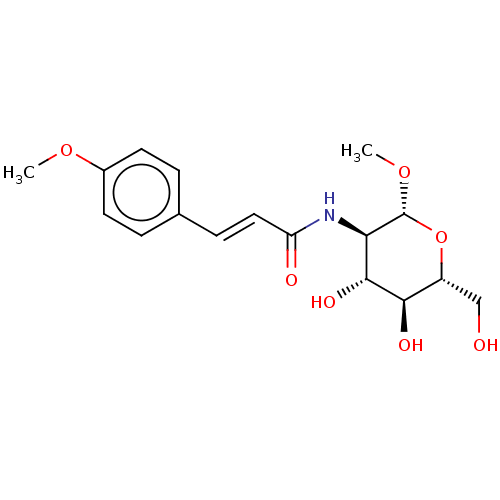
(CHEMBL4072315)Show SMILES CO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1NC(=O)\C=C\c1ccc(OC)cc1 |r| Show InChI InChI=1S/C17H23NO7/c1-23-11-6-3-10(4-7-11)5-8-13(20)18-14-16(22)15(21)12(9-19)25-17(14)24-2/h3-8,12,14-17,19,21-22H,9H2,1-2H3,(H,18,20)/b8-5+/t12-,14-,15-,16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248711

(CHEMBL4072315)Show SMILES CO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1NC(=O)\C=C\c1ccc(OC)cc1 |r| Show InChI InChI=1S/C17H23NO7/c1-23-11-6-3-10(4-7-11)5-8-13(20)18-14-16(22)15(21)12(9-19)25-17(14)24-2/h3-8,12,14-17,19,21-22H,9H2,1-2H3,(H,18,20)/b8-5+/t12-,14-,15-,16-,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50146453
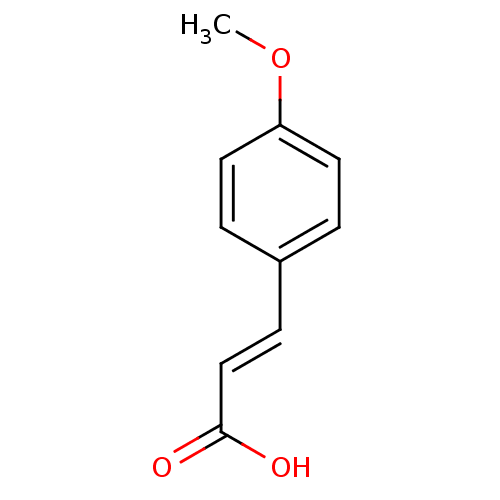
((E)-3-(4-Methoxy-phenyl)-acrylic acid | 3-(4-metho...)Show InChI InChI=1S/C10H10O3/c1-13-9-5-2-8(3-6-9)4-7-10(11)12/h2-7H,1H3,(H,11,12)/b7-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50146453

((E)-3-(4-Methoxy-phenyl)-acrylic acid | 3-(4-metho...)Show InChI InChI=1S/C10H10O3/c1-13-9-5-2-8(3-6-9)4-7-10(11)12/h2-7H,1H3,(H,11,12)/b7-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data