 Found 134 hits with Last Name = 'weinmann' and Initial = 'h'
Found 134 hits with Last Name = 'weinmann' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50180955
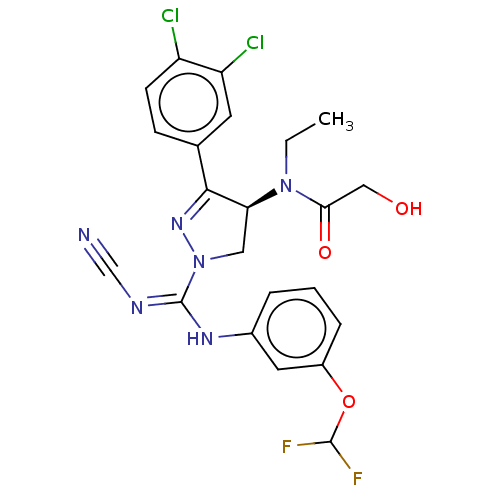
(CHEMBL3818617)Show SMILES CCN([C@H]1CN(N=C1c1ccc(Cl)c(Cl)c1)C(\Nc1cccc(OC(F)F)c1)=N/C#N)C(=O)CO |r,c:6| Show InChI InChI=1S/C22H20Cl2F2N6O3/c1-2-31(19(34)11-33)18-10-32(30-20(18)13-6-7-16(23)17(24)8-13)22(28-12-27)29-14-4-3-5-15(9-14)35-21(25)26/h3-9,18,21,33H,2,10-11H2,1H3,(H,28,29)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length 6xHis-tagged SMYD2 (unknown origin) expressed in Escherichia coli using varying levels of Btn-Ahx-GSRAHSSHLKSKK... |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50180955

(CHEMBL3818617)Show SMILES CCN([C@H]1CN(N=C1c1ccc(Cl)c(Cl)c1)C(\Nc1cccc(OC(F)F)c1)=N/C#N)C(=O)CO |r,c:6| Show InChI InChI=1S/C22H20Cl2F2N6O3/c1-2-31(19(34)11-33)18-10-32(30-20(18)13-6-7-16(23)17(24)8-13)22(28-12-27)29-14-4-3-5-15(9-14)35-21(25)26/h3-9,18,21,33H,2,10-11H2,1H3,(H,28,29)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of full length 6xHis-tagged SMYD2 (unknown origin) expressed in Escherichia coli using fixed levels of Btn-Ahx-GSRAHSSHLKSKK... |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50181506
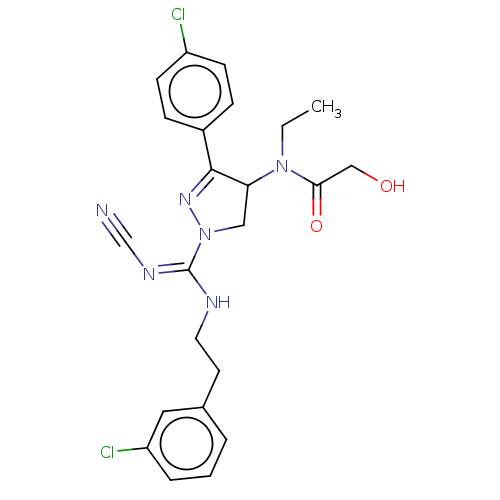
(CHEMBL3819038)Show SMILES CCN(C1CN(N=C1c1ccc(Cl)cc1)C(\NCCc1cccc(Cl)c1)=N/C#N)C(=O)CO |c:6| Show InChI InChI=1S/C23H24Cl2N6O2/c1-2-30(21(33)14-32)20-13-31(29-22(20)17-6-8-18(24)9-7-17)23(28-15-26)27-11-10-16-4-3-5-19(25)12-16/h3-9,12,20,32H,2,10-11,13-14H2,1H3,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Antagonist activity at PAR1 (unknown origin) |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50075102
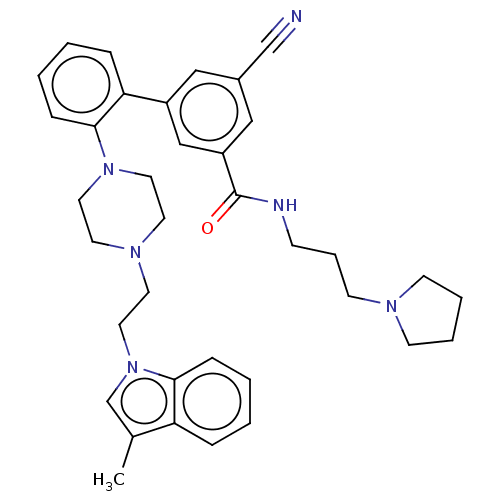
(CHEMBL3414623)Show SMILES Cc1cn(CCN2CCN(CC2)c2ccccc2-c2cc(cc(c2)C(=O)NCCCN2CCCC2)C#N)c2ccccc12 Show InChI InChI=1S/C36H42N6O/c1-28-27-42(34-11-4-2-9-32(28)34)22-19-40-17-20-41(21-18-40)35-12-5-3-10-33(35)30-23-29(26-37)24-31(25-30)36(43)38-13-8-16-39-14-6-7-15-39/h2-5,9-12,23-25,27H,6-8,13-22H2,1H3,(H,38,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <15 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of SMYD2 (unknown origin) expressed in Escherichia coli BL21 (DE3) using Biotinaminohexanoyl-GSRAHSSHLKSKKGQSTSRH as substrate after 75 mi... |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50180967

(CHEMBL3818487)Show SMILES CCN([C@H]1CN(N=C1c1ccc(Cl)c(Cl)c1)C(\Nc1cc(F)cc(OC(F)F)c1)=N/C#N)C(=O)CO |r,c:6| Show InChI InChI=1S/C22H19Cl2F3N6O3/c1-2-32(19(35)10-34)18-9-33(31-20(18)12-3-4-16(23)17(24)5-12)22(29-11-28)30-14-6-13(25)7-15(8-14)36-21(26)27/h3-8,18,21,34H,2,9-10H2,1H3,(H,29,30)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of SMYD2 (unknown origin) using H4 as substrate after 2hrs in presence 3H-SAM of by scintillation proximity assay |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50505787
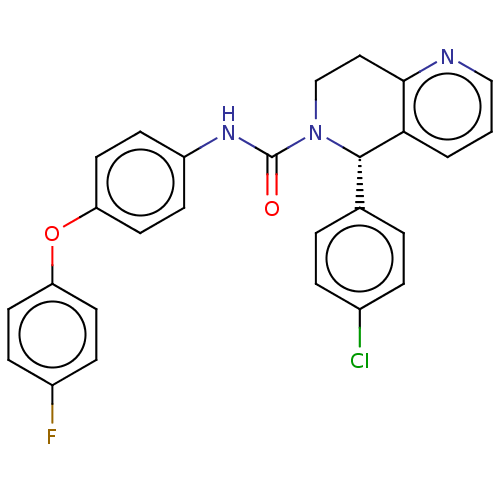
(CHEMBL4537998)Show SMILES Fc1ccc(Oc2ccc(NC(=O)N3CCc4ncccc4[C@@H]3c3ccc(Cl)cc3)cc2)cc1 |r| Show InChI InChI=1S/C27H21ClFN3O2/c28-19-5-3-18(4-6-19)26-24-2-1-16-30-25(24)15-17-32(26)27(33)31-21-9-13-23(14-10-21)34-22-11-7-20(29)8-12-22/h1-14,16,26H,15,17H2,(H,31,33)/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at rat luteinizing hormone receptor expressed in rat ovary granulosa GLHR15 cell suspension assessed as reduction in agonist-indu... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50180955

(CHEMBL3818617)Show SMILES CCN([C@H]1CN(N=C1c1ccc(Cl)c(Cl)c1)C(\Nc1cccc(OC(F)F)c1)=N/C#N)C(=O)CO |r,c:6| Show InChI InChI=1S/C22H20Cl2F2N6O3/c1-2-31(19(34)11-33)18-10-32(30-20(18)13-6-7-16(23)17(24)8-13)22(28-12-27)29-14-4-3-5-15(9-14)35-21(25)26/h3-9,18,21,33H,2,10-11H2,1H3,(H,28,29)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of full length 6xHis-tagged SMYD2 (unknown origin) expressed in Escherichia coli using Btn-Ahx GSRAHSSHLKSKKGQSTSRH-amide as substrate aft... |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50181502
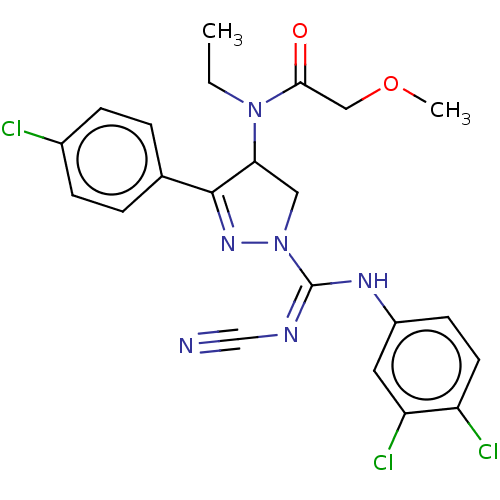
(CHEMBL3818510)Show SMILES CCN(C1CN(N=C1c1ccc(Cl)cc1)C(\Nc1ccc(Cl)c(Cl)c1)=N/C#N)C(=O)COC |c:6| Show InChI InChI=1S/C22H21Cl3N6O2/c1-3-30(20(32)12-33-2)19-11-31(29-21(19)14-4-6-15(23)7-5-14)22(27-13-26)28-16-8-9-17(24)18(25)10-16/h4-10,19H,3,11-12H2,1-2H3,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Antagonist activity at PAR1 (unknown origin) |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50181504
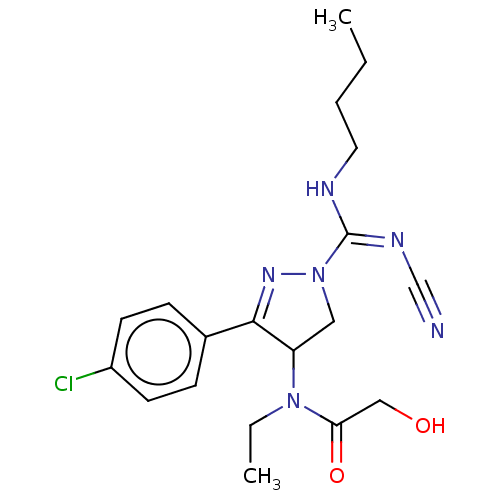
(CHEMBL3818898)Show SMILES CCCCN\C(=N\C#N)N1CC(N(CC)C(=O)CO)C(=N1)c1ccc(Cl)cc1 |c:19| Show InChI InChI=1S/C19H25ClN6O2/c1-3-5-10-22-19(23-13-21)26-11-16(25(4-2)17(28)12-27)18(24-26)14-6-8-15(20)9-7-14/h6-9,16,27H,3-5,10-12H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Antagonist activity at PAR1 (unknown origin) |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50180973
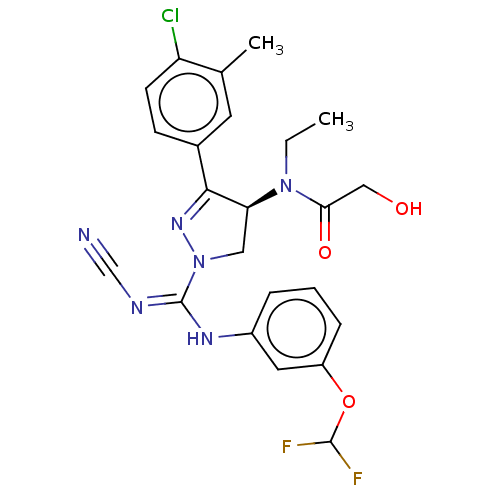
(CHEMBL3819284)Show SMILES CCN([C@H]1CN(N=C1c1ccc(Cl)c(C)c1)C(\Nc1cccc(OC(F)F)c1)=N/C#N)C(=O)CO |r,c:6| Show InChI InChI=1S/C23H23ClF2N6O3/c1-3-31(20(34)12-33)19-11-32(30-21(19)15-7-8-18(24)14(2)9-15)23(28-13-27)29-16-5-4-6-17(10-16)35-22(25)26/h4-10,19,22,33H,3,11-12H2,1-2H3,(H,28,29)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of SMYD2 (unknown origin) using H4 as substrate after 2hrs in presence 3H-SAM of by scintillation proximity assay |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50181505
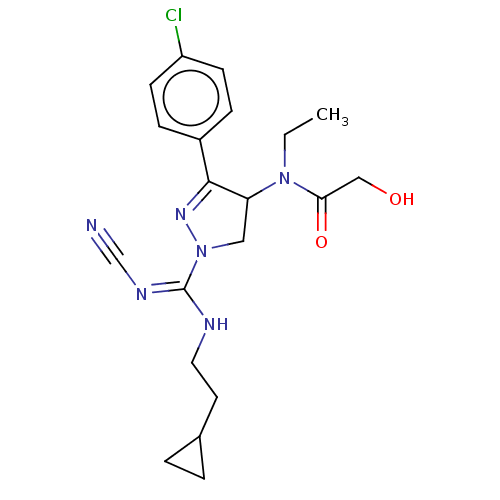
(CHEMBL3819204)Show SMILES CCN(C1CN(N=C1c1ccc(Cl)cc1)C(\NCCC1CC1)=N/C#N)C(=O)CO |c:6| Show InChI InChI=1S/C20H25ClN6O2/c1-2-26(18(29)12-28)17-11-27(20(24-13-22)23-10-9-14-3-4-14)25-19(17)15-5-7-16(21)8-6-15/h5-8,14,17,28H,2-4,9-12H2,1H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Antagonist activity at PAR1 (unknown origin) |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50180968
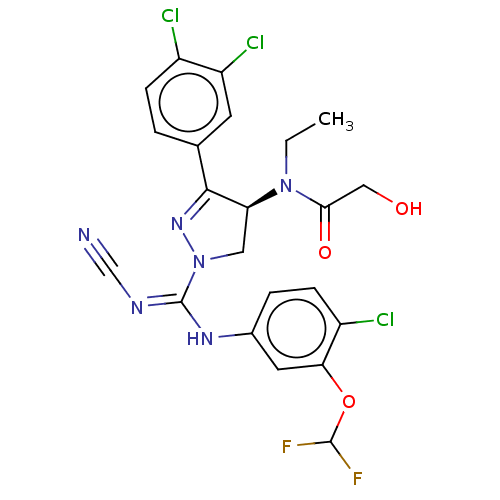
(CHEMBL3818080)Show SMILES CCN([C@H]1CN(N=C1c1ccc(Cl)c(Cl)c1)C(\Nc1ccc(Cl)c(OC(F)F)c1)=N/C#N)C(=O)CO |r,c:6| Show InChI InChI=1S/C22H19Cl3F2N6O3/c1-2-32(19(35)10-34)17-9-33(31-20(17)12-3-5-14(23)16(25)7-12)22(29-11-28)30-13-4-6-15(24)18(8-13)36-21(26)27/h3-8,17,21,34H,2,9-10H2,1H3,(H,29,30)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of SMYD2 (unknown origin) using H4 as substrate after 2hrs in presence 3H-SAM of by scintillation proximity assay |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50505783
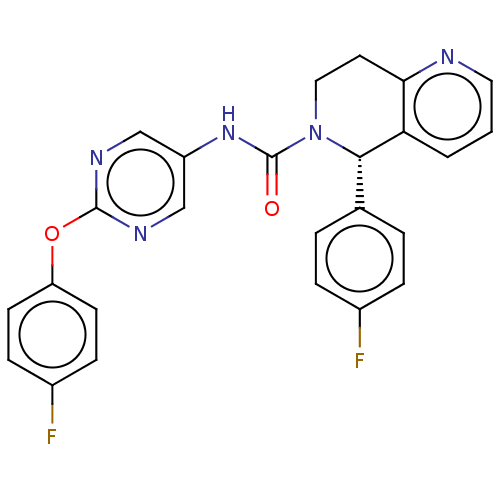
(CHEMBL4458424)Show SMILES Fc1ccc(Oc2ncc(NC(=O)N3CCc4ncccc4[C@@H]3c3ccc(F)cc3)cn2)cc1 |r| Show InChI InChI=1S/C25H19F2N5O2/c26-17-5-3-16(4-6-17)23-21-2-1-12-28-22(21)11-13-32(23)25(33)31-19-14-29-24(30-15-19)34-20-9-7-18(27)8-10-20/h1-10,12,14-15,23H,11,13H2,(H,31,33)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at rat luteinizing hormone receptor expressed in rat ovary granulosa GLHR15 cell suspension assessed as reduction in agonist-indu... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50181513
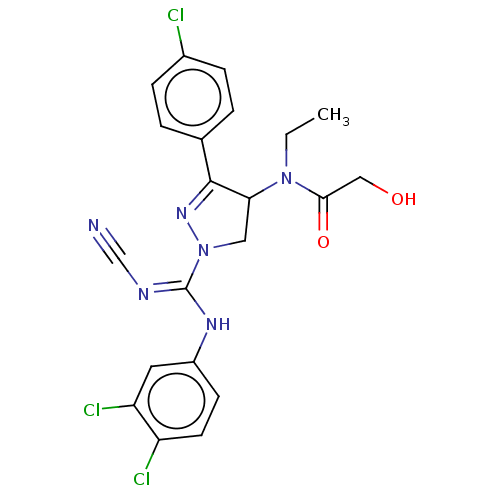
(CHEMBL3819237)Show SMILES CCN(C1CN(N=C1c1ccc(Cl)cc1)C(\Nc1ccc(Cl)c(Cl)c1)=N/C#N)C(=O)CO |c:6| Show InChI InChI=1S/C21H19Cl3N6O2/c1-2-29(19(32)11-31)18-10-30(28-20(18)13-3-5-14(22)6-4-13)21(26-12-25)27-15-7-8-16(23)17(24)9-15/h3-9,18,31H,2,10-11H2,1H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Antagonist activity at PAR1 (unknown origin) |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50180971
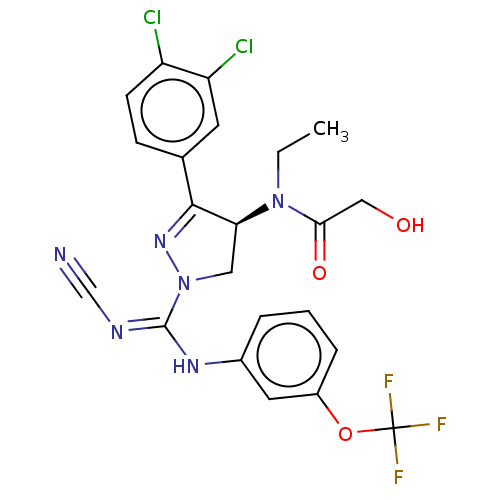
(CHEMBL3818322)Show SMILES CCN([C@H]1CN(N=C1c1ccc(Cl)c(Cl)c1)C(\Nc1cccc(OC(F)(F)F)c1)=N/C#N)C(=O)CO |r,c:6| Show InChI InChI=1S/C22H19Cl2F3N6O3/c1-2-32(19(35)11-34)18-10-33(31-20(18)13-6-7-16(23)17(24)8-13)21(29-12-28)30-14-4-3-5-15(9-14)36-22(25,26)27/h3-9,18,34H,2,10-11H2,1H3,(H,29,30)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of SMYD2 (unknown origin) using H4 as substrate after 2hrs in presence 3H-SAM of by scintillation proximity assay |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50181507
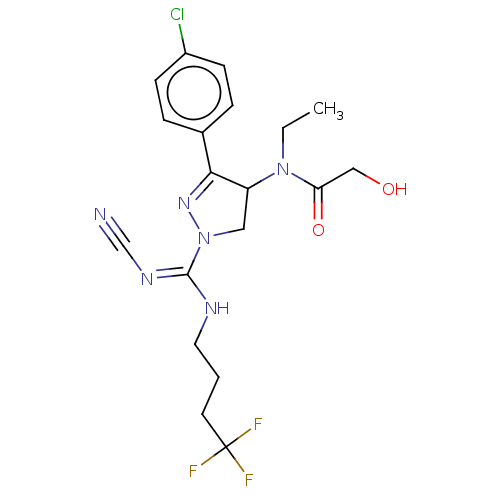
(CHEMBL3818421)Show SMILES CCN(C1CN(N=C1c1ccc(Cl)cc1)C(\NCCCC(F)(F)F)=N/C#N)C(=O)CO |c:6| Show InChI InChI=1S/C19H22ClF3N6O2/c1-2-28(16(31)11-30)15-10-29(27-17(15)13-4-6-14(20)7-5-13)18(26-12-24)25-9-3-8-19(21,22)23/h4-7,15,30H,2-3,8-11H2,1H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Antagonist activity at PAR1 (unknown origin) |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50181511

(CHEMBL3819343)Show SMILES CCN(C1CN(N=C1c1ccc(Cl)cc1)C(\Nc1ccc(OC(F)F)cc1)=N/C#N)C(=O)CO |c:6| Show InChI InChI=1S/C22H21ClF2N6O3/c1-2-30(19(33)12-32)18-11-31(29-20(18)14-3-5-15(23)6-4-14)22(27-13-26)28-16-7-9-17(10-8-16)34-21(24)25/h3-10,18,21,32H,2,11-12H2,1H3,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Antagonist activity at PAR1 (unknown origin) |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50180955

(CHEMBL3818617)Show SMILES CCN([C@H]1CN(N=C1c1ccc(Cl)c(Cl)c1)C(\Nc1cccc(OC(F)F)c1)=N/C#N)C(=O)CO |r,c:6| Show InChI InChI=1S/C22H20Cl2F2N6O3/c1-2-31(19(34)11-33)18-10-32(30-20(18)13-6-7-16(23)17(24)8-13)22(28-12-27)29-14-4-3-5-15(9-14)35-21(25)26/h3-9,18,21,33H,2,10-11H2,1H3,(H,28,29)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 2xc-myc-tagged human SMYD2 transfected in human MDA-MB-231 cells assessed as reduction in AHNAK methylation after 72 hrs by ... |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50181509
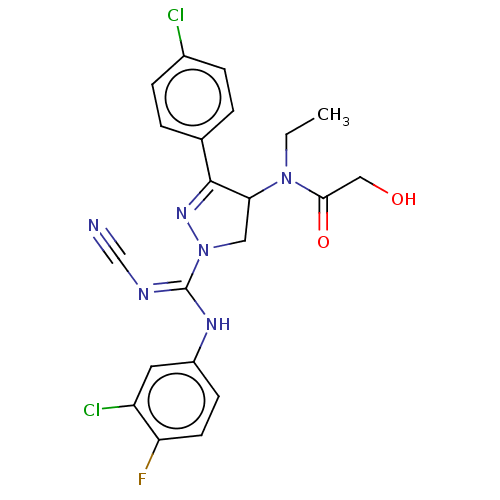
(CHEMBL3817930)Show SMILES CCN(C1CN(N=C1c1ccc(Cl)cc1)C(\Nc1ccc(F)c(Cl)c1)=N/C#N)C(=O)CO |c:6| Show InChI InChI=1S/C21H19Cl2FN6O2/c1-2-29(19(32)11-31)18-10-30(28-20(18)13-3-5-14(22)6-4-13)21(26-12-25)27-15-7-8-17(24)16(23)9-15/h3-9,18,31H,2,10-11H2,1H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Antagonist activity at PAR1 (unknown origin) |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50181512

(CHEMBL3819277 | US10023539, Example WO 2006/07235...)Show SMILES CCN(C1CN(N=C1c1ccc(Cl)cc1)C(\Nc1cccc(OC(F)F)c1)=N/C#N)C(=O)CO |c:6| Show InChI InChI=1S/C22H21ClF2N6O3/c1-2-30(19(33)12-32)18-11-31(29-20(18)14-6-8-15(23)9-7-14)22(27-13-26)28-16-4-3-5-17(10-16)34-21(24)25/h3-10,18,21,32H,2,11-12H2,1H3,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Antagonist activity at PAR1 (unknown origin) |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50180970

(CHEMBL3819554)Show SMILES CCN([C@H]1CN(N=C1c1ccc(Cl)c(Cl)c1)C(\Nc1cccc(OC)c1)=N/C#N)C(=O)CO |r,c:6| Show InChI InChI=1S/C22H22Cl2N6O3/c1-3-29(20(32)12-31)19-11-30(28-21(19)14-7-8-17(23)18(24)9-14)22(26-13-25)27-15-5-4-6-16(10-15)33-2/h4-10,19,31H,3,11-12H2,1-2H3,(H,26,27)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of SMYD2 (unknown origin) using H4 as substrate after 2hrs in presence 3H-SAM of by scintillation proximity assay |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
Luteinizing hormone/choriogondaotropin receptor
(Macaca fascicularis) | BDBM50505787

(CHEMBL4537998)Show SMILES Fc1ccc(Oc2ccc(NC(=O)N3CCc4ncccc4[C@@H]3c3ccc(Cl)cc3)cc2)cc1 |r| Show InChI InChI=1S/C27H21ClFN3O2/c28-19-5-3-18(4-6-19)26-24-2-1-16-30-25(24)15-17-32(26)27(33)31-21-9-13-23(14-10-21)34-22-11-7-20(29)8-12-22/h1-14,16,26H,15,17H2,(H,31,33)/t26-/m0/s1 | PDB
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at cynomolgus monkey luteinizing hormone receptor expressed in HEK293 cells assessed as reduction in agonist-induced cAMP product... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50181508
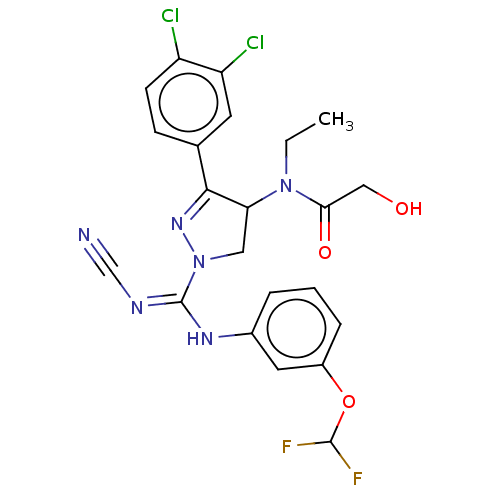
(CHEMBL3818083 | US10023539, Example 4.2)Show SMILES CCN(C1CN(N=C1c1ccc(Cl)c(Cl)c1)C(\Nc1cccc(OC(F)F)c1)=N/C#N)C(=O)CO |c:6| Show InChI InChI=1S/C22H20Cl2F2N6O3/c1-2-31(19(34)11-33)18-10-32(30-20(18)13-6-7-16(23)17(24)8-13)22(28-12-27)29-14-4-3-5-15(9-14)35-21(25)26/h3-9,18,21,33H,2,10-11H2,1H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of full length 6xHis-tagged SMYD2 (unknown origin) expressed in Escherichia coli using Btn-Ahx GSRAHSSHLKSKKGQSTSRH-amide as substrate aft... |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50180974

(CHEMBL3818092 | US10023539, Example 33.2)Show SMILES CCN(C1CN(N=C1c1ccc(Cl)c(C)c1)C(\Nc1cccc(OC(F)F)c1)=N/C#N)C(=O)CO |c:6| Show InChI InChI=1S/C23H23ClF2N6O3/c1-3-31(20(34)12-33)19-11-32(30-21(19)15-7-8-18(24)14(2)9-15)23(28-13-27)29-16-5-4-6-17(10-16)35-22(25)26/h4-10,19,22,33H,3,11-12H2,1-2H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of SMYD2 (unknown origin) using H4 as substrate after 2hrs in presence 3H-SAM of by scintillation proximity assay |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50181510

(CHEMBL3818750)Show SMILES CCN(C1CN(N=C1c1ccc(Cl)cc1)C(\Nc1ccc2OC(F)(F)Oc2c1)=N/C#N)C(=O)CO |c:6| Show InChI InChI=1S/C22H19ClF2N6O4/c1-2-30(19(33)11-32)16-10-31(29-20(16)13-3-5-14(23)6-4-13)21(27-12-26)28-15-7-8-17-18(9-15)35-22(24,25)34-17/h3-9,16,32H,2,10-11H2,1H3,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Antagonist activity at PAR1 (unknown origin) |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Homo sapiens (Human)) | BDBM50505809
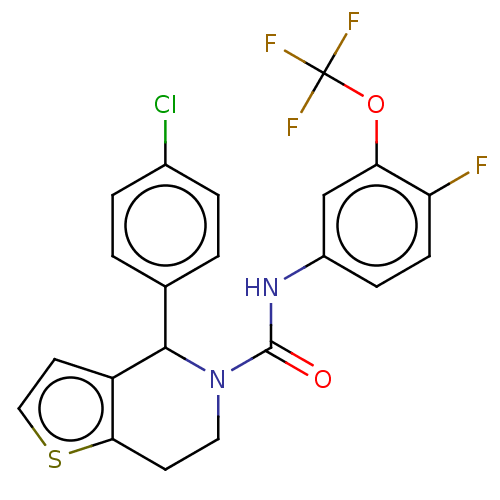
(CHEMBL4460075)Show SMILES Fc1ccc(NC(=O)N2CCc3sccc3C2c2ccc(Cl)cc2)cc1OC(F)(F)F Show InChI InChI=1S/C21H15ClF4N2O2S/c22-13-3-1-12(2-4-13)19-15-8-10-31-18(15)7-9-28(19)20(29)27-14-5-6-16(23)17(11-14)30-21(24,25)26/h1-6,8,10-11,19H,7,9H2,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Homo sapiens (Human)) | BDBM50383229
(CHEMBL2032175)Show InChI InChI=1S/C20H17ClN2OS/c21-15-8-6-14(7-9-15)19-17-11-13-25-18(17)10-12-23(19)20(24)22-16-4-2-1-3-5-16/h1-9,11,13,19H,10,12H2,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Homo sapiens (Human)) | BDBM50505787

(CHEMBL4537998)Show SMILES Fc1ccc(Oc2ccc(NC(=O)N3CCc4ncccc4[C@@H]3c3ccc(Cl)cc3)cc2)cc1 |r| Show InChI InChI=1S/C27H21ClFN3O2/c28-19-5-3-18(4-6-19)26-24-2-1-16-30-25(24)15-17-32(26)27(33)31-21-9-13-23(14-10-21)34-22-11-7-20(29)8-12-22/h1-14,16,26H,15,17H2,(H,31,33)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Homo sapiens (Human)) | BDBM50505787

(CHEMBL4537998)Show SMILES Fc1ccc(Oc2ccc(NC(=O)N3CCc4ncccc4[C@@H]3c3ccc(Cl)cc3)cc2)cc1 |r| Show InChI InChI=1S/C27H21ClFN3O2/c28-19-5-3-18(4-6-19)26-24-2-1-16-30-25(24)15-17-32(26)27(33)31-21-9-13-23(14-10-21)34-22-11-7-20(29)8-12-22/h1-14,16,26H,15,17H2,(H,31,33)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of human luteinizing hormone receptor assessed as reduction in glycoprotein human luteinizing hormone-induced agonist ... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
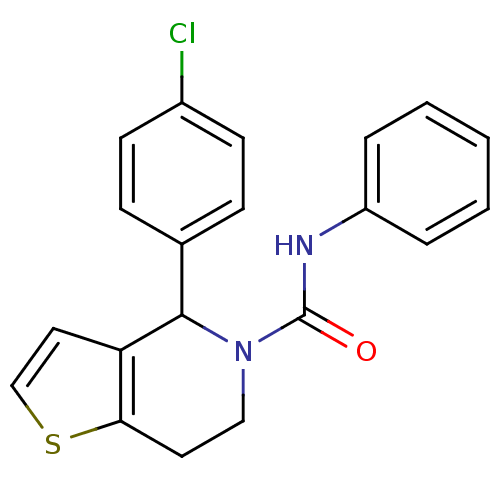
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50180978
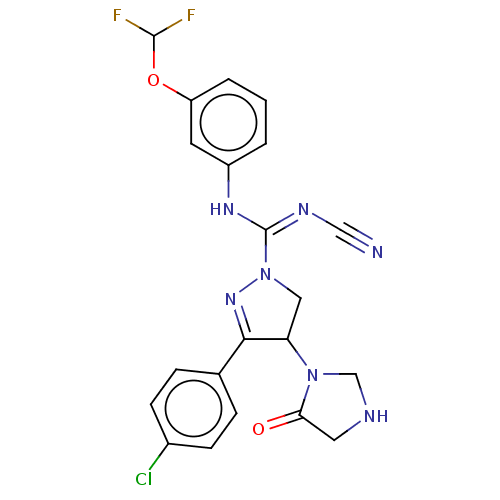
(CHEMBL3819515)Show SMILES FC(F)Oc1cccc(N\C(=N\C#N)N2CC(N3CNCC3=O)C(=N2)c2ccc(Cl)cc2)c1 |c:24| Show InChI InChI=1S/C21H18ClF2N7O2/c22-14-6-4-13(5-7-14)19-17(30-12-26-9-18(30)32)10-31(29-19)21(27-11-25)28-15-2-1-3-16(8-15)33-20(23)24/h1-8,17,20,26H,9-10,12H2,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Antagonist activity at PAR1 (unknown origin) |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Homo sapiens (Human)) | BDBM50505791
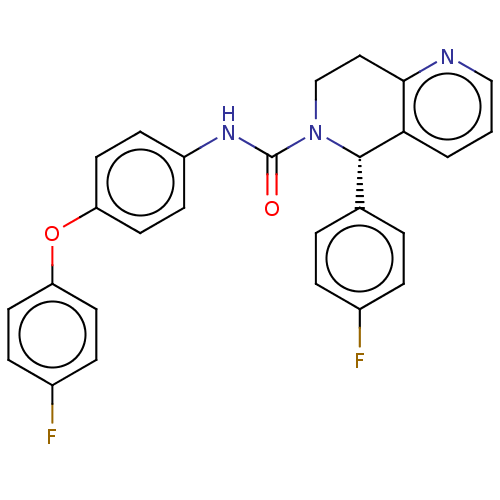
(CHEMBL4587440)Show SMILES Fc1ccc(Oc2ccc(NC(=O)N3CCc4ncccc4[C@@H]3c3ccc(F)cc3)cc2)cc1 |r| Show InChI InChI=1S/C27H21F2N3O2/c28-19-5-3-18(4-6-19)26-24-2-1-16-30-25(24)15-17-32(26)27(33)31-21-9-13-23(14-10-21)34-22-11-7-20(29)8-12-22/h1-14,16,26H,15,17H2,(H,31,33)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Homo sapiens (Human)) | BDBM50505799
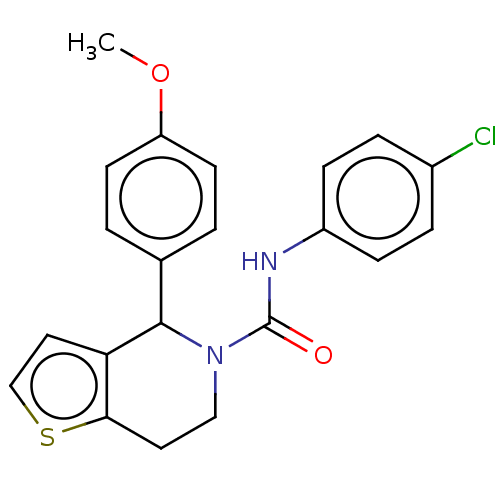
(CHEMBL4573509)Show SMILES COc1ccc(cc1)C1N(CCc2sccc12)C(=O)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C21H19ClN2O2S/c1-26-17-8-2-14(3-9-17)20-18-11-13-27-19(18)10-12-24(20)21(25)23-16-6-4-15(22)5-7-16/h2-9,11,13,20H,10,12H2,1H3,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50180969

(CHEMBL3818526)Show SMILES CCN([C@H]1CN(N=C1c1ccc(Cl)c(Cl)c1)C(\Nc1cccc(OC(F)F)n1)=N/C#N)C(=O)CO |r,c:6| Show InChI InChI=1S/C21H19Cl2F2N7O3/c1-2-31(18(34)10-33)15-9-32(30-19(15)12-6-7-13(22)14(23)8-12)21(27-11-26)29-16-4-3-5-17(28-16)35-20(24)25/h3-8,15,20,33H,2,9-10H2,1H3,(H,27,28,29)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of SMYD2 (unknown origin) using H4 as substrate after 2hrs in presence 3H-SAM of by scintillation proximity assay |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50396022
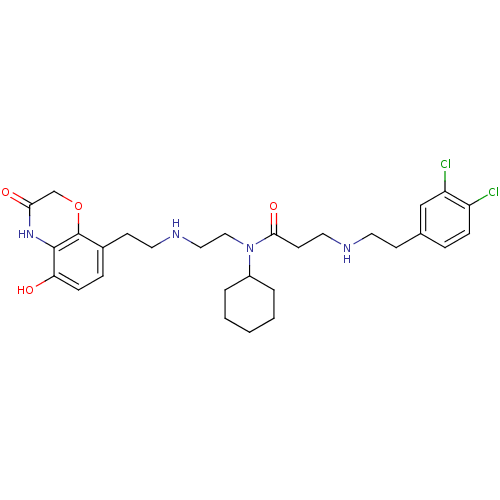
(CHEMBL2169920)Show SMILES Oc1ccc(CCNCCN(C2CCCCC2)C(=O)CCNCCc2ccc(Cl)c(Cl)c2)c2OCC(=O)Nc12 Show InChI InChI=1S/C29H38Cl2N4O4/c30-23-8-6-20(18-24(23)31)10-13-32-15-12-27(38)35(22-4-2-1-3-5-22)17-16-33-14-11-21-7-9-25(36)28-29(21)39-19-26(37)34-28/h6-9,18,22,32-33,36H,1-5,10-17,19H2,(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of SMYD2 (unknown origin) expressed in Escherichia coli BL21 (DE3) using Biotinaminohexanoyl-GSRAHSSHLKSKKGQSTSRH as substrate after 75 mi... |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50396022

(CHEMBL2169920)Show SMILES Oc1ccc(CCNCCN(C2CCCCC2)C(=O)CCNCCc2ccc(Cl)c(Cl)c2)c2OCC(=O)Nc12 Show InChI InChI=1S/C29H38Cl2N4O4/c30-23-8-6-20(18-24(23)31)10-13-32-15-12-27(38)35(22-4-2-1-3-5-22)17-16-33-14-11-21-7-9-25(36)28-29(21)39-19-26(37)34-28/h6-9,18,22,32-33,36H,1-5,10-17,19H2,(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of SMYD2 (unknown origin) expressed in Escherichia coli BL21 (DE3) using Biotinaminohexanoyl-GSRAHSSHLKSKKGQSTSRH as substrate after 75 mi... |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lutropin-choriogonadotropic hormone receptor
(Homo sapiens (Human)) | BDBM50505796
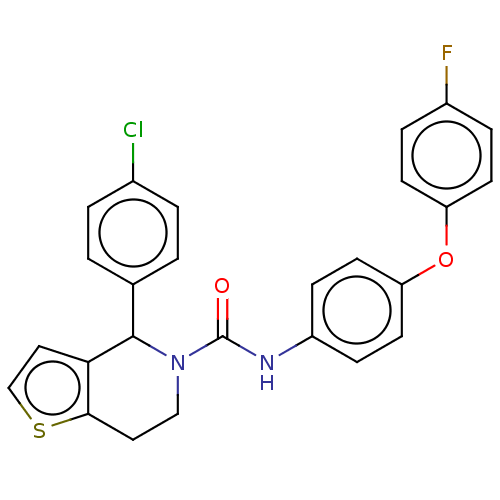
(CHEMBL4531781)Show SMILES Fc1ccc(Oc2ccc(NC(=O)N3CCc4sccc4C3c3ccc(Cl)cc3)cc2)cc1 Show InChI InChI=1S/C26H20ClFN2O2S/c27-18-3-1-17(2-4-18)25-23-14-16-33-24(23)13-15-30(25)26(31)29-20-7-11-22(12-8-20)32-21-9-5-19(28)6-10-21/h1-12,14,16,25H,13,15H2,(H,29,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50181454

(CHEMBL3818932)Show SMILES CCN(C1CN(N=C1c1ccc(Cl)cc1)C(\Nc1ccc(Cl)c(Cl)c1)=N/C#N)C(=O)CN |c:6| Show InChI InChI=1S/C21H20Cl3N7O/c1-2-30(19(32)10-25)18-11-31(29-20(18)13-3-5-14(22)6-4-13)21(27-12-26)28-15-7-8-16(23)17(24)9-15/h3-9,18H,2,10-11,25H2,1H3,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Antagonist activity at PAR1 (unknown origin) |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Homo sapiens (Human)) | BDBM50505785
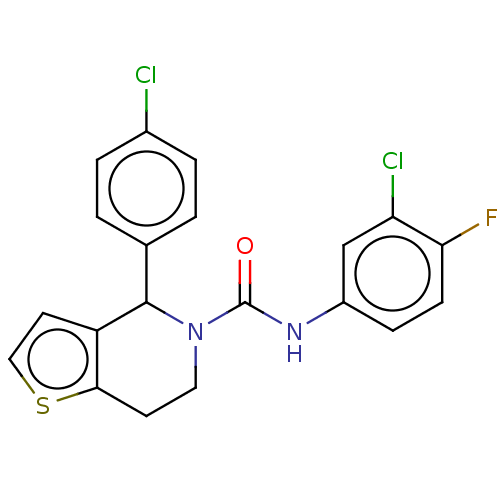
(CHEMBL4443605)Show SMILES Fc1ccc(NC(=O)N2CCc3sccc3C2c2ccc(Cl)cc2)cc1Cl Show InChI InChI=1S/C20H15Cl2FN2OS/c21-13-3-1-12(2-4-13)19-15-8-10-27-18(15)7-9-25(19)20(26)24-14-5-6-17(23)16(22)11-14/h1-6,8,10-11,19H,7,9H2,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Homo sapiens (Human)) | BDBM50505783

(CHEMBL4458424)Show SMILES Fc1ccc(Oc2ncc(NC(=O)N3CCc4ncccc4[C@@H]3c3ccc(F)cc3)cn2)cc1 |r| Show InChI InChI=1S/C25H19F2N5O2/c26-17-5-3-16(4-6-17)23-21-2-1-12-28-22(21)11-13-32(23)25(33)31-19-14-29-24(30-15-19)34-20-9-7-18(27)8-10-20/h1-10,12,14-15,23H,11,13H2,(H,31,33)/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Homo sapiens (Human)) | BDBM50505811
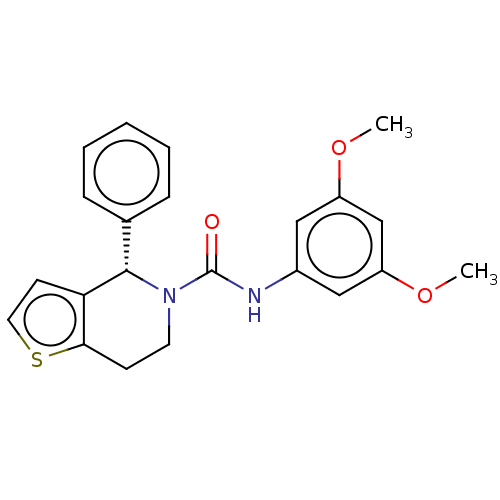
(CHEMBL4461980)Show SMILES COc1cc(NC(=O)N2CCc3sccc3[C@@H]2c2ccccc2)cc(OC)c1 |r| Show InChI InChI=1S/C22H22N2O3S/c1-26-17-12-16(13-18(14-17)27-2)23-22(25)24-10-8-20-19(9-11-28-20)21(24)15-6-4-3-5-7-15/h3-7,9,11-14,21H,8,10H2,1-2H3,(H,23,25)/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 193 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Homo sapiens (Human)) | BDBM50505787

(CHEMBL4537998)Show SMILES Fc1ccc(Oc2ccc(NC(=O)N3CCc4ncccc4[C@@H]3c3ccc(Cl)cc3)cc2)cc1 |r| Show InChI InChI=1S/C27H21ClFN3O2/c28-19-5-3-18(4-6-19)26-24-2-1-16-30-25(24)15-17-32(26)27(33)31-21-9-13-23(14-10-21)34-22-11-7-20(29)8-12-22/h1-14,16,26H,15,17H2,(H,31,33)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of human luteinizing hormone receptor assessed as reduction in glycoprotein human luteinizing hormone-induced agonist ... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Homo sapiens (Human)) | BDBM50505797
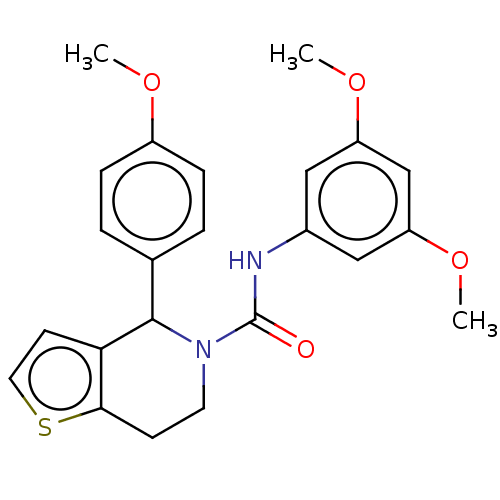
(CHEMBL4522381)Show SMILES COc1ccc(cc1)C1N(CCc2sccc12)C(=O)Nc1cc(OC)cc(OC)c1 Show InChI InChI=1S/C23H24N2O4S/c1-27-17-6-4-15(5-7-17)22-20-9-11-30-21(20)8-10-25(22)23(26)24-16-12-18(28-2)14-19(13-16)29-3/h4-7,9,11-14,22H,8,10H2,1-3H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50505793
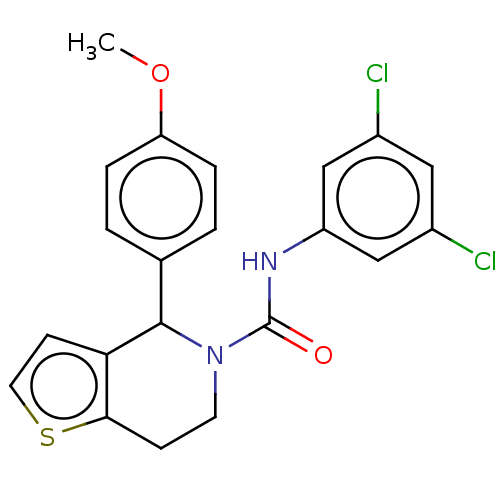
(CHEMBL4593222)Show SMILES COc1ccc(cc1)C1N(CCc2sccc12)C(=O)Nc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C21H18Cl2N2O2S/c1-27-17-4-2-13(3-5-17)20-18-7-9-28-19(18)6-8-25(20)21(26)24-16-11-14(22)10-15(23)12-16/h2-5,7,9-12,20H,6,8H2,1H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 212 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at rat luteinizing hormone receptor expressed in rat ovary granulosa GLHR15 cell suspension assessed as reduction in agonist-indu... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Homo sapiens (Human)) | BDBM50505798
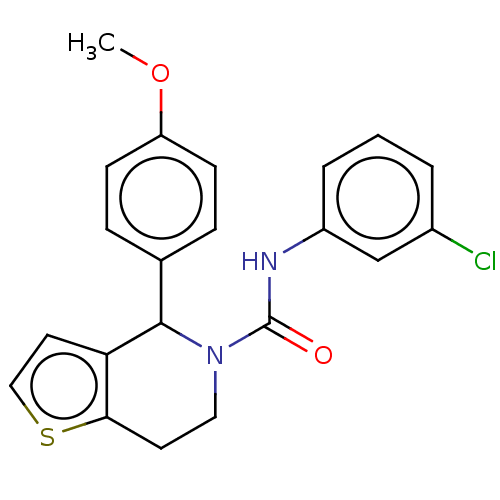
(CHEMBL4581889)Show SMILES COc1ccc(cc1)C1N(CCc2sccc12)C(=O)Nc1cccc(Cl)c1 Show InChI InChI=1S/C21H19ClN2O2S/c1-26-17-7-5-14(6-8-17)20-18-10-12-27-19(18)9-11-24(20)21(25)23-16-4-2-3-15(22)13-16/h2-8,10,12-13,20H,9,11H2,1H3,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Homo sapiens (Human)) | BDBM50505812
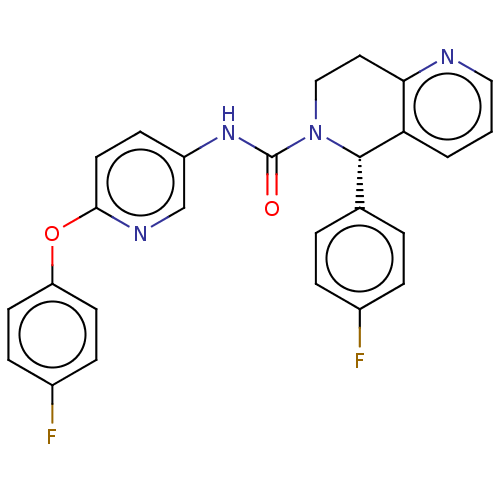
(CHEMBL4555151)Show SMILES Fc1ccc(Oc2ccc(NC(=O)N3CCc4ncccc4[C@@H]3c3ccc(F)cc3)cn2)cc1 |r| Show InChI InChI=1S/C26H20F2N4O2/c27-18-5-3-17(4-6-18)25-22-2-1-14-29-23(22)13-15-32(25)26(33)31-20-9-12-24(30-16-20)34-21-10-7-19(28)8-11-21/h1-12,14,16,25H,13,15H2,(H,31,33)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Homo sapiens (Human)) | BDBM50505795

(CHEMBL4579421)Show SMILES Clc1ccc(cc1)C1N(CCc2sccc12)C(=O)Nc1cccc(Oc2ccccc2)c1 Show InChI InChI=1S/C26H21ClN2O2S/c27-19-11-9-18(10-12-19)25-23-14-16-32-24(23)13-15-29(25)26(30)28-20-5-4-8-22(17-20)31-21-6-2-1-3-7-21/h1-12,14,16-17,25H,13,15H2,(H,28,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 332 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Homo sapiens (Human)) | BDBM50505793

(CHEMBL4593222)Show SMILES COc1ccc(cc1)C1N(CCc2sccc12)C(=O)Nc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C21H18Cl2N2O2S/c1-27-17-4-2-13(3-5-17)20-18-7-9-28-19(18)6-8-25(20)21(26)24-16-11-14(22)10-15(23)12-16/h2-5,7,9-12,20H,6,8H2,1H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 378 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50075102

(CHEMBL3414623)Show SMILES Cc1cn(CCN2CCN(CC2)c2ccccc2-c2cc(cc(c2)C(=O)NCCCN2CCCC2)C#N)c2ccccc12 Show InChI InChI=1S/C36H42N6O/c1-28-27-42(34-11-4-2-9-32(28)34)22-19-40-17-20-41(21-18-40)35-12-5-3-10-33(35)30-23-29(26-37)24-31(25-30)36(43)38-13-8-16-39-14-6-7-15-39/h2-5,9-12,23-25,27H,6-8,13-22H2,1H3,(H,38,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 388 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of SMYD2 (unknown origin) using H4 as substrate after 2hrs in presence 3H-SAM of by scintillation proximity assay |
J Med Chem 59: 4578-600 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01890
BindingDB Entry DOI: 10.7270/Q2RN39S5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lutropin-choriogonadotropic hormone receptor
(Homo sapiens (Human)) | BDBM50505789
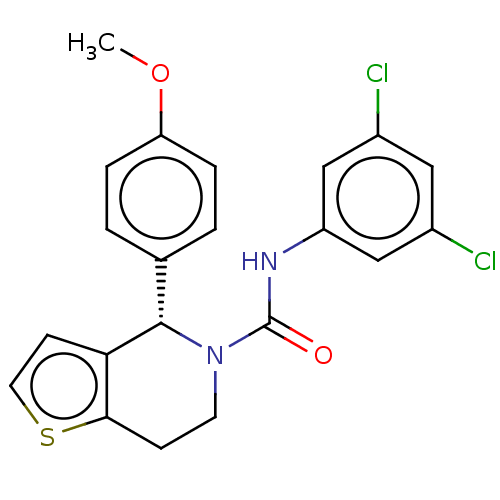
(CHEMBL4471621)Show SMILES COc1ccc(cc1)[C@@H]1N(CCc2sccc12)C(=O)Nc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C21H18Cl2N2O2S/c1-27-17-4-2-13(3-5-17)20-18-7-9-28-19(18)6-8-25(20)21(26)24-16-11-14(22)10-15(23)12-16/h2-5,7,9-12,20H,6,8H2,1H3,(H,24,26)/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 403 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50505789

(CHEMBL4471621)Show SMILES COc1ccc(cc1)[C@@H]1N(CCc2sccc12)C(=O)Nc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C21H18Cl2N2O2S/c1-27-17-4-2-13(3-5-17)20-18-7-9-28-19(18)6-8-25(20)21(26)24-16-11-14(22)10-15(23)12-16/h2-5,7,9-12,20H,6,8H2,1H3,(H,24,26)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 412 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at rat luteinizing hormone receptor expressed in rat ovary granulosa GLHR15 cell suspension assessed as reduction in agonist-indu... |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data