

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10804 ((3R)-3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10807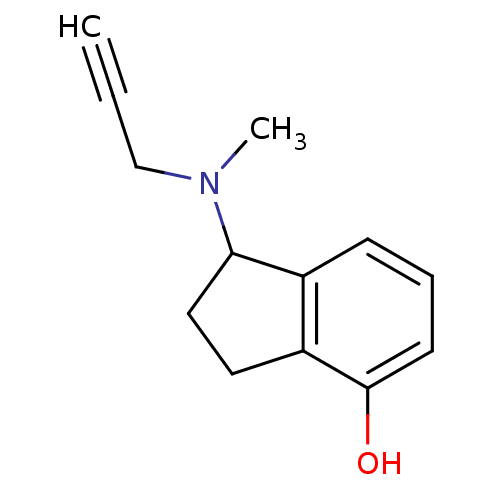 (1-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50400030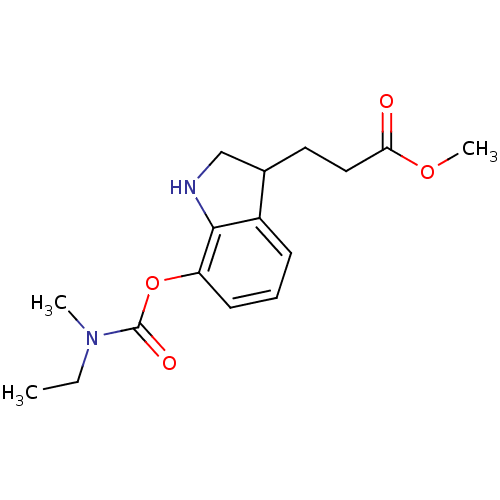 (CHEMBL2177713) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of equine BuChE after 120 mins by Ellman's method | J Med Chem 55: 10700-15 (2012) Article DOI: 10.1021/jm301411g BindingDB Entry DOI: 10.7270/Q23X87S3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10742 (1-amino-2,3-dihydro-1H-inden-4-yl N,N-dimethylcarb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10796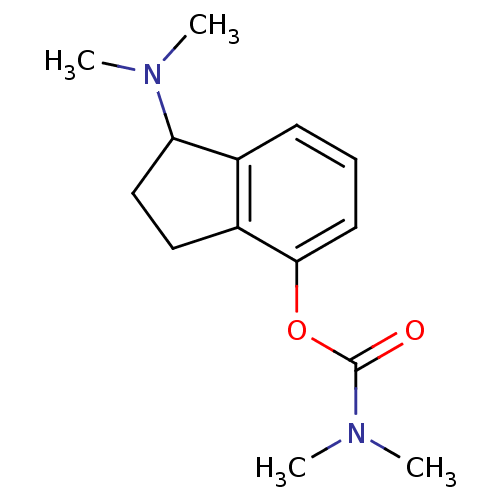 (1-(dimethylamino)-2,3-dihydro-1H-inden-4-yl N,N-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10742 (1-amino-2,3-dihydro-1H-inden-4-yl N,N-dimethylcarb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10803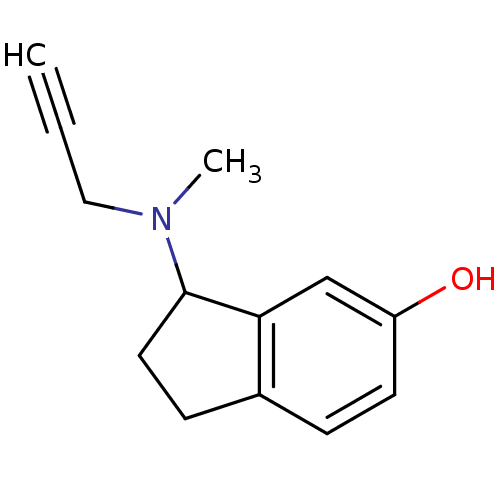 (3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10784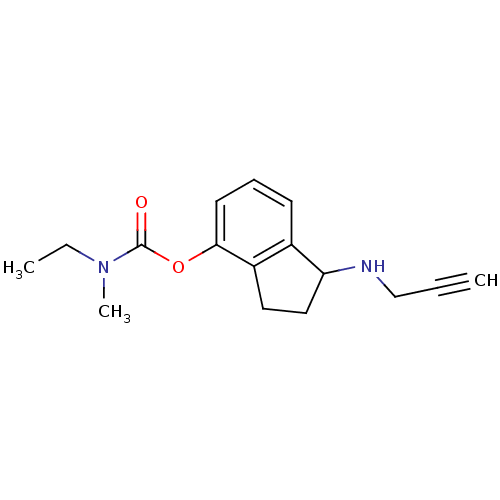 (1-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-4-yl ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10804 ((3R)-3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10743 (1-amino-2,3-dihydro-1H-inden-4-yl N-ethyl-N-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10794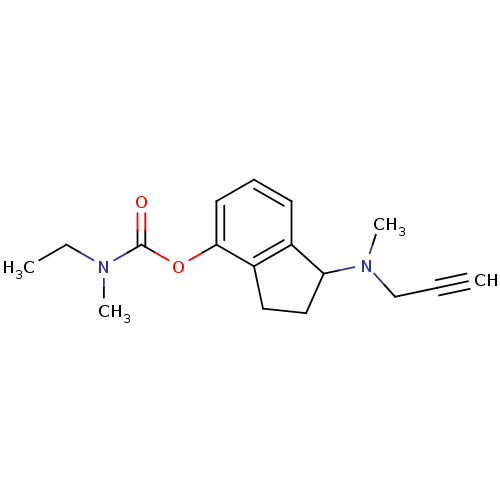 (1-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10803 (3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1H-ind...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10726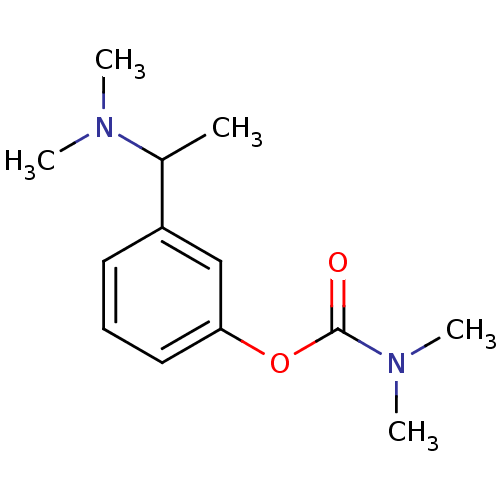 (3-[1-(dimethylamino)ethyl]phenyl N,N-dimethylcarba...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10806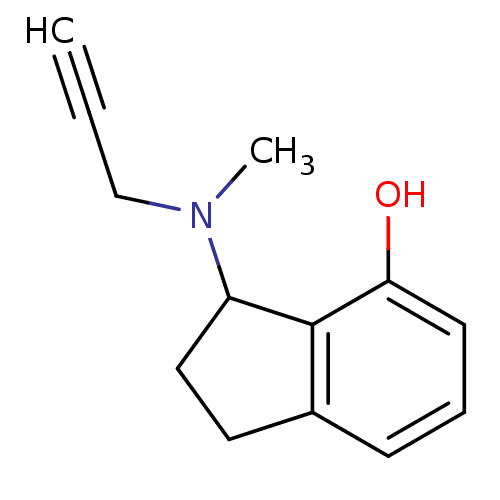 (3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1H-ind...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10783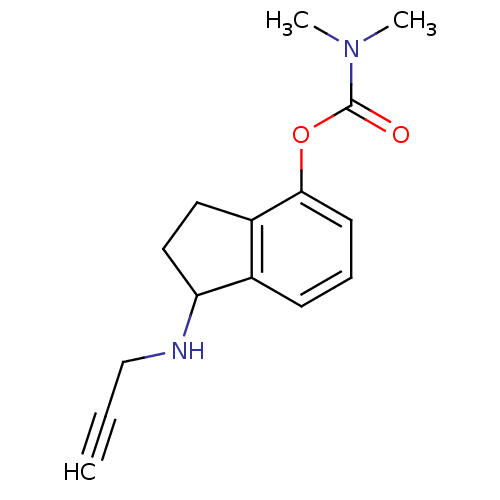 (1-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-4-yl ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10743 (1-amino-2,3-dihydro-1H-inden-4-yl N-ethyl-N-methyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10806 (3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10807 (1-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1H-ind...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10824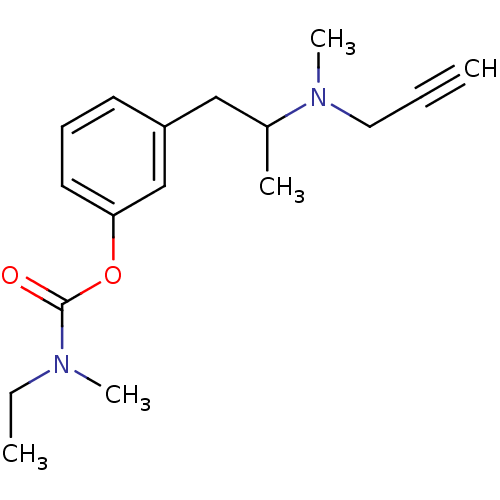 (3-{2-[methyl(prop-2-yn-1-yl)amino]propyl}phenyl N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10824 (3-{2-[methyl(prop-2-yn-1-yl)amino]propyl}phenyl N-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10823 (3-{2-[methyl(prop-2-yn-1-yl)amino]propyl}phenyl N,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10812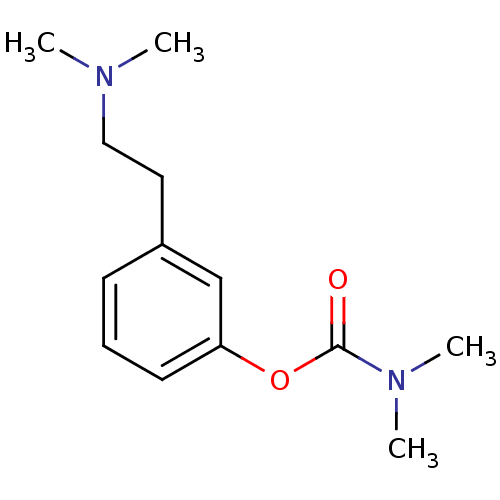 (3-[2-(dimethylamino)ethyl]phenyl N,N-dimethylcarba...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10620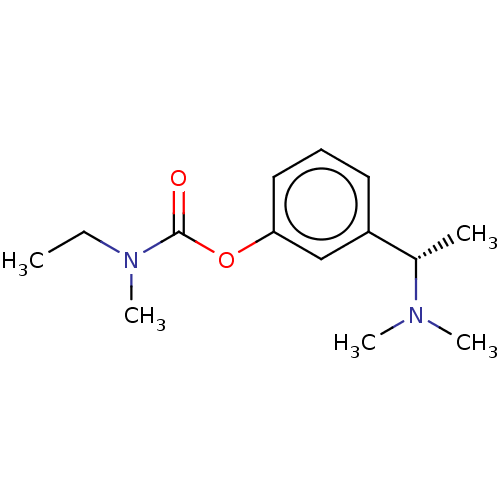 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of equine BuChE after 120 mins by Ellman's method | J Med Chem 55: 10700-15 (2012) Article DOI: 10.1021/jm301411g BindingDB Entry DOI: 10.7270/Q23X87S3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10820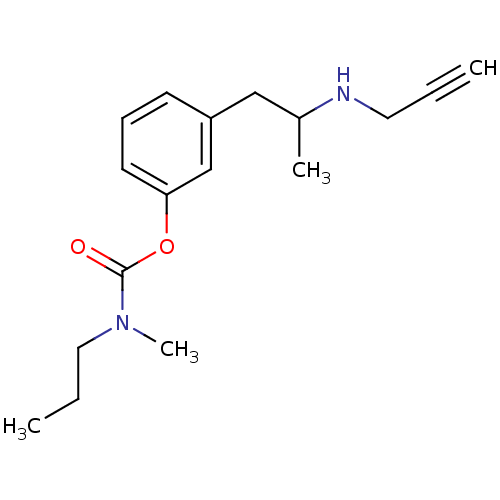 (3-[2-(prop-2-yn-1-ylamino)propyl]phenyl N-methyl-N...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10832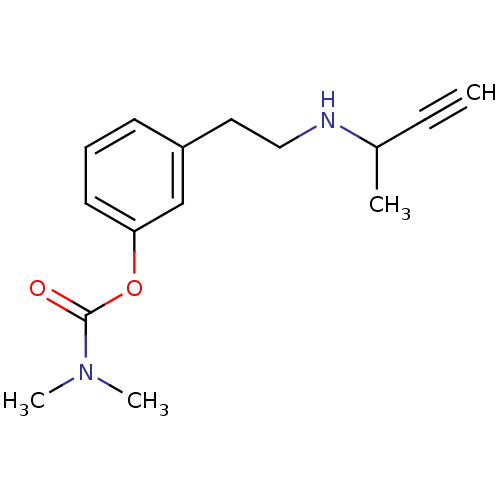 (3-[2-(but-3-yn-2-ylamino)ethyl]phenyl N,N-dimethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50400024 (CHEMBL2177719) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50400024 (CHEMBL2177719) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of equine BuChE after 120 mins by Ellman's method | J Med Chem 55: 10700-15 (2012) Article DOI: 10.1021/jm301411g BindingDB Entry DOI: 10.7270/Q23X87S3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10813 (3-[2-(prop-2-yn-1-ylamino)ethyl]phenyl N,N-dimethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10813 (3-[2-(prop-2-yn-1-ylamino)ethyl]phenyl N,N-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10830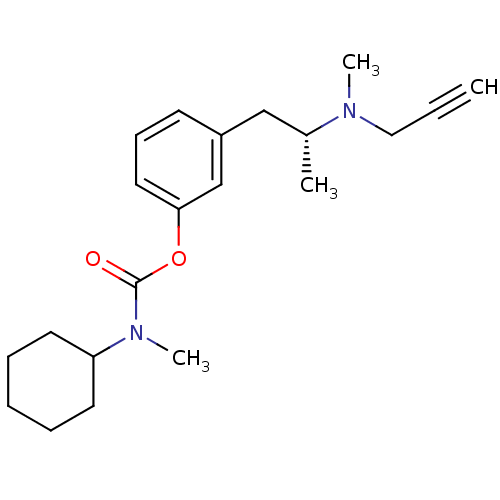 (3-[(2R)-2-[methyl(prop-2-yn-1-yl)amino]propyl]phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10797 (3-(2-aminoethyl)phenyl N,N-dimethylcarbamate | Phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10799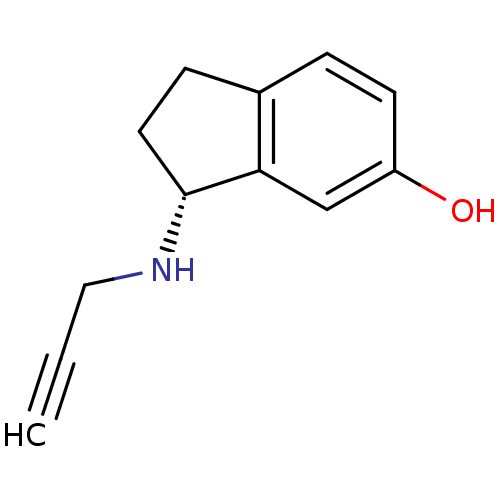 ((3R)-3-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10821 (3-[2-(prop-2-yn-1-ylamino)propyl]phenyl N-cyclohex...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10829 (3-(N-Methyl,Ncyclohexylcarbamyloxy)-N-methyl-N-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10832 (3-[2-(but-3-yn-2-ylamino)ethyl]phenyl N,N-dimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10833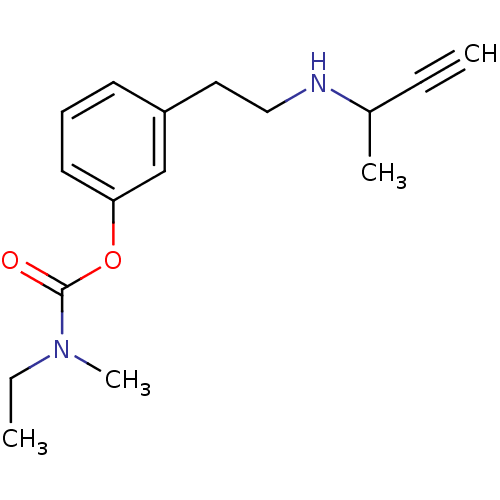 (3-[2-(but-3-yn-2-ylamino)ethyl]phenyl N-ethyl-N-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10783 (1-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-4-yl ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10810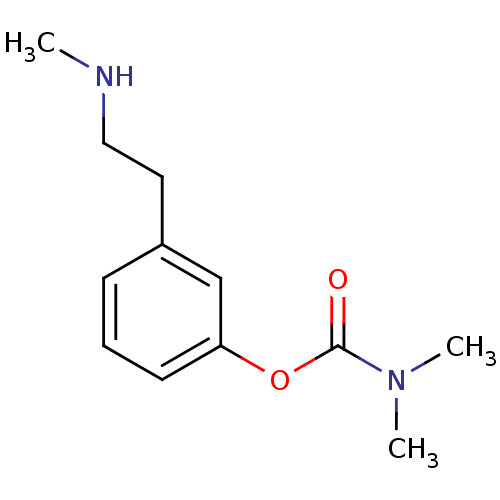 (3-[2-(methylamino)ethyl]phenyl N,N-dimethylcarbama...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10831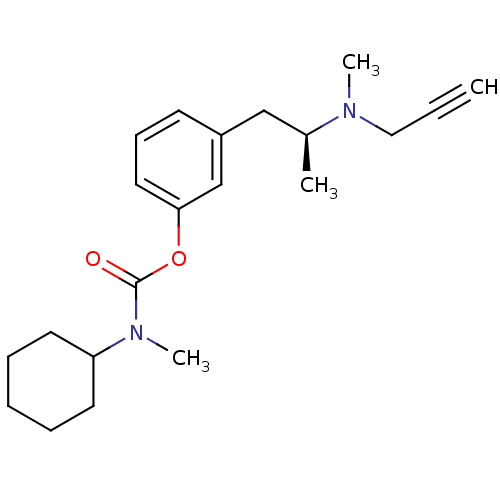 (3-[(2S)-2-[methyl(prop-2-yn-1-yl)amino]propyl]phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10815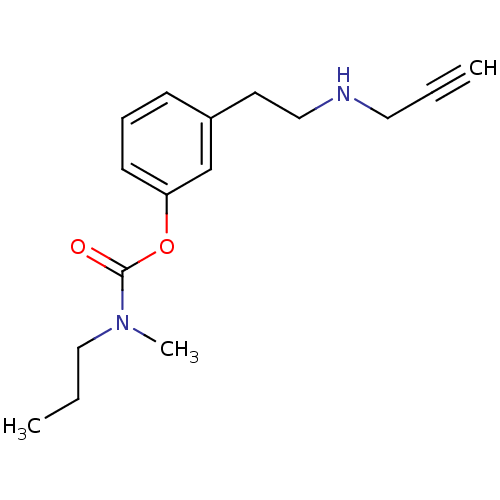 (3-[2-(prop-2-yn-1-ylamino)ethyl]phenyl N-methyl-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10793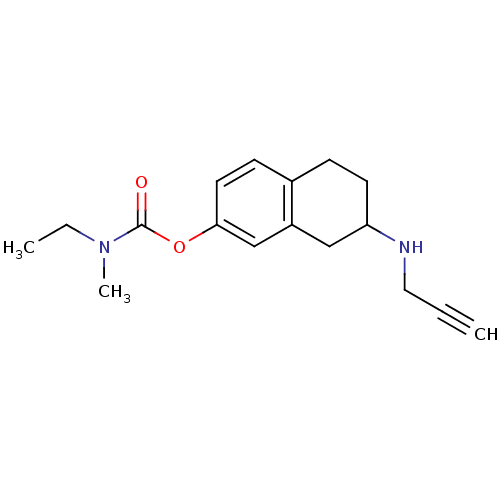 (7-(prop-2-yn-1-ylamino)-5,6,7,8-tetrahydronaphthal...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50400017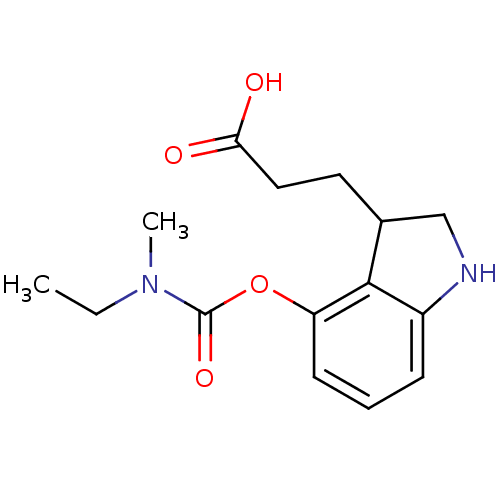 (CHEMBL2177700) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of equine BuChE after 120 mins by Ellman's method | J Med Chem 55: 10700-15 (2012) Article DOI: 10.1021/jm301411g BindingDB Entry DOI: 10.7270/Q23X87S3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50400020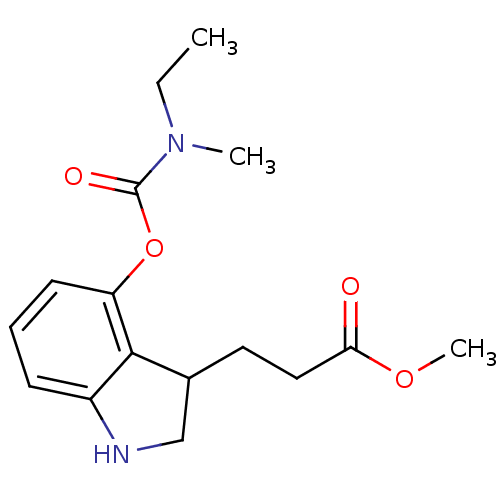 (CHEMBL2181475) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10732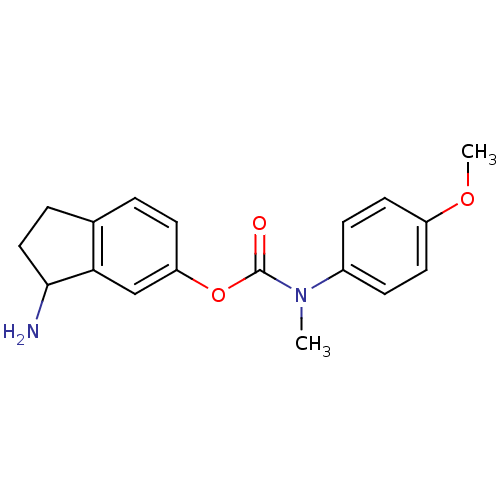 (3-amino-2,3-dihydro-1H-inden-5-yl N-(4-methoxyphen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10799 ((3R)-3-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10816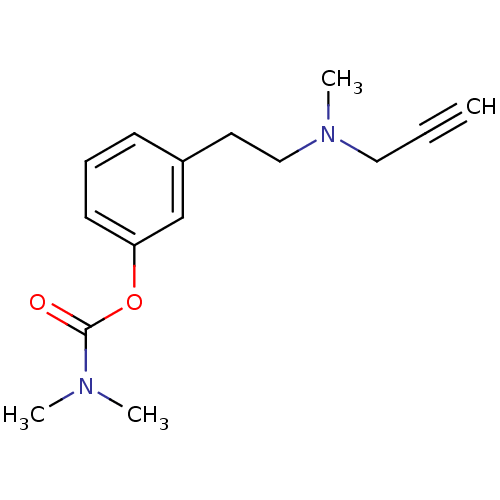 (3-{2-[methyl(prop-2-yn-1-yl)amino]ethyl}phenyl N,N...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50400020 (CHEMBL2181475) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of equine BuChE after 120 mins by Ellman's method | J Med Chem 55: 10700-15 (2012) Article DOI: 10.1021/jm301411g BindingDB Entry DOI: 10.7270/Q23X87S3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10778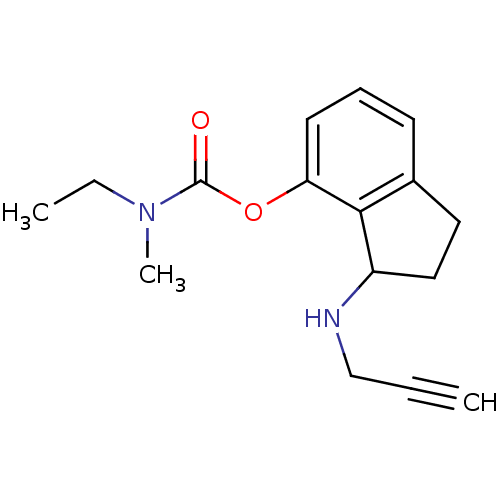 (3-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-4-yl ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50013328 (CHEMBL3263347) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50400024 (CHEMBL2177719) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 371 total ) | Next | Last >> |