

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539662 (CHEMBL4641579) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM50045830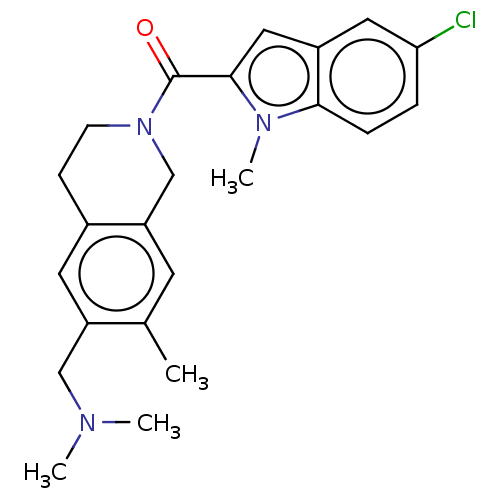 (CHEMBL3314362) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]QRFP43 from human GPR103 receptor expressed in HEK cells after 90 mins incubation by scintillation counting | J Med Chem 57: 5935-48 (2014) Article DOI: 10.1021/jm401951t BindingDB Entry DOI: 10.7270/Q2MP54W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350728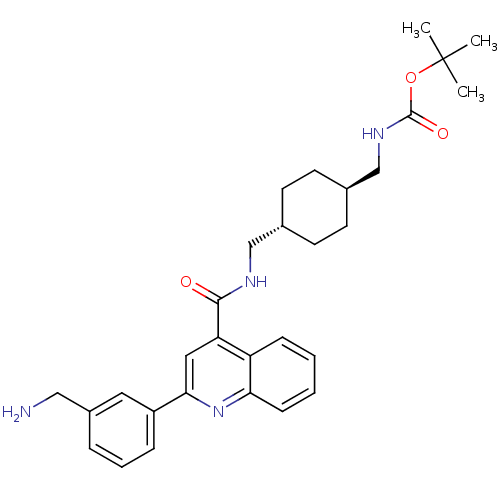 (CHEMBL1818291) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM50045833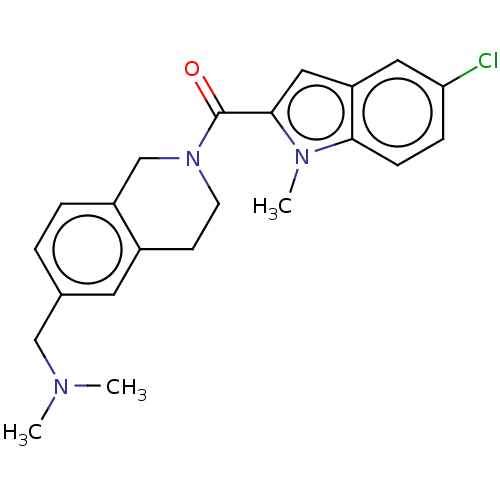 (CHEMBL3314349) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]QRFP43 from human GPR103 receptor expressed in HEK cells after 90 mins incubation by scintillation counting | J Med Chem 57: 5935-48 (2014) Article DOI: 10.1021/jm401951t BindingDB Entry DOI: 10.7270/Q2MP54W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM50045833 (CHEMBL3314349) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human GPR103 receptor expressed in CHOK1 cells by IP-1 assay | J Med Chem 57: 5935-48 (2014) Article DOI: 10.1021/jm401951t BindingDB Entry DOI: 10.7270/Q2MP54W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350748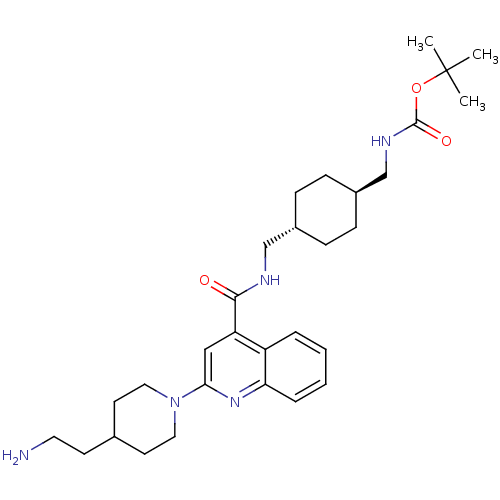 (CHEMBL1818297) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350750 (CHEMBL1818300) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350747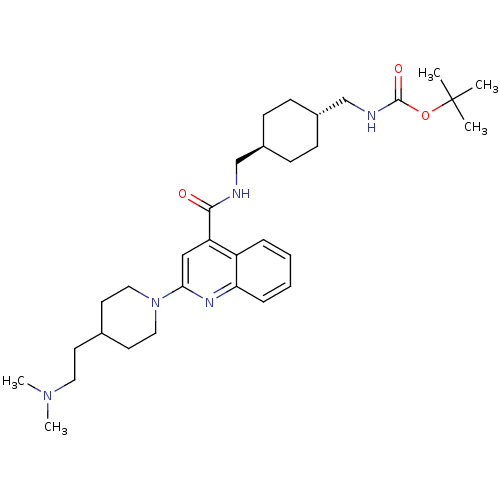 (CHEMBL1818296) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM50045838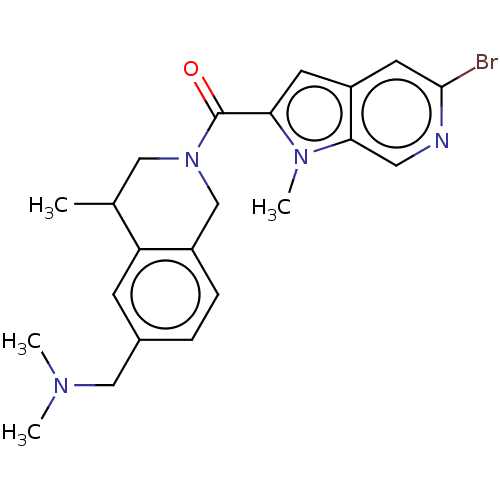 (CHEMBL3314358) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human GPR103 receptor expressed in CHOK1 cells by IP-1 assay | J Med Chem 57: 5935-48 (2014) Article DOI: 10.1021/jm401951t BindingDB Entry DOI: 10.7270/Q2MP54W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350753 (CHEMBL1818303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM50045842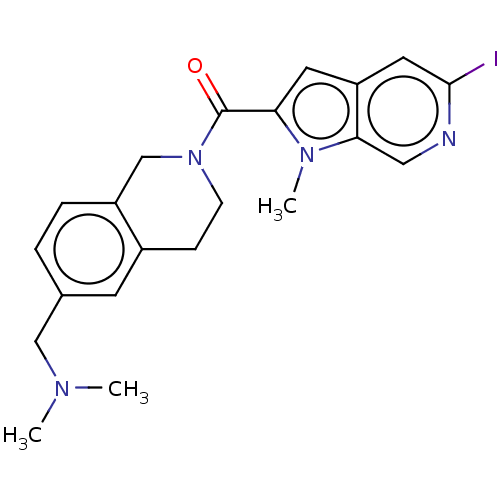 (CHEMBL3314354) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human GPR103 receptor expressed in CHOK1 cells by IP-1 assay | J Med Chem 57: 5935-48 (2014) Article DOI: 10.1021/jm401951t BindingDB Entry DOI: 10.7270/Q2MP54W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase (Rattus norvegicus (Rat)) | BDBM50350750 (CHEMBL1818300) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of ACC2 in obese Zucker rat assessed as reduction in hepatic malonyl-coA level preincubated for 15 mins measured after 1.5 hrs using Malac... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase (Rattus norvegicus (Rat)) | BDBM50189617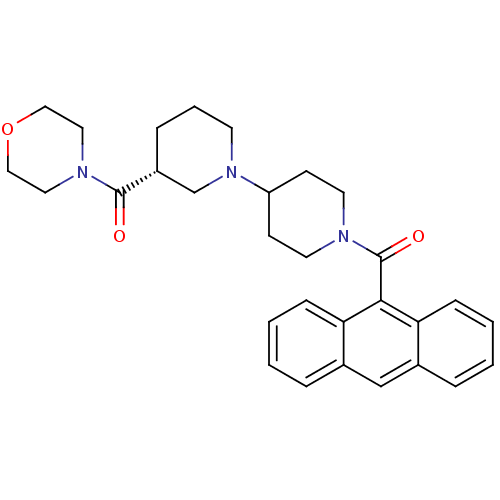 ((3R)-1'-(9-anthrylcarbonyl)-3-(morpholin-4-ylcarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of ACC2 in obese Zucker rat assessed as reduction in hepatic malonyl-coA level preincubated for 15 mins measured after 1.5 hrs using Malac... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetyl-CoA carboxylase (Rattus norvegicus (Rat)) | BDBM50350747 (CHEMBL1818296) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of ACC2 in obese Zucker rat assessed as reduction in hepatic malonyl-coA level preincubated for 15 mins measured after 1.5 hrs using Malac... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350739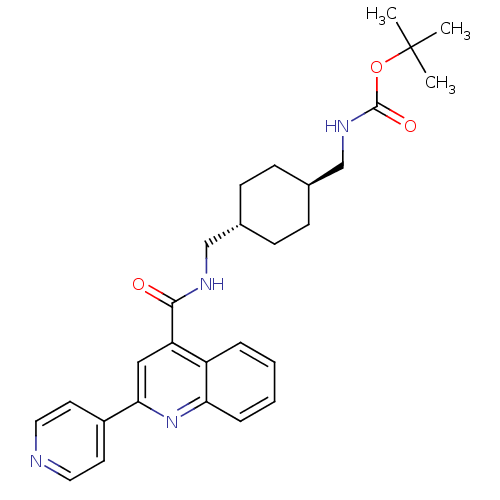 (CHEMBL1818194) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350732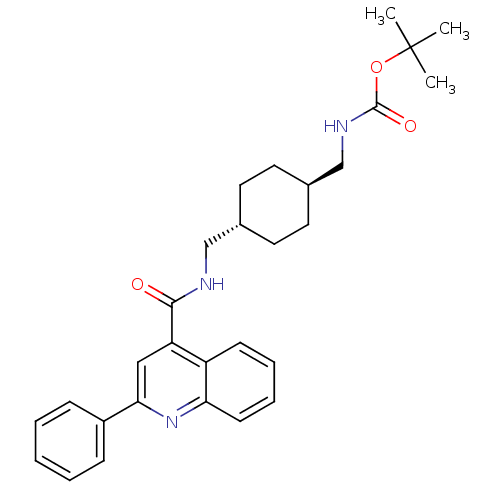 (CHEMBL1818187) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350746 (CHEMBL1818295) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50189617 ((3R)-1'-(9-anthrylcarbonyl)-3-(morpholin-4-ylcarbo...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of obese Zucker rat ACC1 assessed as reduction in hepatic malonyl-coA level preincubated for 15 mins measured after 1.5 hrs using Malachit... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50350728 (CHEMBL1818291) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC1 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350744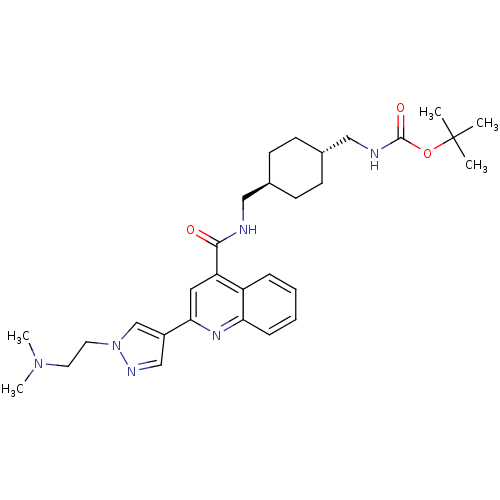 (CHEMBL1818293) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM50045831 (CHEMBL3314352) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human GPR103 receptor expressed in CHOK1 cells by IP-1 assay | J Med Chem 57: 5935-48 (2014) Article DOI: 10.1021/jm401951t BindingDB Entry DOI: 10.7270/Q2MP54W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50350750 (CHEMBL1818300) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of obese Zucker rat ACC1 assessed as reduction in hepatic malonyl-coA level preincubated for 15 mins measured after 1.5 hrs using Malachit... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM50045831 (CHEMBL3314352) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]QRFP43 from human GPR103 receptor expressed in HEK cells after 90 mins incubation by scintillation counting | J Med Chem 57: 5935-48 (2014) Article DOI: 10.1021/jm401951t BindingDB Entry DOI: 10.7270/Q2MP54W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350742 (CHEMBL1615283) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539689 (CHEMBL4642043) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM50045834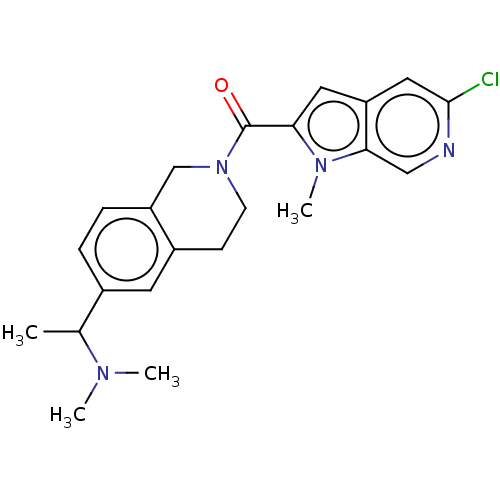 (CHEMBL3314361) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human GPR103 receptor expressed in CHOK1 cells by IP-1 assay | J Med Chem 57: 5935-48 (2014) Article DOI: 10.1021/jm401951t BindingDB Entry DOI: 10.7270/Q2MP54W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50350747 (CHEMBL1818296) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of obese Zucker rat ACC1 assessed as reduction in hepatic malonyl-coA level preincubated for 15 mins measured after 1.5 hrs using Malachit... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM50045837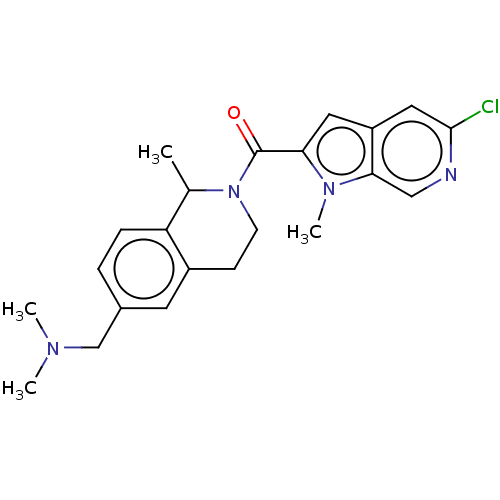 (CHEMBL3314359) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human GPR103 receptor expressed in CHOK1 cells by IP-1 assay | J Med Chem 57: 5935-48 (2014) Article DOI: 10.1021/jm401951t BindingDB Entry DOI: 10.7270/Q2MP54W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350740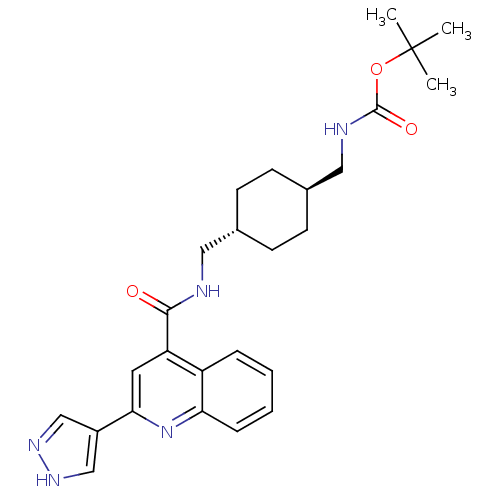 (CHEMBL1818195) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM50045846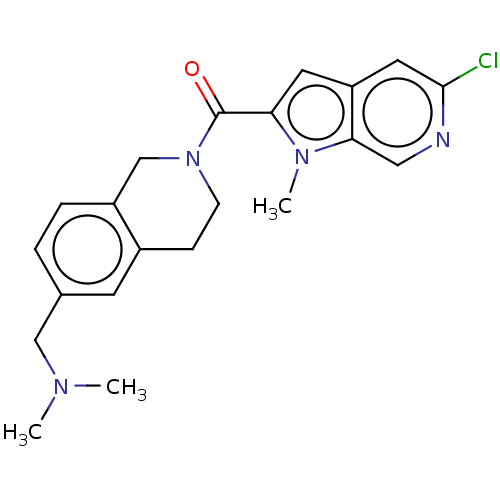 (CHEMBL3314350) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human GPR103 receptor expressed in CHOK1 cells by IP-1 assay | J Med Chem 57: 5935-48 (2014) Article DOI: 10.1021/jm401951t BindingDB Entry DOI: 10.7270/Q2MP54W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350755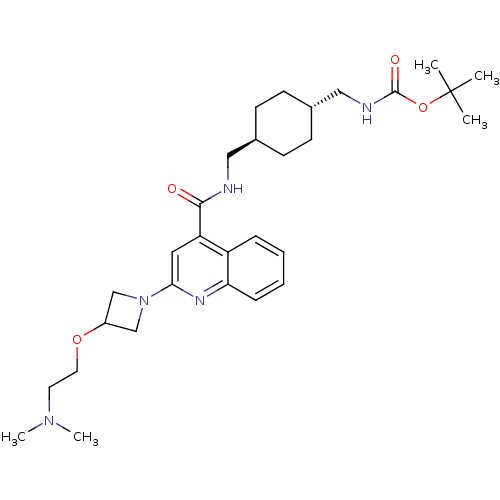 (CHEMBL1818299) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350752 (CHEMBL1818302) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350731 (CHEMBL1818186) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350749 (CHEMBL1818298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM50045836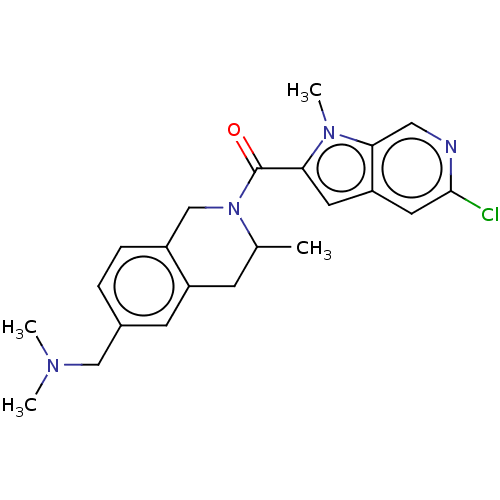 (CHEMBL3314360) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human GPR103 receptor expressed in CHOK1 cells by IP-1 assay | J Med Chem 57: 5935-48 (2014) Article DOI: 10.1021/jm401951t BindingDB Entry DOI: 10.7270/Q2MP54W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350745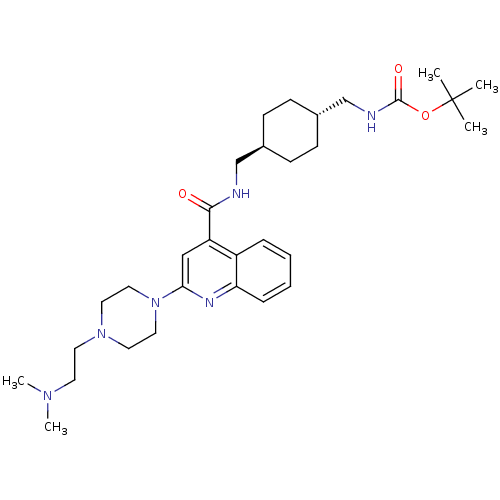 (CHEMBL1818294) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350743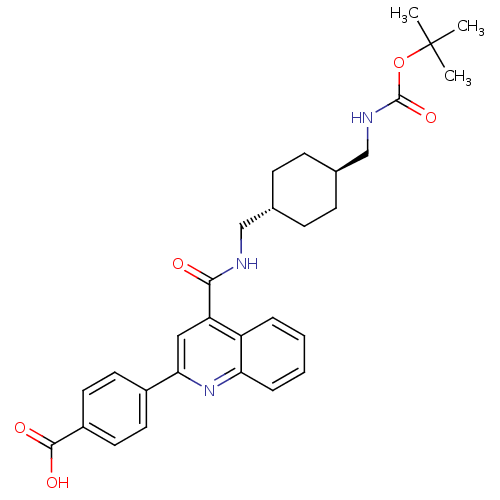 (CHEMBL1818292) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350736 (CHEMBL1818191) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50350748 (CHEMBL1818297) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC1 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50350728 (CHEMBL1818291) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C9 | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM50045830 (CHEMBL3314362) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human GPR103 receptor expressed in CHOK1 cells by IP-1 assay | J Med Chem 57: 5935-48 (2014) Article DOI: 10.1021/jm401951t BindingDB Entry DOI: 10.7270/Q2MP54W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM50045830 (CHEMBL3314362) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human GPR103 receptor expressed in CHOK1 cells by IP-1 assay | J Med Chem 57: 5935-48 (2014) Article DOI: 10.1021/jm401951t BindingDB Entry DOI: 10.7270/Q2MP54W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539663 (CHEMBL4632411) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350733 (CHEMBL1818188) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50350753 (CHEMBL1818303) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC1 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50350750 (CHEMBL1818300) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC1 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50350739 (CHEMBL1818194) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC1 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50389657 (CHEMBL2069803) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2C19 coexpressed in Escherichia coli | J Med Chem 55: 1817-30 (2012) Article DOI: 10.1021/jm2013248 BindingDB Entry DOI: 10.7270/Q2XK8GMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50350747 (CHEMBL1818296) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC1 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350741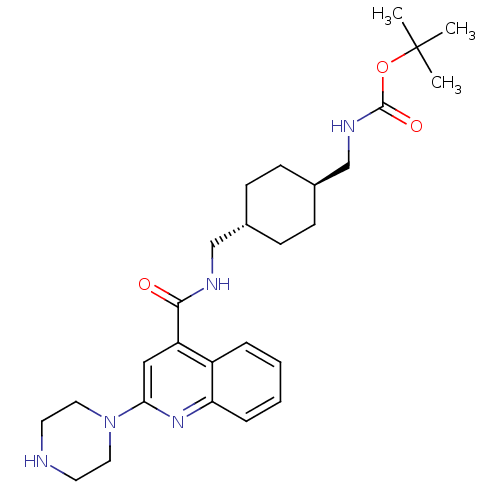 (CHEMBL1818290) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 238 total ) | Next | Last >> |