 Found 353 hits with Last Name = 'wernic' and Initial = 'd'
Found 353 hits with Last Name = 'wernic' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM729

((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-3-[(2S)-...)Show SMILES CC(C)[C@H](NC(=O)c1ccc2ccccc2n1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1CC[C@H](C[C@H]1C(=O)NC(C)(C)C)OCc1ccncc1 |r| Show InChI InChI=1S/C41H52N6O5/c1-27(2)37(45-38(49)33-16-15-30-13-9-10-14-32(30)43-33)40(51)44-34(23-28-11-7-6-8-12-28)36(48)25-47-22-19-31(52-26-29-17-20-42-21-18-29)24-35(47)39(50)46-41(3,4)5/h6-18,20-21,27,31,34-37,48H,19,22-26H2,1-5H3,(H,44,51)(H,45,49)(H,46,50)/t31-,34+,35+,36-,37+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0310 | -62.4 | 4 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... |
J Med Chem 43: 1094-108 (2000)
Article DOI: 10.1021/jm990336n
BindingDB Entry DOI: 10.7270/Q2BZ647F |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [514-612]
(Human immunodeficiency virus type 2) | BDBM729

((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-3-[(2S)-...)Show SMILES CC(C)[C@H](NC(=O)c1ccc2ccccc2n1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1CC[C@H](C[C@H]1C(=O)NC(C)(C)C)OCc1ccncc1 |r| Show InChI InChI=1S/C41H52N6O5/c1-27(2)37(45-38(49)33-16-15-30-13-9-10-14-32(30)43-33)40(51)44-34(23-28-11-7-6-8-12-28)36(48)25-47-22-19-31(52-26-29-17-20-42-21-18-29)24-35(47)39(50)46-41(3,4)5/h6-18,20-21,27,31,34-37,48H,19,22-26H2,1-5H3,(H,44,51)(H,45,49)(H,46,50)/t31-,34+,35+,36-,37+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | -58.7 | 10 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... |
J Med Chem 43: 1094-108 (2000)
Article DOI: 10.1021/jm990336n
BindingDB Entry DOI: 10.7270/Q2BZ647F |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50093010
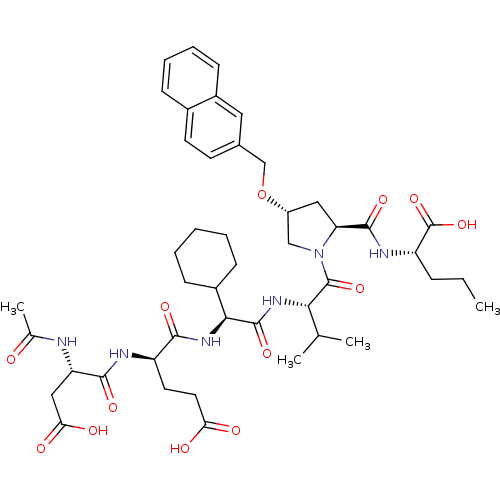
((S)-2-{[(2S,4R)-1-((S)-2-{(S)-2-[(R)-2-((S)-2-Acet...)Show SMILES CCC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C1CCCCC1)C(C)C)OCc1ccc2ccccc2c1)C(O)=O Show InChI InChI=1S/C45H62N6O13/c1-5-11-33(45(62)63)48-42(59)35-21-31(64-24-27-16-17-28-12-9-10-15-30(28)20-27)23-51(35)44(61)38(25(2)3)49-43(60)39(29-13-7-6-8-14-29)50-40(57)32(18-19-36(53)54)47-41(58)34(22-37(55)56)46-26(4)52/h9-10,12,15-17,20,25,29,31-35,38-39H,5-8,11,13-14,18-19,21-24H2,1-4H3,(H,46,52)(H,47,58)(H,48,59)(H,49,60)(H,50,57)(H,53,54)(H,55,56)(H,62,63)/t31-,32-,33+,34+,35+,38+,39+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against NS3 protease complexed with NS4A cofactor peptide (NS3-4A pep) |
Bioorg Med Chem Lett 10: 2267-70 (2001)
BindingDB Entry DOI: 10.7270/Q2S1831T |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50071982
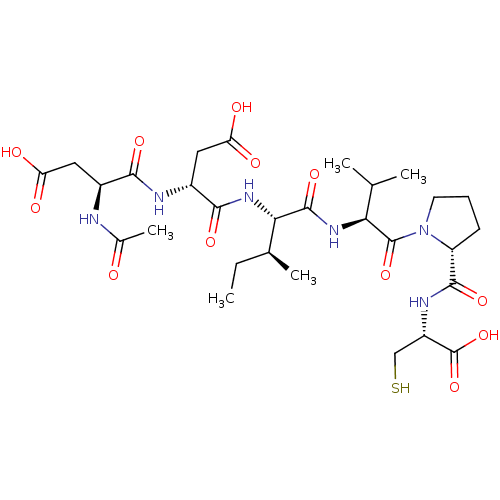
((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CS)C(O)=O Show InChI InChI=1S/C29H46N6O12S/c1-6-14(4)23(34-25(42)17(11-21(39)40)31-24(41)16(10-20(37)38)30-15(5)36)27(44)33-22(13(2)3)28(45)35-9-7-8-19(35)26(43)32-18(12-48)29(46)47/h13-14,16-19,22-23,48H,6-12H2,1-5H3,(H,30,36)(H,31,41)(H,32,43)(H,33,44)(H,34,42)(H,37,38)(H,39,40)(H,46,47)/t14-,16-,17+,18-,19+,22-,23-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of hepatitis C virus (HCV) NS3 protease. |
Bioorg Med Chem Lett 8: 2719-24 (1999)
BindingDB Entry DOI: 10.7270/Q2V69HRX |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50070797

(CHEMBL2370476 | Hexapeptide analogue)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CS)C(O)=O Show InChI InChI=1S/C29H46N6O12S/c1-6-14(4)23(34-25(42)17(11-21(39)40)31-24(41)16(10-20(37)38)30-15(5)36)27(44)33-22(13(2)3)28(45)35-9-7-8-19(35)26(43)32-18(12-48)29(46)47/h13-14,16-19,22-23,48H,6-12H2,1-5H3,(H,30,36)(H,31,41)(H,32,43)(H,33,44)(H,34,42)(H,37,38)(H,39,40)(H,46,47)/t14-,16-,17+,18-,19-,22-,23-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd.
Curated by ChEMBL
| Assay Description
The apparent Ki value against NS3-4Apep protease |
Bioorg Med Chem Lett 8: 1713-8 (1999)
BindingDB Entry DOI: 10.7270/Q2Z89CX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50366517
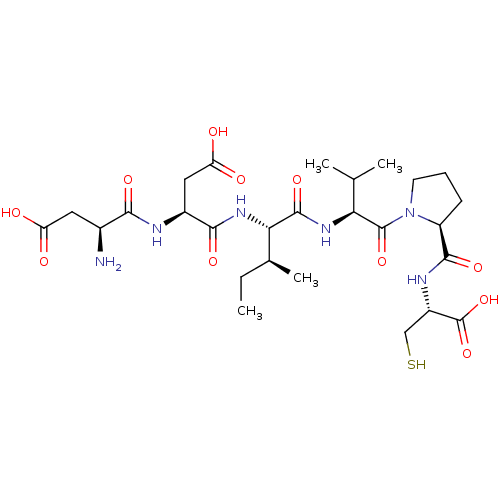
(CHEMBL1790303)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CS)C(O)=O Show InChI InChI=1S/C27H44N6O11S/c1-5-13(4)21(32-23(39)15(10-19(36)37)29-22(38)14(28)9-18(34)35)25(41)31-20(12(2)3)26(42)33-8-6-7-17(33)24(40)30-16(11-45)27(43)44/h12-17,20-21,45H,5-11,28H2,1-4H3,(H,29,38)(H,30,40)(H,31,41)(H,32,39)(H,34,35)(H,36,37)(H,43,44)/t13-,14-,15-,16-,17-,20-,21-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd.
Curated by ChEMBL
| Assay Description
The apparent Ki value against NS3-4Apep protease |
Bioorg Med Chem Lett 8: 1713-8 (1999)
BindingDB Entry DOI: 10.7270/Q2Z89CX4 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50228710
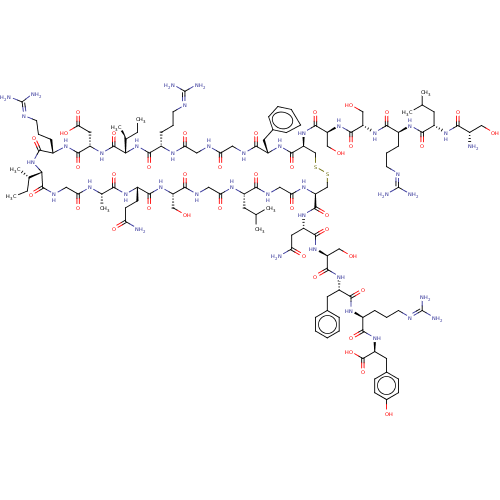
(CHEMBL3349899)Show SMILES [H][C@]1([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6]1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#8])[#6@@H](-[#6])-[#6]-[#6])[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C122H193N41O38S2/c1-10-61(7)95-116(198)142-49-90(173)143-63(9)97(179)147-73(34-35-87(124)170)104(186)157-81(53-165)101(183)141-50-92(175)145-74(40-59(3)4)99(181)140-51-93(176)146-85(114(196)154-78(45-88(125)171)109(191)159-82(54-166)111(193)153-77(43-65-24-16-13-17-25-65)108(190)149-70(27-19-37-135-120(128)129)102(184)156-80(118(200)201)44-66-30-32-67(169)33-31-66)57-202-203-58-86(161-113(195)84(56-168)160-112(194)83(55-167)158-103(185)71(28-20-38-136-121(130)131)148-107(189)75(41-60(5)6)151-98(180)68(123)52-164)115(197)152-76(42-64-22-14-12-15-23-64)100(182)139-47-89(172)138-48-91(174)144-69(26-18-36-134-119(126)127)105(187)163-96(62(8)11-2)117(199)155-79(46-94(177)178)110(192)150-72(106(188)162-95)29-21-39-137-122(132)133/h12-17,22-25,30-33,59-63,68-86,95-96,164-169H,10-11,18-21,26-29,34-58,123H2,1-9H3,(H2,124,170)(H2,125,171)(H,138,172)(H,139,182)(H,140,181)(H,141,183)(H,142,198)(H,143,173)(H,144,174)(H,145,175)(H,146,176)(H,147,179)(H,148,189)(H,149,190)(H,150,192)(H,151,180)(H,152,197)(H,153,193)(H,154,196)(H,155,199)(H,156,184)(H,157,186)(H,158,185)(H,159,191)(H,160,194)(H,161,195)(H,162,188)(H,163,187)(H,177,178)(H,200,201)(H4,126,127,134)(H4,128,129,135)(H4,130,131,136)(H4,132,133,137)/t61-,62-,63-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,95-,96-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50228710

(CHEMBL3349899)Show SMILES [H][C@]1([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6]1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#8])[#6@@H](-[#6])-[#6]-[#6])[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C122H193N41O38S2/c1-10-61(7)95-116(198)142-49-90(173)143-63(9)97(179)147-73(34-35-87(124)170)104(186)157-81(53-165)101(183)141-50-92(175)145-74(40-59(3)4)99(181)140-51-93(176)146-85(114(196)154-78(45-88(125)171)109(191)159-82(54-166)111(193)153-77(43-65-24-16-13-17-25-65)108(190)149-70(27-19-37-135-120(128)129)102(184)156-80(118(200)201)44-66-30-32-67(169)33-31-66)57-202-203-58-86(161-113(195)84(56-168)160-112(194)83(55-167)158-103(185)71(28-20-38-136-121(130)131)148-107(189)75(41-60(5)6)151-98(180)68(123)52-164)115(197)152-76(42-64-22-14-12-15-23-64)100(182)139-47-89(172)138-48-91(174)144-69(26-18-36-134-119(126)127)105(187)163-96(62(8)11-2)117(199)155-79(46-94(177)178)110(192)150-72(106(188)162-95)29-21-39-137-122(132)133/h12-17,22-25,30-33,59-63,68-86,95-96,164-169H,10-11,18-21,26-29,34-58,123H2,1-9H3,(H2,124,170)(H2,125,171)(H,138,172)(H,139,182)(H,140,181)(H,141,183)(H,142,198)(H,143,173)(H,144,174)(H,145,175)(H,146,176)(H,147,179)(H,148,189)(H,149,190)(H,150,192)(H,151,180)(H,152,197)(H,153,193)(H,154,196)(H,155,199)(H,156,184)(H,157,186)(H,158,185)(H,159,191)(H,160,194)(H,161,195)(H,162,188)(H,163,187)(H,177,178)(H,200,201)(H4,126,127,134)(H4,128,129,135)(H4,130,131,136)(H4,132,133,137)/t61-,62-,63-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,95-,96-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on mouse fibroblasts (NIH 3T3) cells (Atrionatriu... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013340
(CHEMBL3349626)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#16])-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C103H158N34O33S/c1-8-51(5)81(136-91(159)61(25-18-34-115-102(111)112)127-94(162)67(41-80(149)150)130-97(165)82(52(6)9-2)137-90(158)59(23-16-32-113-100(107)108)122-77(146)43-116-75(144)42-117-86(154)64(128-84(152)58(104)49-171)36-54-19-12-10-13-20-54)96(164)120-44-76(145)121-53(7)83(151)125-62(30-31-72(105)141)89(157)132-70(47-138)87(155)119-45-78(147)124-63(35-50(3)4)85(153)118-46-79(148)135-103(170)134-69(99(168)169)40-74(143)123-66(39-73(106)142)93(161)133-71(48-139)95(163)129-65(37-55-21-14-11-15-22-55)92(160)126-60(24-17-33-114-101(109)110)88(156)131-68(98(166)167)38-56-26-28-57(140)29-27-56/h10-15,19-22,26-29,50-53,58-71,81-82,138-140,171H,8-9,16-18,23-25,30-49,104H2,1-7H3,(H2,105,141)(H2,106,142)(H,116,144)(H,117,154)(H,118,153)(H,119,155)(H,120,164)(H,121,145)(H,122,146)(H,123,143)(H,124,147)(H,125,151)(H,126,160)(H,127,162)(H,128,152)(H,129,163)(H,130,165)(H,131,156)(H,132,157)(H,133,161)(H,136,159)(H,137,158)(H,149,150)(H,166,167)(H,168,169)(H4,107,108,113)(H4,109,110,114)(H4,111,112,115)(H2,134,135,148,170)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,81-,82-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01031
BindingDB Entry DOI: 10.7270/Q2FF3XF8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013343
(CHEMBL3349621 | deamino [Mpr105,Cys121] r-ANF (99-...)Show SMILES [H][C@]1([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6]1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)[#6@@H](-[#6])-[#6]-[#6])[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C101H154N32O30S2/c1-8-52(5)81-96(160)117-45-76(141)118-54(7)83(147)123-62(30-31-72(102)137)88(152)130-69(48-134)86(150)116-46-78(143)121-63(37-51(3)4)84(148)115-47-79(144)122-71(95(159)127-66(41-73(103)138)92(156)131-70(49-135)94(158)126-65(39-56-21-14-11-15-22-56)91(155)124-60(24-17-34-111-100(106)107)87(151)129-68(98(162)163)40-57-26-28-58(136)29-27-57)50-165-164-36-32-74(139)120-64(38-55-19-12-10-13-20-55)85(149)114-43-75(140)113-44-77(142)119-59(23-16-33-110-99(104)105)89(153)133-82(53(6)9-2)97(161)128-67(42-80(145)146)93(157)125-61(90(154)132-81)25-18-35-112-101(108)109/h10-15,19-22,26-29,51-54,59-71,81-82,134-136H,8-9,16-18,23-25,30-50H2,1-7H3,(H2,102,137)(H2,103,138)(H,113,140)(H,114,149)(H,115,148)(H,116,150)(H,117,160)(H,118,141)(H,119,142)(H,120,139)(H,121,143)(H,122,144)(H,123,147)(H,124,155)(H,125,157)(H,126,158)(H,127,159)(H,128,161)(H,129,151)(H,130,152)(H,131,156)(H,132,154)(H,133,153)(H,145,146)(H,162,163)(H4,104,105,110)(H4,106,107,111)(H4,108,109,112)/t52-,53-,54-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69+,70-,71-,81-,82-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013341
(CHEMBL3349629)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)C([#6])([#6])[#16] |r| Show InChI InChI=1S/C105H161N33O34S/c1-10-52(5)81(137-91(160)61(27-20-36-116-103(112)113)127-94(163)67(42-79(149)150)129-97(166)82(53(6)11-2)138-90(159)59(25-18-34-114-101(108)109)123-76(146)45-117-74(144)44-118-86(155)64(38-55-21-14-12-15-22-55)134-104(172)135-69(100(170)171)43-80(151)152)96(165)121-46-75(145)122-54(7)84(153)125-62(32-33-72(106)142)89(158)132-70(49-139)87(156)120-47-77(147)124-63(37-51(3)4)85(154)119-48-78(148)136-83(105(8,9)173)98(167)130-66(41-73(107)143)93(162)133-71(50-140)95(164)128-65(39-56-23-16-13-17-24-56)92(161)126-60(26-19-35-115-102(110)111)88(157)131-68(99(168)169)40-57-28-30-58(141)31-29-57/h12-17,21-24,28-31,51-54,59-71,81-83,139-141,173H,10-11,18-20,25-27,32-50H2,1-9H3,(H2,106,142)(H2,107,143)(H,117,144)(H,118,155)(H,119,154)(H,120,156)(H,121,165)(H,122,145)(H,123,146)(H,124,147)(H,125,153)(H,126,161)(H,127,163)(H,128,164)(H,129,166)(H,130,167)(H,131,157)(H,132,158)(H,133,162)(H,136,148)(H,137,160)(H,138,159)(H,149,150)(H,151,152)(H,168,169)(H,170,171)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116)(H2,134,135,172)/t52-,53-,54-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,81-,82-,83+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013338
(CHEMBL413659 | r-ANF (103-126)(Atrial Natriuretic ...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)CNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CSSC[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)CNC1=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CO)[C@@H](C)CC Show InChI InChI=1S/C107H165N35O34S2/c1-8-53(5)84-102(173)124-43-79(151)125-55(7)86(157)129-64(30-31-76(109)148)92(163)138-71(47-144)90(161)123-44-81(153)127-65(35-52(3)4)88(159)122-45-82(154)128-74(100(171)134-68(39-77(110)149)96(167)139-73(49-146)98(169)133-67(37-57-21-14-11-15-22-57)95(166)130-62(24-17-33-118-106(113)114)91(162)136-70(104(175)176)38-58-26-28-59(147)29-27-58)50-177-178-51-75(140-99(170)72(48-145)137-87(158)60(108)46-143)101(172)132-66(36-56-19-12-10-13-20-56)89(160)121-41-78(150)120-42-80(152)126-61(23-16-32-117-105(111)112)93(164)142-85(54(6)9-2)103(174)135-69(40-83(155)156)97(168)131-63(94(165)141-84)25-18-34-119-107(115)116/h10-15,19-22,26-29,52-55,60-75,84-85,143-147H,8-9,16-18,23-25,30-51,108H2,1-7H3,(H2,109,148)(H2,110,149)(H,120,150)(H,121,160)(H,122,159)(H,123,161)(H,124,173)(H,125,151)(H,126,152)(H,127,153)(H,128,154)(H,129,157)(H,130,166)(H,131,168)(H,132,172)(H,133,169)(H,134,171)(H,135,174)(H,136,162)(H,137,158)(H,138,163)(H,139,167)(H,140,170)(H,141,165)(H,142,164)(H,155,156)(H,175,176)(H4,111,112,117)(H4,113,114,118)(H4,115,116,119)/t53-,54-,55-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71+,72-,73-,74-,75-,84-,85-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013342

(CHEMBL3349625)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](=O)-[#8]-[#6])-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#16])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C104H159N33O34S/c1-9-52(5)82(136-91(158)61(26-19-35-115-103(111)112)127-94(161)67(41-80(148)149)130-98(165)83(53(6)10-2)137-90(157)59(24-17-33-113-101(107)108)122-77(145)44-116-75(143)43-117-86(153)64(37-55-20-13-11-14-21-55)134-104(170)135-69(100(168)169)42-81(150)171-8)97(164)120-45-76(144)121-54(7)84(151)125-62(31-32-73(105)141)89(156)132-70(48-138)87(154)119-46-78(146)123-63(36-51(3)4)85(152)118-47-79(147)124-72(50-172)96(163)129-66(40-74(106)142)93(160)133-71(49-139)95(162)128-65(38-56-22-15-12-16-23-56)92(159)126-60(25-18-34-114-102(109)110)88(155)131-68(99(166)167)39-57-27-29-58(140)30-28-57/h11-16,20-23,27-30,51-54,59-72,82-83,138-140,172H,9-10,17-19,24-26,31-50H2,1-8H3,(H2,105,141)(H2,106,142)(H,116,143)(H,117,153)(H,118,152)(H,119,154)(H,120,164)(H,121,144)(H,122,145)(H,123,146)(H,124,147)(H,125,151)(H,126,159)(H,127,161)(H,128,162)(H,129,163)(H,130,165)(H,131,155)(H,132,156)(H,133,160)(H,136,158)(H,137,157)(H,148,149)(H,166,167)(H,168,169)(H4,107,108,113)(H4,109,110,114)(H4,111,112,115)(H2,134,135,170)/t52-,53-,54-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,72-,82-,83-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50228711

(CHEMBL3349900)Show SMILES [H][C@]1([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6]1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)[#6@@H](-[#6])-[#6]-[#6])[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C101H155N33O30S2/c1-8-51(5)80-96(161)118-43-75(141)119-53(7)82(147)123-62(30-31-72(103)138)88(153)131-69(46-135)86(151)117-44-77(143)121-63(35-50(3)4)84(149)116-45-78(144)122-71(95(160)128-66(39-73(104)139)92(157)132-70(47-136)94(159)127-65(37-55-21-14-11-15-22-55)91(156)124-60(24-17-33-112-100(107)108)87(152)130-68(98(163)164)38-56-26-28-57(137)29-27-56)49-166-165-48-58(102)83(148)126-64(36-54-19-12-10-13-20-54)85(150)115-41-74(140)114-42-76(142)120-59(23-16-32-111-99(105)106)89(154)134-81(52(6)9-2)97(162)129-67(40-79(145)146)93(158)125-61(90(155)133-80)25-18-34-113-101(109)110/h10-15,19-22,26-29,50-53,58-71,80-81,135-137H,8-9,16-18,23-25,30-49,102H2,1-7H3,(H2,103,138)(H2,104,139)(H,114,140)(H,115,150)(H,116,149)(H,117,151)(H,118,161)(H,119,141)(H,120,142)(H,121,143)(H,122,144)(H,123,147)(H,124,156)(H,125,158)(H,126,148)(H,127,159)(H,128,160)(H,129,162)(H,130,152)(H,131,153)(H,132,157)(H,133,155)(H,134,154)(H,145,146)(H,163,164)(H4,105,106,111)(H4,107,108,112)(H4,109,110,113)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,80-,81-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on mouse fibroblasts (NIH 3T3) cells (Atrionatriu... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013344
(CHEMBL3349628)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#16])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C103H157N33O34S/c1-8-51(5)81(135-90(158)60(25-18-34-114-102(110)111)126-93(161)66(40-79(147)148)129-97(165)82(52(6)9-2)136-89(157)58(23-16-32-112-100(106)107)121-76(144)43-115-74(142)42-116-85(153)63(36-54-19-12-10-13-20-54)133-103(170)134-68(99(168)169)41-80(149)150)96(164)119-44-75(143)120-53(7)83(151)124-61(30-31-72(104)140)88(156)131-69(47-137)86(154)118-45-77(145)122-62(35-50(3)4)84(152)117-46-78(146)123-71(49-171)95(163)128-65(39-73(105)141)92(160)132-70(48-138)94(162)127-64(37-55-21-14-11-15-22-55)91(159)125-59(24-17-33-113-101(108)109)87(155)130-67(98(166)167)38-56-26-28-57(139)29-27-56/h10-15,19-22,26-29,50-53,58-71,81-82,137-139,171H,8-9,16-18,23-25,30-49H2,1-7H3,(H2,104,140)(H2,105,141)(H,115,142)(H,116,153)(H,117,152)(H,118,154)(H,119,164)(H,120,143)(H,121,144)(H,122,145)(H,123,146)(H,124,151)(H,125,159)(H,126,161)(H,127,162)(H,128,163)(H,129,165)(H,130,155)(H,131,156)(H,132,160)(H,135,158)(H,136,157)(H,147,148)(H,149,150)(H,166,167)(H,168,169)(H4,106,107,112)(H4,108,109,113)(H4,110,111,114)(H2,133,134,170)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69+,70-,71-,81-,82-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01031
BindingDB Entry DOI: 10.7270/Q2FF3XF8 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50053967
((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...)Show SMILES CCC(CC)NC(=O)N[C@H](C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)(C)C)C(O)=O)C(C)(C)C Show InChI InChI=1S/C31H54N6O9/c1-9-18(10-2)32-29(46)36-24(31(6,7)8)27(43)34-19(15-22(38)37-13-11-12-14-37)25(41)33-20(16-23(39)40)26(42)35-21(28(44)45)17-30(3,4)5/h18-21,24H,9-17H2,1-8H3,(H,33,41)(H,34,43)(H,35,42)(H,39,40)(H,44,45)(H2,32,36,46)/t19-,20-,21-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50228712
(CHEMBL3350078)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]\[#6](=[#6]\[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](=O)-[#7]\[#6](=[#6]\[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C105H154N34O37/c1-8-51(5)82(138-91(162)60(25-18-34-116-103(112)113)128-94(165)66(41-80(151)152)130-97(168)83(52(6)9-2)139-90(161)58(23-16-32-114-101(108)109)123-77(148)44-117-75(146)43-118-86(157)63(36-54-19-12-10-13-20-54)134-104(175)136-69(100(173)174)42-81(153)154)96(167)121-45-76(147)122-53(7)84(155)126-61(30-31-72(106)143)89(160)132-70(48-140)87(158)120-46-78(149)125-62(35-50(3)4)85(156)119-47-79(150)137-105(176)135-68(99(171)172)40-74(145)124-65(39-73(107)144)93(164)133-71(49-141)95(166)129-64(37-55-21-14-11-15-22-55)92(163)127-59(24-17-33-115-102(110)111)88(159)131-67(98(169)170)38-56-26-28-57(142)29-27-56/h10-15,19-22,26-29,40,42,50-53,58-67,70-71,82-83,140-142H,8-9,16-18,23-25,30-39,41,43-49H2,1-7H3,(H2,106,143)(H2,107,144)(H,117,146)(H,118,157)(H,119,156)(H,120,158)(H,121,167)(H,122,147)(H,123,148)(H,124,145)(H,125,149)(H,126,155)(H,127,163)(H,128,165)(H,129,166)(H,130,168)(H,131,159)(H,132,160)(H,133,164)(H,138,162)(H,139,161)(H,151,152)(H,153,154)(H,169,170)(H,171,172)(H,173,174)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116)(H2,134,136,175)(H2,135,137,150,176)/b68-40+,69-42+/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,70-,71-,82-,83-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on mouse fibroblasts (NIH 3T3) cells (Atrionatriu... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01031
BindingDB Entry DOI: 10.7270/Q2FF3XF8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013339

(CHEMBL3349627)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C105H158N34O37/c1-8-51(5)82(138-91(162)60(25-18-34-116-103(112)113)128-94(165)66(41-80(151)152)130-97(168)83(52(6)9-2)139-90(161)58(23-16-32-114-101(108)109)123-77(148)44-117-75(146)43-118-86(157)63(36-54-19-12-10-13-20-54)134-104(175)136-69(100(173)174)42-81(153)154)96(167)121-45-76(147)122-53(7)84(155)126-61(30-31-72(106)143)89(160)132-70(48-140)87(158)120-46-78(149)125-62(35-50(3)4)85(156)119-47-79(150)137-105(176)135-68(99(171)172)40-74(145)124-65(39-73(107)144)93(164)133-71(49-141)95(166)129-64(37-55-21-14-11-15-22-55)92(163)127-59(24-17-33-115-102(110)111)88(159)131-67(98(169)170)38-56-26-28-57(142)29-27-56/h10-15,19-22,26-29,50-53,58-71,82-83,140-142H,8-9,16-18,23-25,30-49H2,1-7H3,(H2,106,143)(H2,107,144)(H,117,146)(H,118,157)(H,119,156)(H,120,158)(H,121,167)(H,122,147)(H,123,148)(H,124,145)(H,125,149)(H,126,155)(H,127,163)(H,128,165)(H,129,166)(H,130,168)(H,131,159)(H,132,160)(H,133,164)(H,138,162)(H,139,161)(H,151,152)(H,153,154)(H,169,170)(H,171,172)(H,173,174)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116)(H2,134,136,175)(H2,135,137,150,176)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,82-,83-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01031
BindingDB Entry DOI: 10.7270/Q2FF3XF8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50013343

(CHEMBL3349621 | deamino [Mpr105,Cys121] r-ANF (99-...)Show SMILES [H][C@]1([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6]1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)[#6@@H](-[#6])-[#6]-[#6])[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C101H154N32O30S2/c1-8-52(5)81-96(160)117-45-76(141)118-54(7)83(147)123-62(30-31-72(102)137)88(152)130-69(48-134)86(150)116-46-78(143)121-63(37-51(3)4)84(148)115-47-79(144)122-71(95(159)127-66(41-73(103)138)92(156)131-70(49-135)94(158)126-65(39-56-21-14-11-15-22-56)91(155)124-60(24-17-34-111-100(106)107)87(151)129-68(98(162)163)40-57-26-28-58(136)29-27-57)50-165-164-36-32-74(139)120-64(38-55-19-12-10-13-20-55)85(149)114-43-75(140)113-44-77(142)119-59(23-16-33-110-99(104)105)89(153)133-82(53(6)9-2)97(161)128-67(42-80(145)146)93(157)125-61(90(154)132-81)25-18-35-112-101(108)109/h10-15,19-22,26-29,51-54,59-71,81-82,134-136H,8-9,16-18,23-25,30-50H2,1-7H3,(H2,102,137)(H2,103,138)(H,113,140)(H,114,149)(H,115,148)(H,116,150)(H,117,160)(H,118,141)(H,119,142)(H,120,139)(H,121,143)(H,122,144)(H,123,147)(H,124,155)(H,125,157)(H,126,158)(H,127,159)(H,128,161)(H,129,151)(H,130,152)(H,131,156)(H,132,154)(H,133,153)(H,145,146)(H,162,163)(H4,104,105,110)(H4,106,107,111)(H4,108,109,112)/t52-,53-,54-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69+,70-,71-,81-,82-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens) | BDBM50277545

(4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...)Show SMILES COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |c:11| Show InChI InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01031
BindingDB Entry DOI: 10.7270/Q2FF3XF8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50013338

(CHEMBL413659 | r-ANF (103-126)(Atrial Natriuretic ...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)CNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CSSC[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)CNC1=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CO)[C@@H](C)CC Show InChI InChI=1S/C107H165N35O34S2/c1-8-53(5)84-102(173)124-43-79(151)125-55(7)86(157)129-64(30-31-76(109)148)92(163)138-71(47-144)90(161)123-44-81(153)127-65(35-52(3)4)88(159)122-45-82(154)128-74(100(171)134-68(39-77(110)149)96(167)139-73(49-146)98(169)133-67(37-57-21-14-11-15-22-57)95(166)130-62(24-17-33-118-106(113)114)91(162)136-70(104(175)176)38-58-26-28-59(147)29-27-58)50-177-178-51-75(140-99(170)72(48-145)137-87(158)60(108)46-143)101(172)132-66(36-56-19-12-10-13-20-56)89(160)121-41-78(150)120-42-80(152)126-61(23-16-32-117-105(111)112)93(164)142-85(54(6)9-2)103(174)135-69(40-83(155)156)97(168)131-63(94(165)141-84)25-18-34-119-107(115)116/h10-15,19-22,26-29,52-55,60-75,84-85,143-147H,8-9,16-18,23-25,30-51,108H2,1-7H3,(H2,109,148)(H2,110,149)(H,120,150)(H,121,160)(H,122,159)(H,123,161)(H,124,173)(H,125,151)(H,126,152)(H,127,153)(H,128,154)(H,129,157)(H,130,166)(H,131,168)(H,132,172)(H,133,169)(H,134,171)(H,135,174)(H,136,162)(H,137,158)(H,138,163)(H,139,167)(H,140,170)(H,141,165)(H,142,164)(H,155,156)(H,175,176)(H4,111,112,117)(H4,113,114,118)(H4,115,116,119)/t53-,54-,55-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71+,72-,73-,74-,75-,84-,85-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM748
((2S,4R)-N-tert-butyl-1-[(2R,3S)-3-[2-(2,6-dimethyl...)Show SMILES Cc1cc(S[C@@H]2CCN(C[C@@H](O)[C@H](Cc3ccccc3)NC(=O)COc3c(C)cccc3C)[C@@H](C2)C(=O)NC(C)(C)C)nc(C)n1 |r| Show InChI InChI=1S/C36H49N5O4S/c1-23-12-11-13-24(2)34(23)45-22-32(43)39-29(19-27-14-9-8-10-15-27)31(42)21-41-17-16-28(20-30(41)35(44)40-36(5,6)7)46-33-18-25(3)37-26(4)38-33/h8-15,18,28-31,42H,16-17,19-22H2,1-7H3,(H,39,43)(H,40,44)/t28-,29+,30+,31-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... |
J Med Chem 43: 1094-108 (2000)
Article DOI: 10.1021/jm990336n
BindingDB Entry DOI: 10.7270/Q2BZ647F |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM747
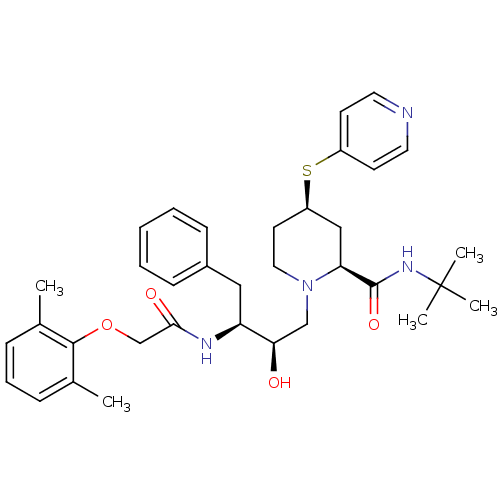
((2S,4R)-N-tert-butyl-1-[(2R,3S)-3-[2-(2,6-dimethyl...)Show SMILES Cc1cccc(C)c1OCC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1CC[C@H](C[C@H]1C(=O)NC(C)(C)C)Sc1ccncc1 |r| Show InChI InChI=1S/C35H46N4O4S/c1-24-10-9-11-25(2)33(24)43-23-32(41)37-29(20-26-12-7-6-8-13-26)31(40)22-39-19-16-28(44-27-14-17-36-18-15-27)21-30(39)34(42)38-35(3,4)5/h6-15,17-18,28-31,40H,16,19-23H2,1-5H3,(H,37,41)(H,38,42)/t28-,29+,30+,31-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... |
J Med Chem 43: 1094-108 (2000)
Article DOI: 10.1021/jm990336n
BindingDB Entry DOI: 10.7270/Q2BZ647F |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM746
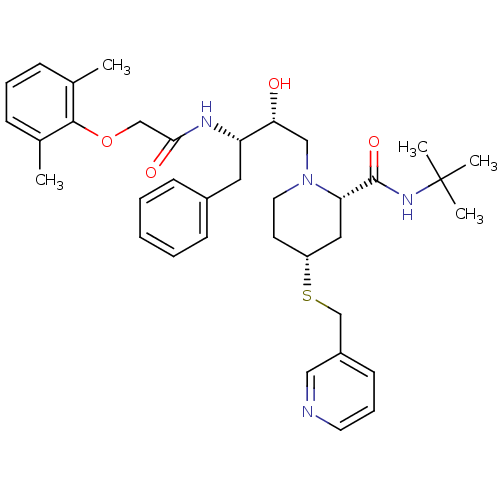
((2S,4R)-N-tert-butyl-1-[(2R,3S)-3-[2-(2,6-dimethyl...)Show SMILES Cc1cccc(C)c1OCC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1CC[C@H](C[C@H]1C(=O)NC(C)(C)C)SCc1cccnc1 |r| Show InChI InChI=1S/C36H48N4O4S/c1-25-11-9-12-26(2)34(25)44-23-33(42)38-30(19-27-13-7-6-8-14-27)32(41)22-40-18-16-29(45-24-28-15-10-17-37-21-28)20-31(40)35(43)39-36(3,4)5/h6-15,17,21,29-32,41H,16,18-20,22-24H2,1-5H3,(H,38,42)(H,39,43)/t29-,30+,31+,32-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... |
J Med Chem 43: 1094-108 (2000)
Article DOI: 10.1021/jm990336n
BindingDB Entry DOI: 10.7270/Q2BZ647F |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM745
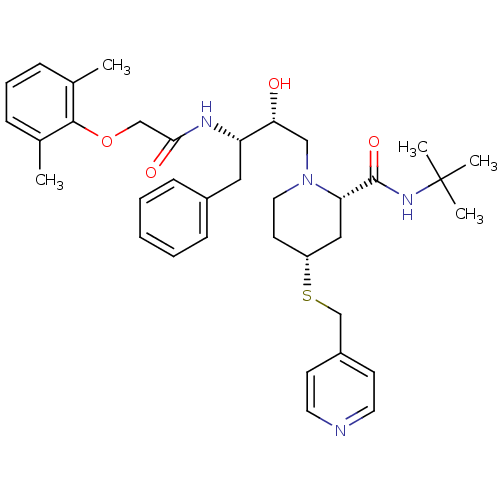
((2S,4R)-N-tert-butyl-1-[(2R,3S)-3-[2-(2,6-dimethyl...)Show SMILES Cc1cccc(C)c1OCC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1CC[C@H](C[C@H]1C(=O)NC(C)(C)C)SCc1ccncc1 |r| Show InChI InChI=1S/C36H48N4O4S/c1-25-10-9-11-26(2)34(25)44-23-33(42)38-30(20-27-12-7-6-8-13-27)32(41)22-40-19-16-29(45-24-28-14-17-37-18-15-28)21-31(40)35(43)39-36(3,4)5/h6-15,17-18,29-32,41H,16,19-24H2,1-5H3,(H,38,42)(H,39,43)/t29-,30+,31+,32-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... |
J Med Chem 43: 1094-108 (2000)
Article DOI: 10.1021/jm990336n
BindingDB Entry DOI: 10.7270/Q2BZ647F |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1250
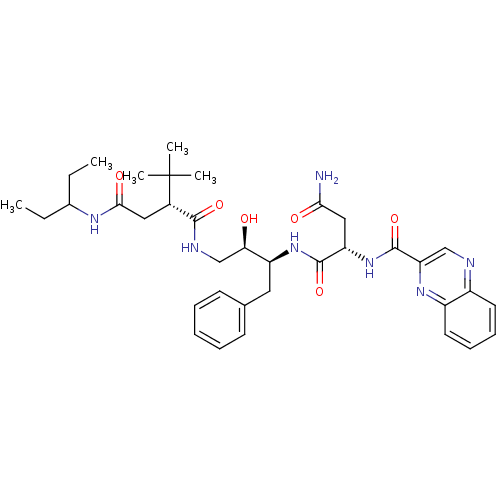
((2S)-N-[(2S,3R)-4-[(2R)-2-tert-butyl-N-(pentan-3-y...)Show SMILES CCC(CC)NC(=O)C[C@@H](C(=O)NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1cnc2ccccc2n1)C(C)(C)C |r| Show InChI InChI=1S/C36H49N7O6/c1-6-23(7-2)40-32(46)18-24(36(3,4)5)33(47)39-21-30(44)27(17-22-13-9-8-10-14-22)42-34(48)28(19-31(37)45)43-35(49)29-20-38-25-15-11-12-16-26(25)41-29/h8-16,20,23-24,27-28,30,44H,6-7,17-19,21H2,1-5H3,(H2,37,45)(H,39,47)(H,40,46)(H,42,48)(H,43,49)/t24-,27-,28-,30+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim (Canada) Ltd.
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... |
J Med Chem 40: 2164-76 (1997)
Article DOI: 10.1021/jm9606608
BindingDB Entry DOI: 10.7270/Q2R20ZJW |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50369164

(CHEMBL1169533)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CC(=O)N1CCCC1)NC(=O)[C@H](NC(=O)NC1[C@@H](C)CCC[C@H]1C)C(C)C)C(O)=O |r| Show InChI InChI=1S/C32H54N6O9/c1-17(2)14-23(31(45)46)35-29(43)22(16-25(40)41)33-28(42)21(15-24(39)38-12-7-8-13-38)34-30(44)26(18(3)4)36-32(47)37-27-19(5)10-9-11-20(27)6/h17-23,26-27H,7-16H2,1-6H3,(H,33,42)(H,34,44)(H,35,43)(H,40,41)(H,45,46)(H2,36,37,47)/t19-,20+,21-,22+,23+,26+,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50369164

(CHEMBL1169533)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CC(=O)N1CCCC1)NC(=O)[C@H](NC(=O)NC1[C@@H](C)CCC[C@H]1C)C(C)C)C(O)=O |r| Show InChI InChI=1S/C32H54N6O9/c1-17(2)14-23(31(45)46)35-29(43)22(16-25(40)41)33-28(42)21(15-24(39)38-12-7-8-13-38)34-30(44)26(18(3)4)36-32(47)37-27-19(5)10-9-11-20(27)6/h17-23,26-27H,7-16H2,1-6H3,(H,33,42)(H,34,44)(H,35,43)(H,40,41)(H,45,46)(H2,36,37,47)/t19-,20+,21-,22+,23+,26+,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D3
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01031
BindingDB Entry DOI: 10.7270/Q2FF3XF8 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1248

((2R)-2-tert-butyl-N-[(2R,3S)-2-hydroxy-3-[(2S)-3-m...)Show SMILES CCC(CC)NC(=O)C[C@@H](C(=O)NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1cnc2ccccc2n1)C(C)C)C(C)(C)C |r| Show InChI InChI=1S/C37H52N6O5/c1-8-25(9-2)40-32(45)20-26(37(5,6)7)34(46)39-22-31(44)29(19-24-15-11-10-12-16-24)42-36(48)33(23(3)4)43-35(47)30-21-38-27-17-13-14-18-28(27)41-30/h10-18,21,23,25-26,29,31,33,44H,8-9,19-20,22H2,1-7H3,(H,39,46)(H,40,45)(H,42,48)(H,43,47)/t26-,29-,31+,33-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim (Canada) Ltd.
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... |
J Med Chem 40: 2164-76 (1997)
Article DOI: 10.1021/jm9606608
BindingDB Entry DOI: 10.7270/Q2R20ZJW |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1249

((2S)-N-[(2S,3R)-4-[(2R)-2-tert-butyl-N-(pentan-3-y...)Show SMILES CCC(CC)NC(=O)C[C@@H](C(=O)NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc2ccccc2n1)C(C)(C)C |r| Show InChI InChI=1S/C37H50N6O6/c1-6-25(7-2)40-33(46)20-26(37(3,4)5)34(47)39-22-31(44)29(19-23-13-9-8-10-14-23)42-36(49)30(21-32(38)45)43-35(48)28-18-17-24-15-11-12-16-27(24)41-28/h8-18,25-26,29-31,44H,6-7,19-22H2,1-5H3,(H2,38,45)(H,39,47)(H,40,46)(H,42,49)(H,43,48)/t26-,29-,30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim (Canada) Ltd.
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... |
J Med Chem 40: 2164-76 (1997)
Article DOI: 10.1021/jm9606608
BindingDB Entry DOI: 10.7270/Q2R20ZJW |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1247
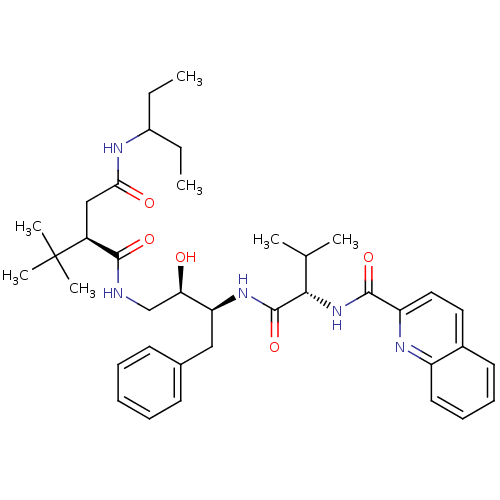
((2R)-2-tert-butyl-N-[(2R,3S)-2-hydroxy-3-[(2S)-3-m...)Show SMILES CCC(CC)NC(=O)C[C@@H](C(=O)NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccc2ccccc2n1)C(C)C)C(C)(C)C |r| Show InChI InChI=1S/C38H53N5O5/c1-8-27(9-2)40-33(45)22-28(38(5,6)7)35(46)39-23-32(44)31(21-25-15-11-10-12-16-25)42-37(48)34(24(3)4)43-36(47)30-20-19-26-17-13-14-18-29(26)41-30/h10-20,24,27-28,31-32,34,44H,8-9,21-23H2,1-7H3,(H,39,46)(H,40,45)(H,42,48)(H,43,47)/t28-,31-,32+,34-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim (Canada) Ltd.
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... |
J Med Chem 40: 2164-76 (1997)
Article DOI: 10.1021/jm9606608
BindingDB Entry DOI: 10.7270/Q2R20ZJW |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM577

((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C25H35N3O6S/c1-18(2)15-28(35(31,32)22-10-8-20(26)9-11-22)16-24(29)23(14-19-6-4-3-5-7-19)27-25(30)34-21-12-13-33-17-21/h3-11,18,21,23-24,29H,12-17,26H2,1-2H3,(H,27,30)/t21-,23-,24+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... |
J Med Chem 43: 1094-108 (2000)
Article DOI: 10.1021/jm990336n
BindingDB Entry DOI: 10.7270/Q2BZ647F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1244
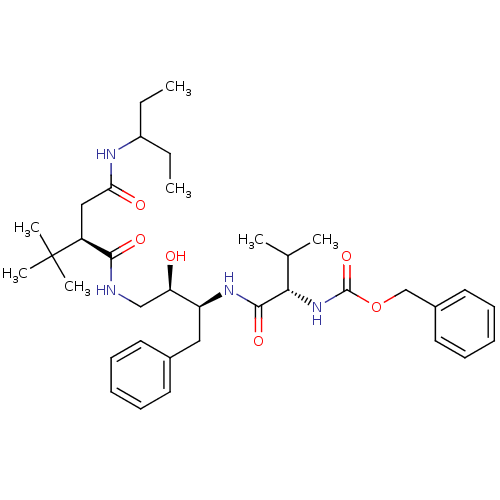
((Hydroxyethyl)amidosuccinoyl deriv. 16 | benzyl N-...)Show SMILES CCC(CC)NC(=O)C[C@@H](C(=O)NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C)C(C)(C)C |r| Show InChI InChI=1S/C36H54N4O6/c1-8-27(9-2)38-31(42)21-28(36(5,6)7)33(43)37-22-30(41)29(20-25-16-12-10-13-17-25)39-34(44)32(24(3)4)40-35(45)46-23-26-18-14-11-15-19-26/h10-19,24,27-30,32,41H,8-9,20-23H2,1-7H3,(H,37,43)(H,38,42)(H,39,44)(H,40,45)/t28-,29-,30+,32-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim (Canada) Ltd.
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... |
J Med Chem 40: 2164-76 (1997)
Article DOI: 10.1021/jm9606608
BindingDB Entry DOI: 10.7270/Q2R20ZJW |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050831
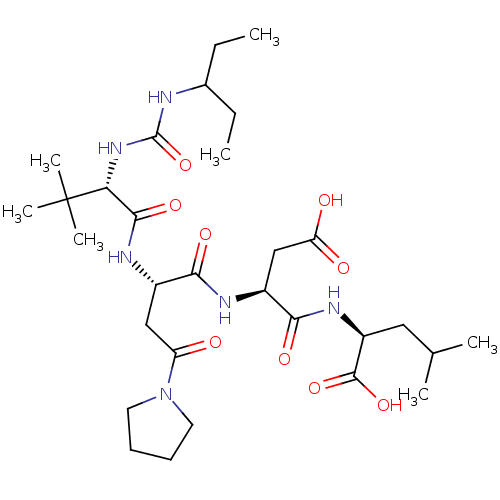
((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...)Show SMILES CCC(CC)NC(=O)N[C@H](C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O)C(C)(C)C Show InChI InChI=1S/C30H52N6O9/c1-8-18(9-2)31-29(45)35-24(30(5,6)7)27(42)33-19(15-22(37)36-12-10-11-13-36)25(40)32-20(16-23(38)39)26(41)34-21(28(43)44)14-17(3)4/h17-21,24H,8-16H2,1-7H3,(H,32,40)(H,33,42)(H,34,41)(H,38,39)(H,43,44)(H2,31,35,45)/t19-,20-,21-,24+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) |
J Med Chem 39: 2178-87 (1996)
Article DOI: 10.1021/jm950825x
BindingDB Entry DOI: 10.7270/Q2BG2N22 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1231

((Hydroxyethyl)amidosuccinoyl deriv. 3 | benzyl N-[...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC(=O)[C@H](CC(=O)NC(C)(C)C)C(C)(C)C |r| Show InChI InChI=1S/C35H52N4O6/c1-23(2)30(38-33(44)45-22-25-17-13-10-14-18-25)32(43)37-27(19-24-15-11-9-12-16-24)28(40)21-36-31(42)26(34(3,4)5)20-29(41)39-35(6,7)8/h9-18,23,26-28,30,40H,19-22H2,1-8H3,(H,36,42)(H,37,43)(H,38,44)(H,39,41)/t26-,27-,28+,30-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim (Canada) Ltd.
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... |
J Med Chem 40: 2164-76 (1997)
Article DOI: 10.1021/jm9606608
BindingDB Entry DOI: 10.7270/Q2R20ZJW |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM26326
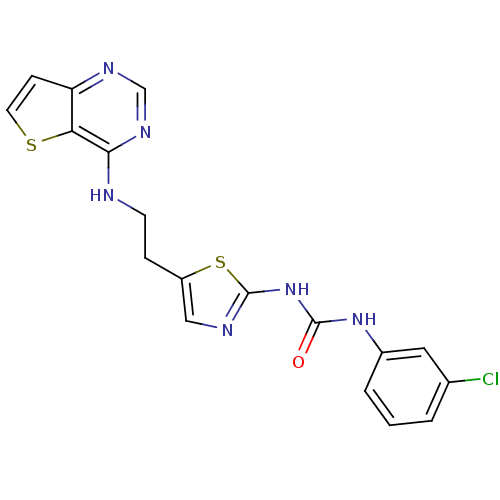
(1-(3-chlorophenyl)-3-[5-(2-{thieno[3,2-d]pyrimidin...)Show SMILES Clc1cccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C18H15ClN6OS2/c19-11-2-1-3-12(8-11)24-17(26)25-18-21-9-13(28-18)4-6-20-16-15-14(5-7-27-15)22-10-23-16/h1-3,5,7-10H,4,6H2,(H,20,22,23)(H2,21,24,25,26) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01031
BindingDB Entry DOI: 10.7270/Q2FF3XF8 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50053968
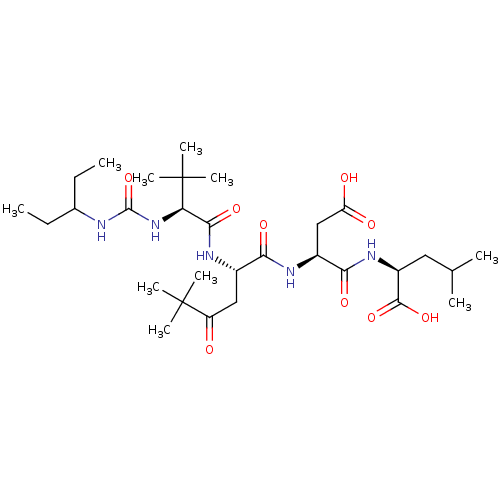
((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...)Show SMILES CCC(CC)NC(=O)N[C@H](C(=O)N[C@@H](CC(=O)C(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O)C(C)(C)C Show InChI InChI=1S/C30H53N5O9/c1-11-17(12-2)31-28(44)35-23(30(8,9)10)26(41)33-18(14-21(36)29(5,6)7)24(39)32-19(15-22(37)38)25(40)34-20(27(42)43)13-16(3)4/h16-20,23H,11-15H2,1-10H3,(H,32,39)(H,33,41)(H,34,40)(H,37,38)(H,42,43)(H2,31,35,44)/t18-,19-,20-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50050831

((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...)Show SMILES CCC(CC)NC(=O)N[C@H](C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O)C(C)(C)C Show InChI InChI=1S/C30H52N6O9/c1-8-18(9-2)31-29(45)35-24(30(5,6)7)27(42)33-19(15-22(37)36-12-10-11-13-36)25(40)32-20(16-23(38)39)26(41)34-21(28(43)44)14-17(3)4/h17-21,24H,8-16H2,1-7H3,(H,32,40)(H,33,42)(H,34,41)(H,38,39)(H,43,44)(H2,31,35,45)/t19-,20-,21-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1252
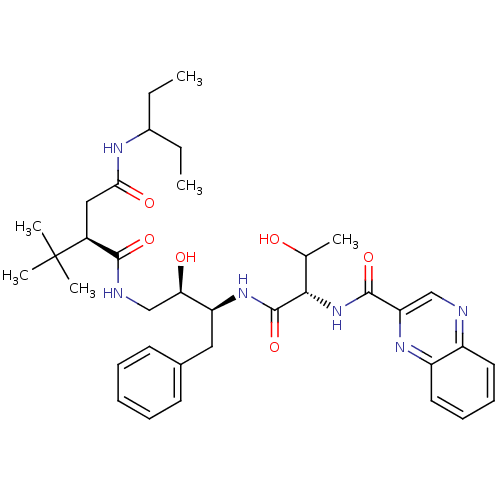
((2R)-2-tert-butyl-N-[(2R,3S)-2-hydroxy-3-[(2S,3R)-...)Show SMILES CCC(CC)NC(=O)C[C@@H](C(=O)NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1cnc2ccccc2n1)C(C)O)C(C)(C)C |r| Show InChI InChI=1S/C36H50N6O6/c1-7-24(8-2)39-31(45)19-25(36(4,5)6)33(46)38-21-30(44)28(18-23-14-10-9-11-15-23)41-35(48)32(22(3)43)42-34(47)29-20-37-26-16-12-13-17-27(26)40-29/h9-17,20,22,24-25,28,30,32,43-44H,7-8,18-19,21H2,1-6H3,(H,38,46)(H,39,45)(H,41,48)(H,42,47)/t22?,25-,28-,30+,32-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim (Canada) Ltd.
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... |
J Med Chem 40: 2164-76 (1997)
Article DOI: 10.1021/jm9606608
BindingDB Entry DOI: 10.7270/Q2R20ZJW |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01031
BindingDB Entry DOI: 10.7270/Q2FF3XF8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50228711

(CHEMBL3349900)Show SMILES [H][C@]1([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6]1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)[#6@@H](-[#6])-[#6]-[#6])[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C101H155N33O30S2/c1-8-51(5)80-96(161)118-43-75(141)119-53(7)82(147)123-62(30-31-72(103)138)88(153)131-69(46-135)86(151)117-44-77(143)121-63(35-50(3)4)84(149)116-45-78(144)122-71(95(160)128-66(39-73(104)139)92(157)132-70(47-136)94(159)127-65(37-55-21-14-11-15-22-55)91(156)124-60(24-17-33-112-100(107)108)87(152)130-68(98(163)164)38-56-26-28-57(137)29-27-56)49-166-165-48-58(102)83(148)126-64(36-54-19-12-10-13-20-54)85(150)115-41-74(140)114-42-76(142)120-59(23-16-32-111-99(105)106)89(154)134-81(52(6)9-2)97(162)129-67(40-79(145)146)93(158)125-61(90(155)133-80)25-18-34-113-101(109)110/h10-15,19-22,26-29,50-53,58-71,80-81,135-137H,8-9,16-18,23-25,30-49,102H2,1-7H3,(H2,103,138)(H2,104,139)(H,114,140)(H,115,150)(H,116,149)(H,117,151)(H,118,161)(H,119,141)(H,120,142)(H,121,143)(H,122,144)(H,123,147)(H,124,156)(H,125,158)(H,126,148)(H,127,159)(H,128,160)(H,129,162)(H,130,152)(H,131,153)(H,132,157)(H,133,155)(H,134,154)(H,145,146)(H,163,164)(H4,105,106,111)(H4,107,108,112)(H4,109,110,113)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,80-,81-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1251
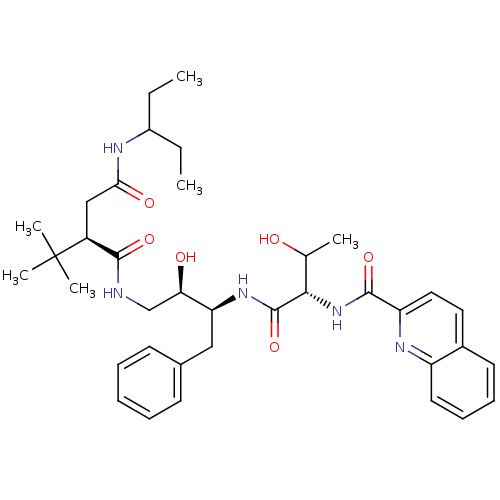
((2R)-2-tert-butyl-N-[(2R,3S)-2-hydroxy-3-[(2S,3R)-...)Show SMILES CCC(CC)NC(=O)C[C@@H](C(=O)NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccc2ccccc2n1)C(C)O)C(C)(C)C |r| Show InChI InChI=1S/C37H51N5O6/c1-7-26(8-2)39-32(45)21-27(37(4,5)6)34(46)38-22-31(44)30(20-24-14-10-9-11-15-24)41-36(48)33(23(3)43)42-35(47)29-19-18-25-16-12-13-17-28(25)40-29/h9-19,23,26-27,30-31,33,43-44H,7-8,20-22H2,1-6H3,(H,38,46)(H,39,45)(H,41,48)(H,42,47)/t23?,27-,30-,31+,33-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim (Canada) Ltd.
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... |
J Med Chem 40: 2164-76 (1997)
Article DOI: 10.1021/jm9606608
BindingDB Entry DOI: 10.7270/Q2R20ZJW |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1245

((Hydroxyethyl)amidosuccinoyl deriv. 17 | benzyl N-...)Show SMILES CCC(CC)NC(=O)C[C@@H](C(=O)NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)OCc1ccccc1)C(C)(C)C |r| Show InChI InChI=1S/C35H51N5O7/c1-6-25(7-2)38-31(43)19-26(35(3,4)5)32(44)37-21-29(41)27(18-23-14-10-8-11-15-23)39-33(45)28(20-30(36)42)40-34(46)47-22-24-16-12-9-13-17-24/h8-17,25-29,41H,6-7,18-22H2,1-5H3,(H2,36,42)(H,37,44)(H,38,43)(H,39,45)(H,40,46)/t26-,27-,28-,29+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim (Canada) Ltd.
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... |
J Med Chem 40: 2164-76 (1997)
Article DOI: 10.1021/jm9606608
BindingDB Entry DOI: 10.7270/Q2R20ZJW |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM3149

(2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCCNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H26N2O10/c31-16-8-6-14(7-9-16)26(36)30-18-13-29-10-2-5-22(18)40-28(39)15-11-20(33)24(21(34)12-15)25(35)23-17(27(37)38)3-1-4-19(23)32/h1,3-4,6-9,11-12,18,22,29,31-34H,2,5,10,13H2,(H,30,36)(H,37,38)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01031
BindingDB Entry DOI: 10.7270/Q2FF3XF8 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit gamma
(Homo sapiens (Human)) | BDBM3149

(2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCCNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H26N2O10/c31-16-8-6-14(7-9-16)26(36)30-18-13-29-10-2-5-22(18)40-28(39)15-11-20(33)24(21(34)12-15)25(35)23-17(27(37)38)3-1-4-19(23)32/h1,3-4,6-9,11-12,18,22,29,31-34H,2,5,10,13H2,(H,30,36)(H,37,38)/t18-,22-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01031
BindingDB Entry DOI: 10.7270/Q2FF3XF8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data



















































