

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412656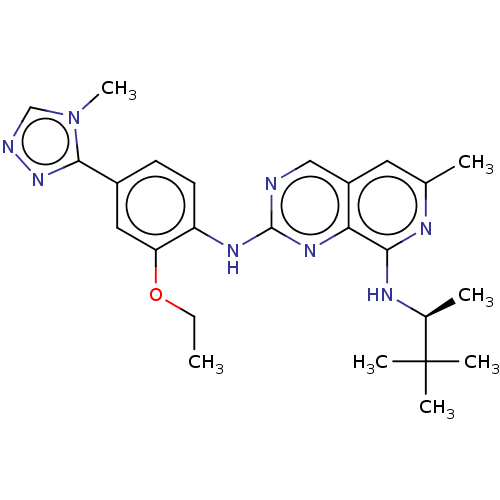 (US10399974, Example 54) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241208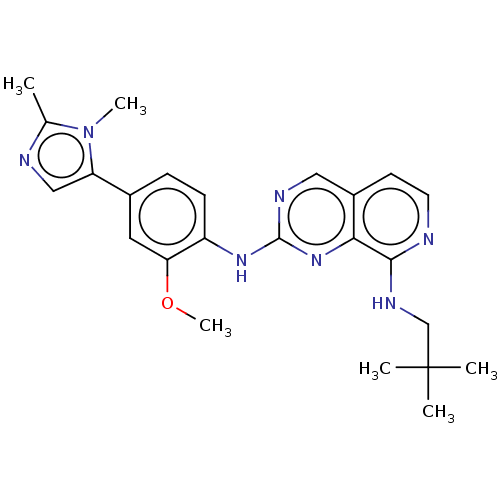 (US11046688, Example 50 | US9409907, 50) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464039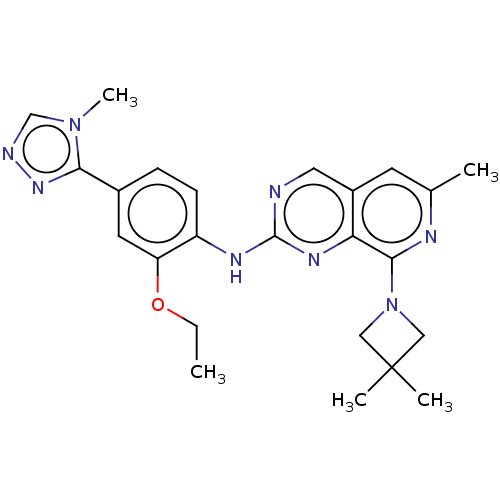 (CHEMBL4245639) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241333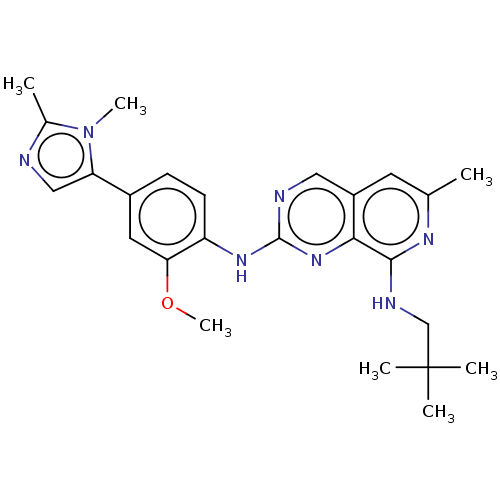 (US10479788, Example 177 | US11046688, Example 177 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241338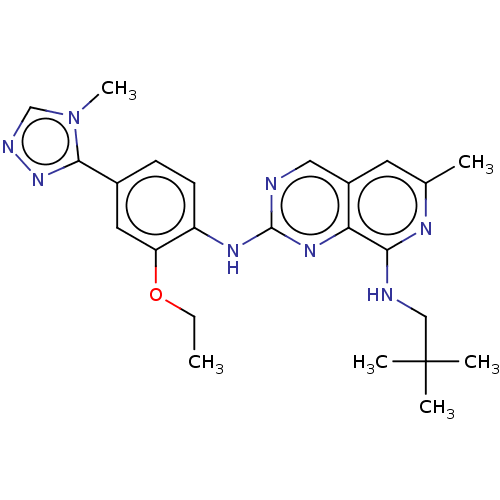 (US10479788, Example 182 | US11046688, Example 182 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412611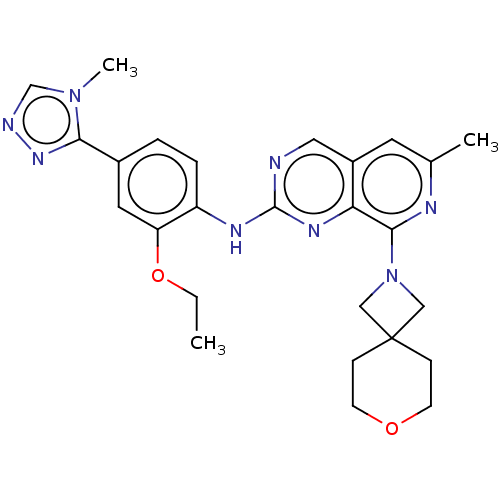 (N-(2-ethoxy-4-(4-methyl-4H-1,2,4-triazol-3-yl)phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412614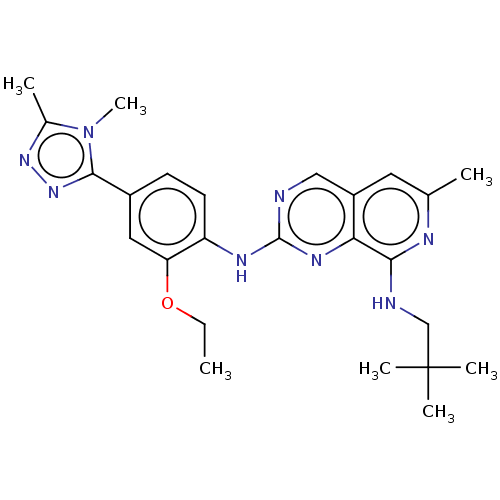 (N2-(4-(4,5-dimethyl-4H-1,2,4-triazol-3-yl)-2-ethox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464037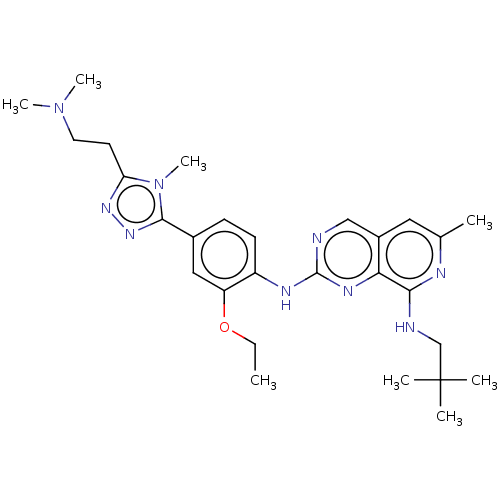 (CHEMBL4240502) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241335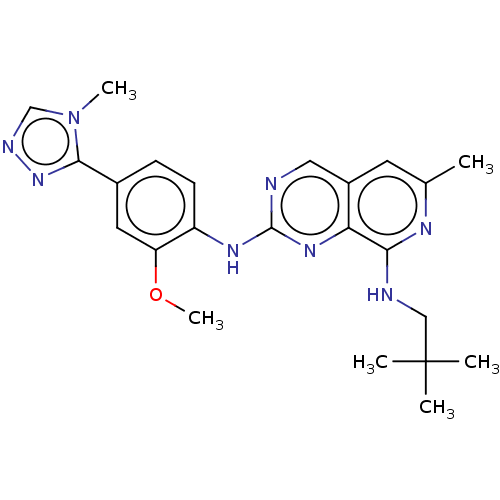 (US9409907, 179) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464040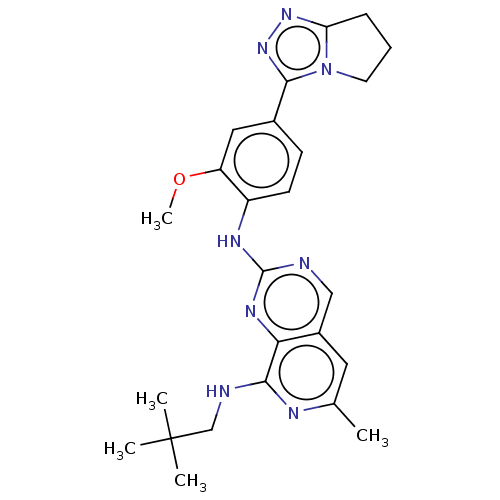 (CHEMBL4251352) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412610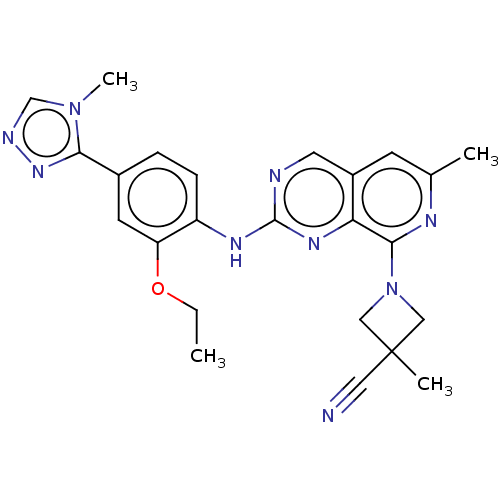 (1-(2-((2-ethoxy-4-(4-methyl-4H-1,2,4-triazol-3-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241235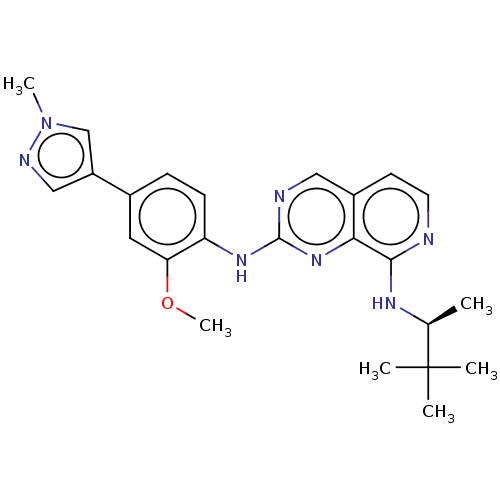 (US10479788, Example 77 | US11046688, Example 77 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50311512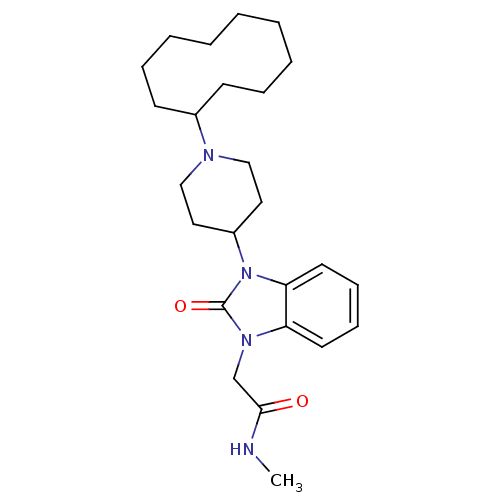 (2-(3-(1-cyclodecylpiperidin-4-yl)-2-oxo-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 6441-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.028 BindingDB Entry DOI: 10.7270/Q21J99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464038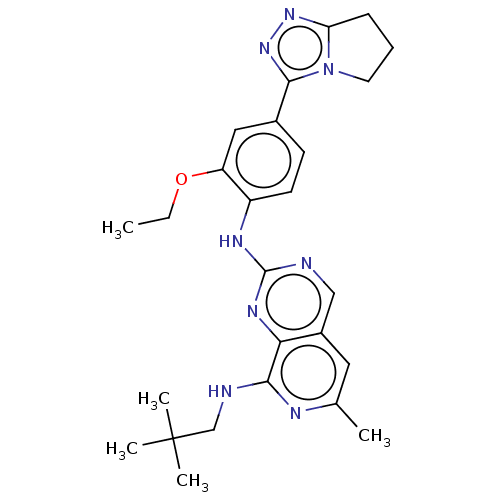 (CHEMBL4250961) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241226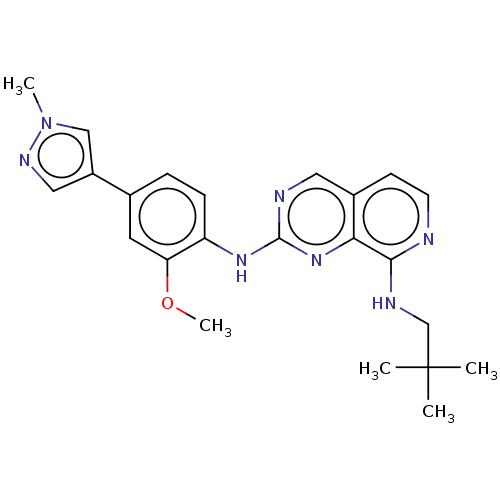 (US10479788, Example 68 | US11046688, Example 68 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241226 (US10479788, Example 68 | US11046688, Example 68 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50311486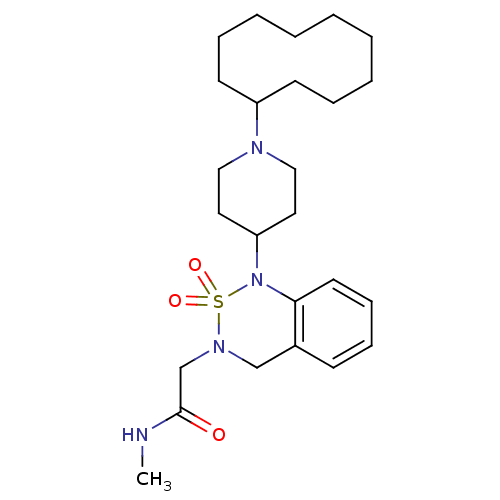 (2-[1-(1-Cyclodecyl-piperidin-4-yl)-2,2-dioxo-1,4-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 6441-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.028 BindingDB Entry DOI: 10.7270/Q21J99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50311475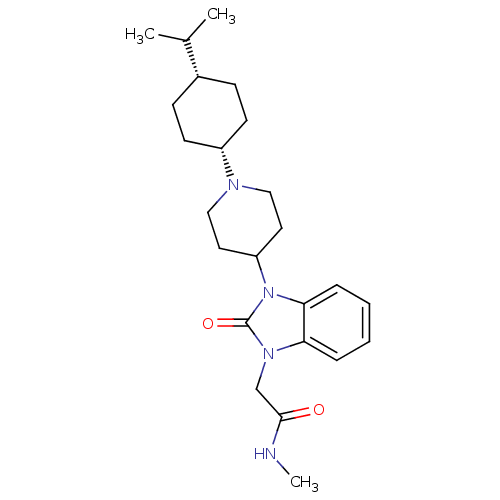 (2-(3-(1-((1s,4s)-4-isopropylcyclohexyl)piperidin-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 6441-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.028 BindingDB Entry DOI: 10.7270/Q21J99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241207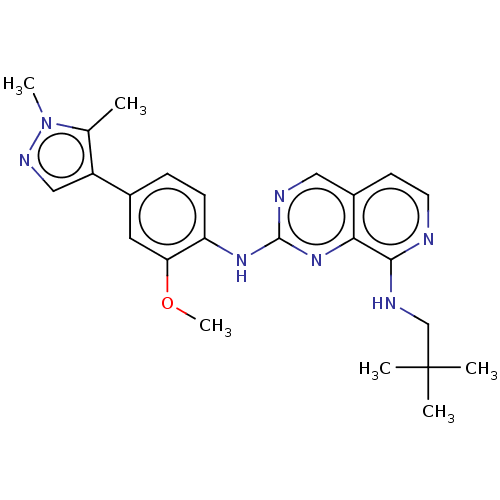 (US11046688, Example 49 | US9409907, 49) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM419188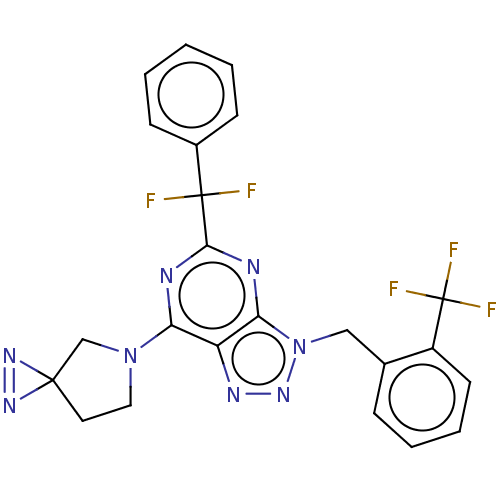 (5-[Difluoro(phenyl)methyl]-7-(1,2,5-triazaspiro[2....) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The affinity of the compounds of the invention for cannabinoid receptors was determined using recommended amounts of membrane preparations (PerkinElm... | US Patent US10457684 (2019) BindingDB Entry DOI: 10.7270/Q2HM5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412658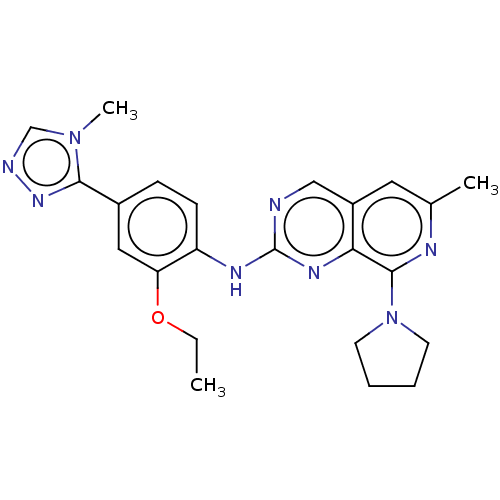 (US10399974, Example 56) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50200594 (1-{1-[3-(5-methoxy-2-methyl-phenoxy)-4-methylpenty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem 15: 1828-47 (2007) Article DOI: 10.1016/j.bmc.2006.11.030 BindingDB Entry DOI: 10.7270/Q27S7NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50200613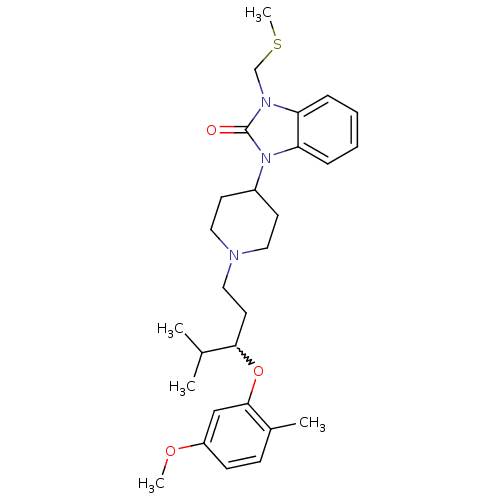 (1-{1-[3-(5-methoxy-2-methyl-phenoxy)-4-methylpenty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem 15: 1828-47 (2007) Article DOI: 10.1016/j.bmc.2006.11.030 BindingDB Entry DOI: 10.7270/Q27S7NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM419309 (2-[[5-tert-Butyl-7-(3,3-difluoropyrrolidin-1-yl)tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description he affinity of the compounds of the invention for cannabinoid receptors was determined using recommended amounts of membrane preparations (PerkinElme... | US Patent US10457685 (2019) BindingDB Entry DOI: 10.7270/Q2CV4M4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50311487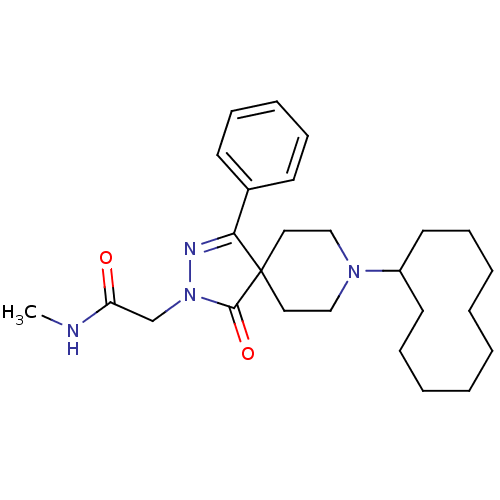 (2-(8-cyclodecyl-1-oxo-4-phenyl-2,3,8-triazaspiro[4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human MOP receptor expressed in CHOK1 cells | Bioorg Med Chem Lett 19: 6441-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.028 BindingDB Entry DOI: 10.7270/Q21J99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241213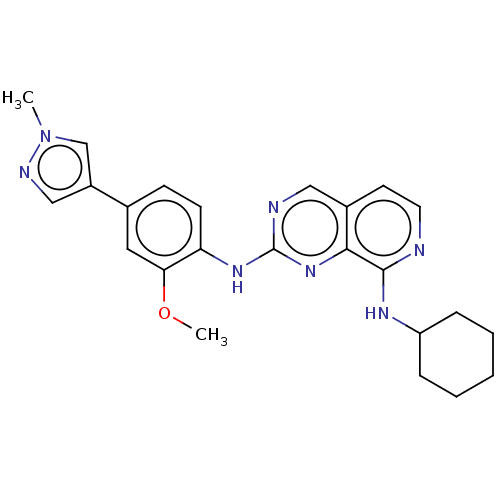 (US10479788, Example 55 | US11046688, Example 55 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50311511 (CHEMBL1088171 | N-methyl-2-(2-oxo-3-(1-(spiro[5.5]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 6441-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.028 BindingDB Entry DOI: 10.7270/Q21J99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50417599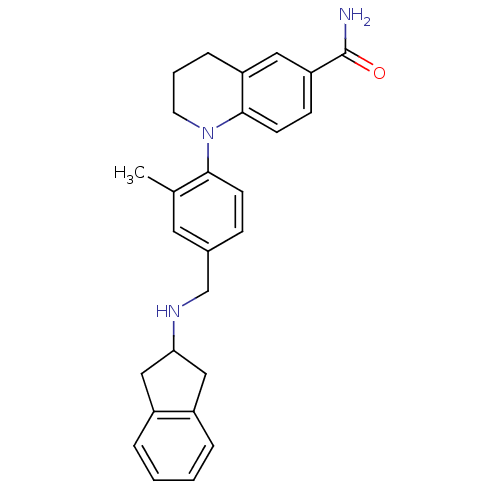 (CHEMBL1642758) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA | Bioorg Med Chem Lett 21: 670-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.010 BindingDB Entry DOI: 10.7270/Q2KD2051 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50311475 (2-(3-(1-((1s,4s)-4-isopropylcyclohexyl)piperidin-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 6441-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.028 BindingDB Entry DOI: 10.7270/Q21J99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50200589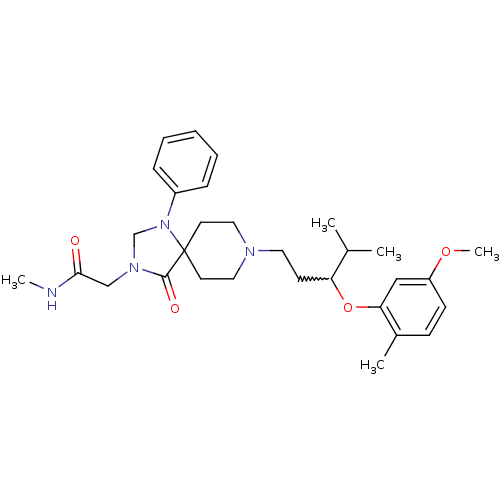 (2-{8-[3-(5-methoxy-2-methyl-phenoxy)-4-methylpenty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem 15: 1828-47 (2007) Article DOI: 10.1016/j.bmc.2006.11.030 BindingDB Entry DOI: 10.7270/Q27S7NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50200611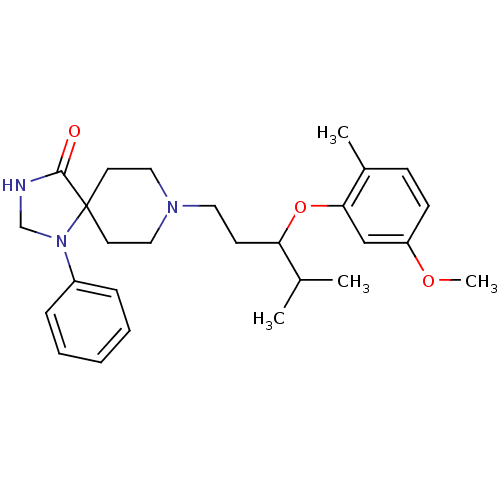 (8-[3-(5-methoxy-2-methyl-phenoxy)-4-methylpentyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem 15: 1828-47 (2007) Article DOI: 10.1016/j.bmc.2006.11.030 BindingDB Entry DOI: 10.7270/Q27S7NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50200601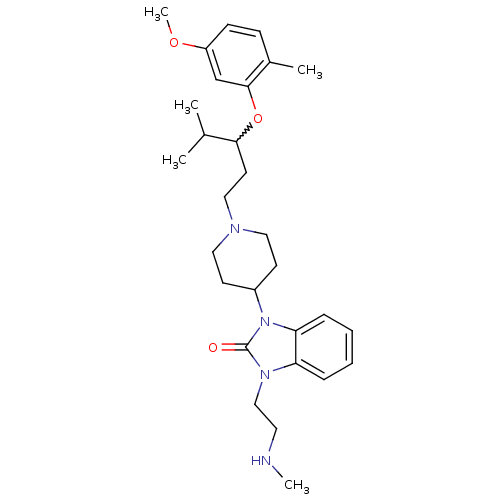 (1-{1-[3-(5-methoxy-2-methyl-phenoxy)-4-methylpenty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem 15: 1828-47 (2007) Article DOI: 10.1016/j.bmc.2006.11.030 BindingDB Entry DOI: 10.7270/Q27S7NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50200597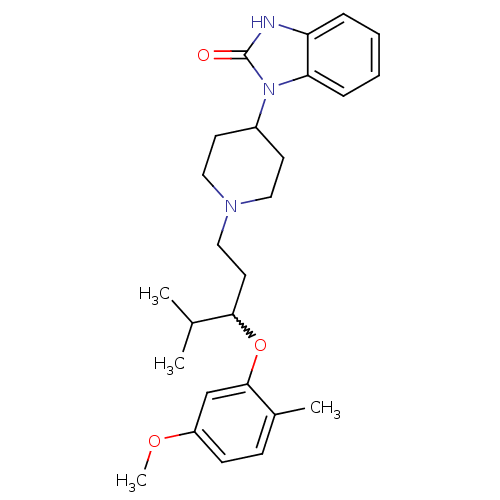 (1-(1-(3-(5-methoxy-2-methylphenoxy)-4-methylpentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem 15: 1828-47 (2007) Article DOI: 10.1016/j.bmc.2006.11.030 BindingDB Entry DOI: 10.7270/Q27S7NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50000787 ((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10,17-dihydr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA | Bioorg Med Chem Lett 21: 670-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.010 BindingDB Entry DOI: 10.7270/Q2KD2051 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50311486 (2-[1-(1-Cyclodecyl-piperidin-4-yl)-2,2-dioxo-1,4-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human MOP receptor expressed in CHOK1 cells | Bioorg Med Chem Lett 19: 6441-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.028 BindingDB Entry DOI: 10.7270/Q21J99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50311487 (2-(8-cyclodecyl-1-oxo-4-phenyl-2,3,8-triazaspiro[4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 6441-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.028 BindingDB Entry DOI: 10.7270/Q21J99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50311485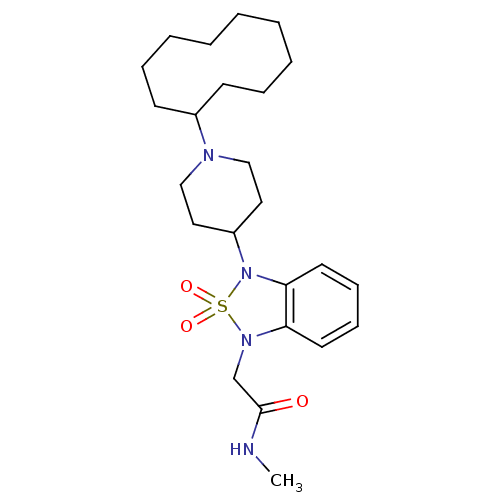 (2-[3-(1-Cyclodecyl-piperidin-4-yl)-2,2-dioxo-2,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 6441-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.028 BindingDB Entry DOI: 10.7270/Q21J99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50311497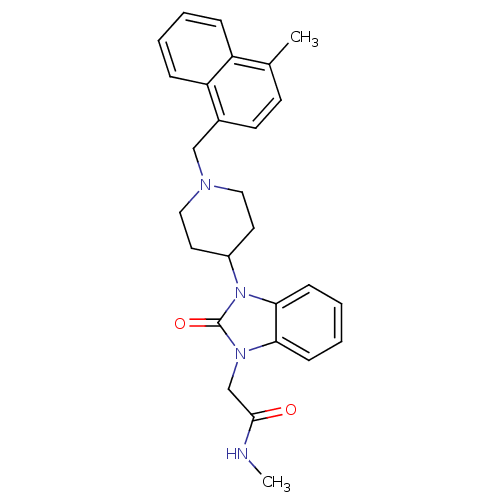 (CHEMBL1080508 | N-methyl-2-(3-(1-((4-methylnaphtha...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 6441-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.028 BindingDB Entry DOI: 10.7270/Q21J99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241216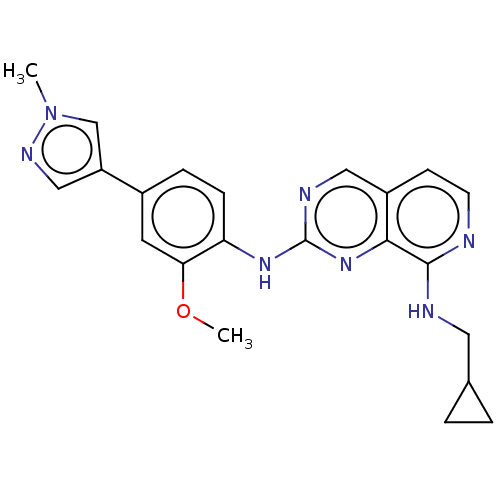 (US10479788, Example 58 | US11046688, Example 58 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50311472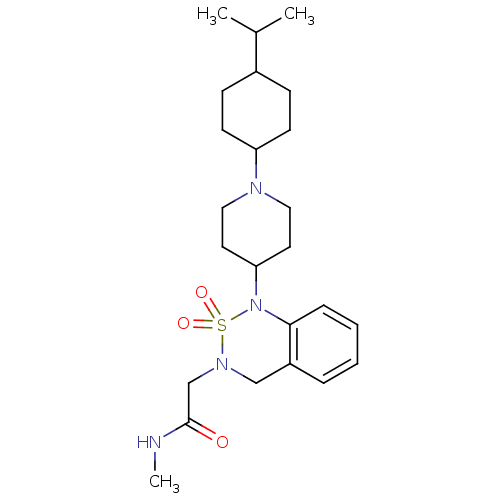 (2-{1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 6441-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.028 BindingDB Entry DOI: 10.7270/Q21J99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50200608 (CHEMBL238818 | N-cyclopropyl-2-(3-{1-[3-(5-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem 15: 1828-47 (2007) Article DOI: 10.1016/j.bmc.2006.11.030 BindingDB Entry DOI: 10.7270/Q27S7NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50200607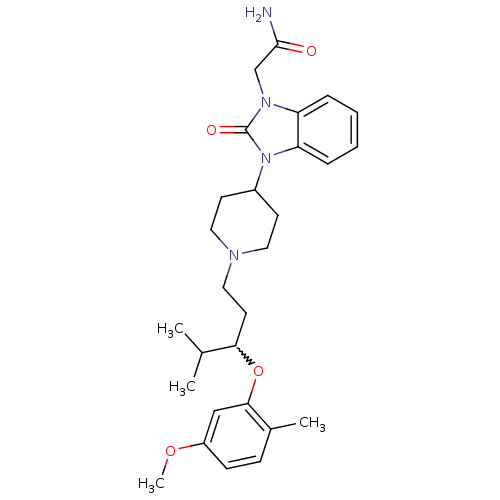 (2-(3-{1-[3-(5-methoxy-2-methyl-phenoxy)-4-methylpe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem 15: 1828-47 (2007) Article DOI: 10.1016/j.bmc.2006.11.030 BindingDB Entry DOI: 10.7270/Q27S7NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50200614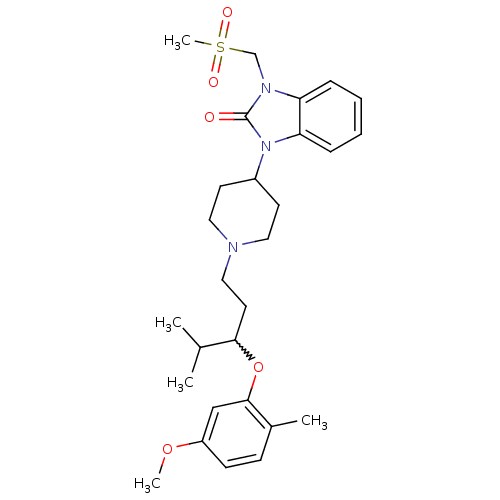 (1-methanesulfonylmethyl-3-{1-[3-(5-methoxy-2-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem 15: 1828-47 (2007) Article DOI: 10.1016/j.bmc.2006.11.030 BindingDB Entry DOI: 10.7270/Q27S7NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50159903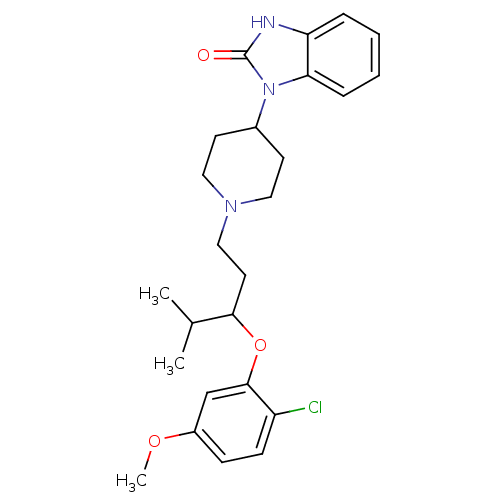 (1-{1-[3-(2-Chloro-5-methoxy-phenoxy)-4-methyl-pent...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd Curated by ChEMBL | Assay Description Binding affinity towards human opioid receptor like 1 was determined by using [3H]-nociceptin as radioligand expressed in Chinese hamster ovary (CHO)... | Bioorg Med Chem Lett 15: 589-93 (2005) Article DOI: 10.1016/j.bmcl.2004.11.049 BindingDB Entry DOI: 10.7270/Q2K35T50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50200610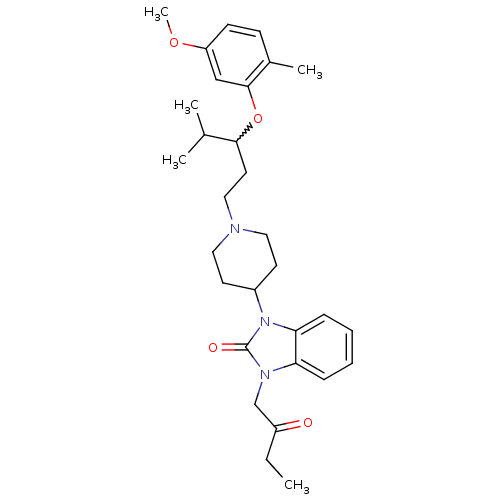 (1-{1-[3-(5-methoxy-2-methyl-phenoxy)-4-methylpenty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem 15: 1828-47 (2007) Article DOI: 10.1016/j.bmc.2006.11.030 BindingDB Entry DOI: 10.7270/Q27S7NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241195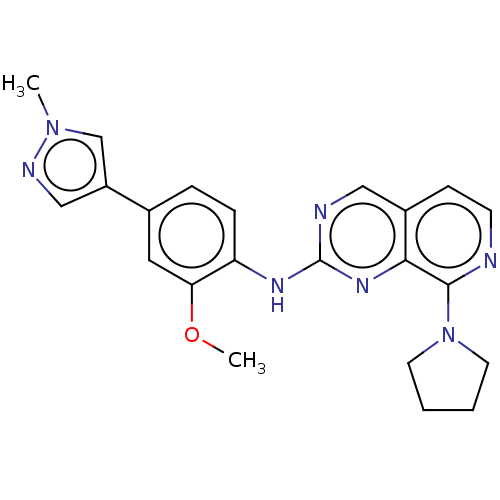 (US10479788, Example 37 | US11046688, Example 37 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM419190 (5-tert-Butyl-3-[(2-chlorophenyl)methyl]-7-(1,2,5-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The affinity of the compounds of the invention for cannabinoid receptors was determined using recommended amounts of membrane preparations (PerkinElm... | US Patent US10457684 (2019) BindingDB Entry DOI: 10.7270/Q2HM5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50311505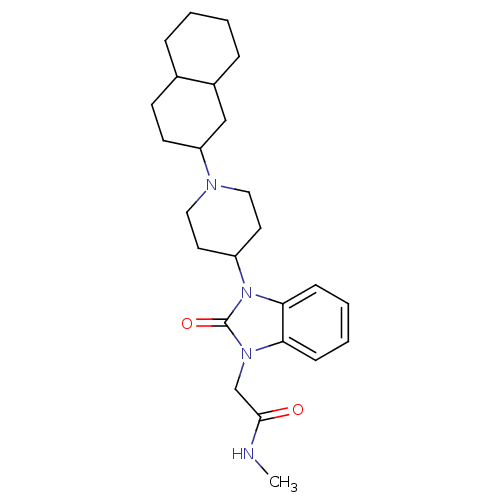 (2-(3-(1-(decahydronaphthalen-2-yl)piperidin-4-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 6441-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.028 BindingDB Entry DOI: 10.7270/Q21J99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241223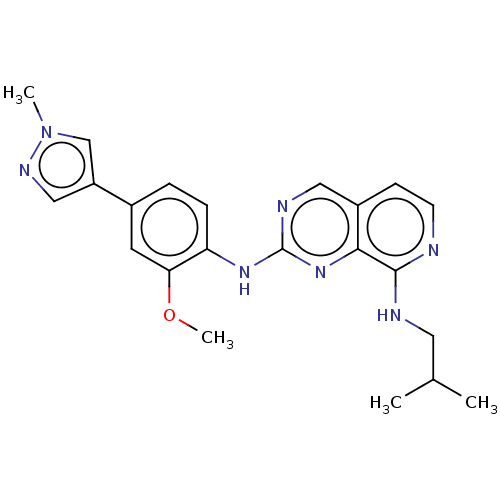 (US10479788, Example 65 | US11046688, Example 65 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50311491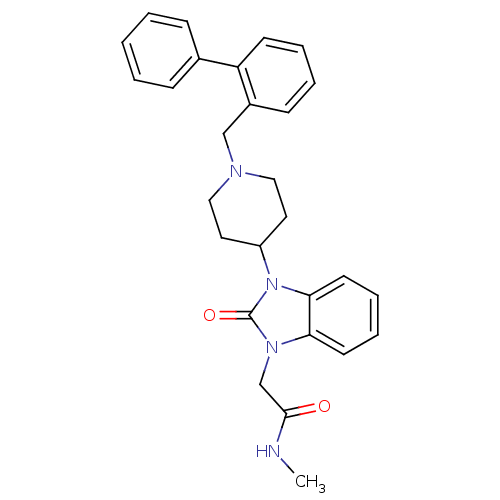 (2-(3-(1-(biphenyl-2-ylmethyl)piperidin-4-yl)-2-oxo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human MOP receptor expressed in CHOK1 cells | Bioorg Med Chem Lett 19: 6441-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.028 BindingDB Entry DOI: 10.7270/Q21J99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4318 total ) | Next | Last >> |