

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9182 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <0.00500 | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9180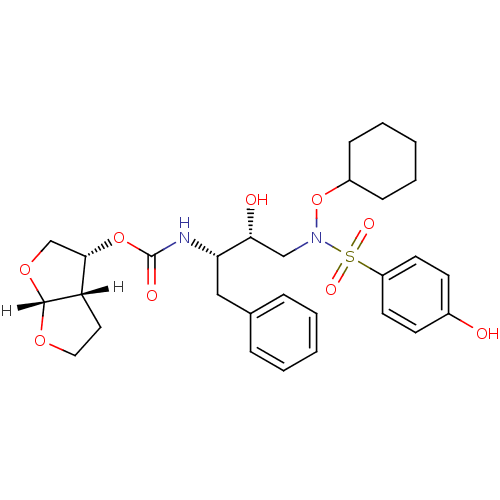 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <0.00500 | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9171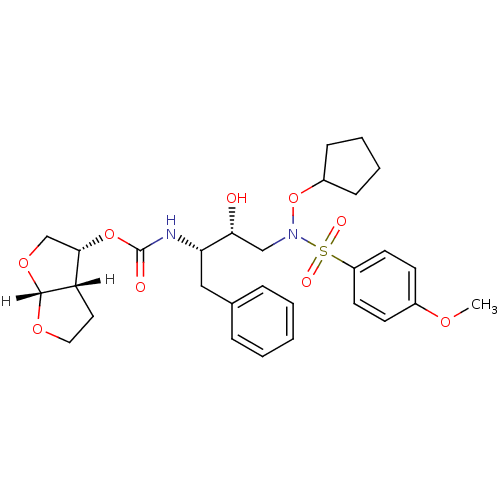 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <0.00500 | <-64.5 | 6 | n/a | n/a | n/a | n/a | 6.8 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9175 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.00500 | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9176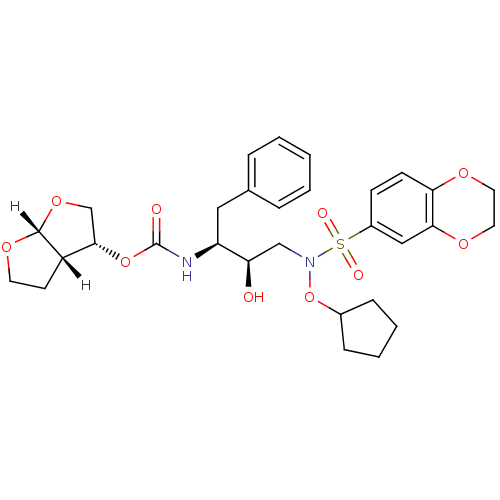 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.00500 | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9173 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.00500 | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9178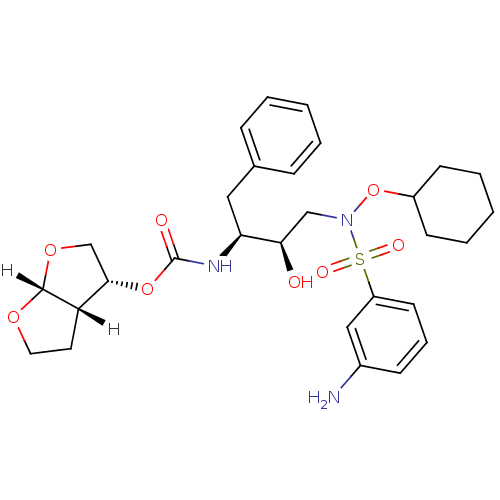 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00500 | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9174 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <0.00500 | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9181 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <0.00500 | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9172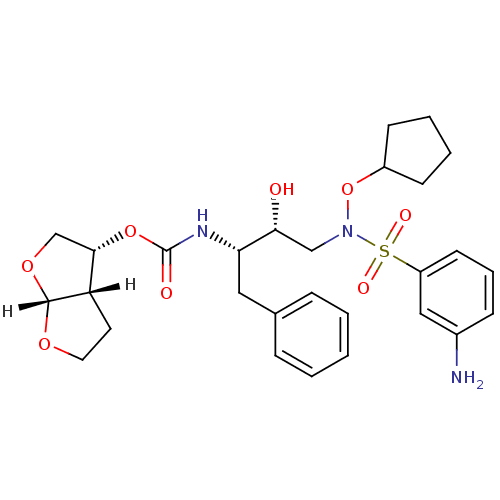 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <0.00500 | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9177 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.00500 | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9170 ((2S)-N-[(2S,3R)-4-[(cyclopentyloxy)(4-methoxybenze...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.00500 | <-64.5 | 24 | n/a | n/a | n/a | n/a | 6.8 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9179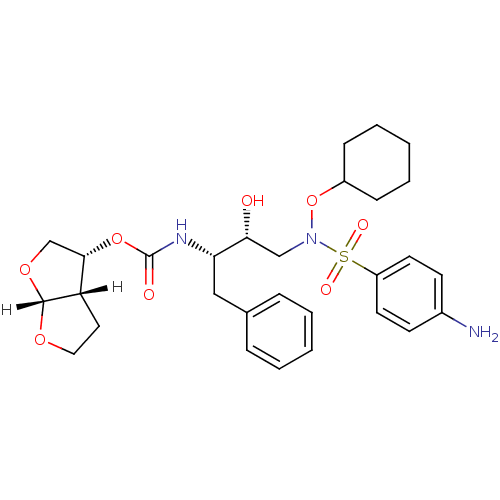 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0180 | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0400 | -59.3 | 150 | n/a | n/a | n/a | n/a | 6.8 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9169 (N-alkoxysulfonamide analog 9 | N-alkoxysulfonamide...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | -57.3 | 203 | n/a | n/a | n/a | n/a | 6.8 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9168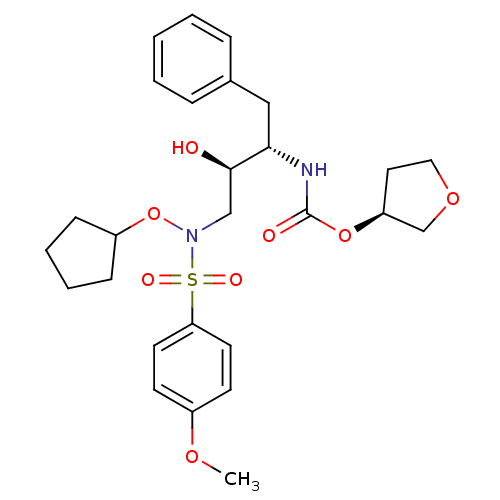 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(cyclopentyloxy)(4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.190 | -55.5 | 738 | n/a | n/a | n/a | n/a | 6.8 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9165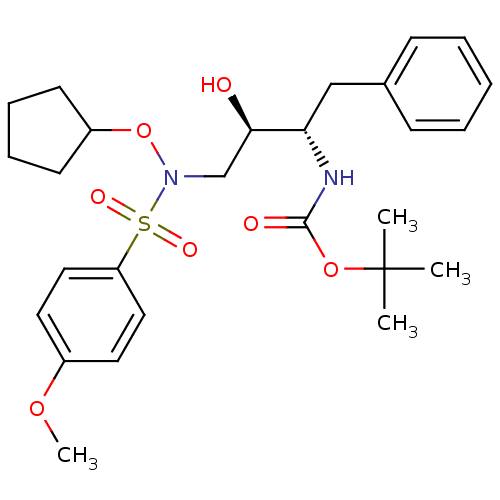 (N-alkoxysulfonamide analog 5 | N-alkoxysulfonamide...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | -50.2 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9164 (N-alkoxysulfonamide analog 4 | N-alkoxysulfonamide...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | -48.8 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9162 (N-alkoxysulfonamide analog 2 | N-alkoxysulfonamide...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | -48.5 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9163 (N-alkoxysulfonamide analog 3 | N-alkoxysulfonamide...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | -47.9 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9166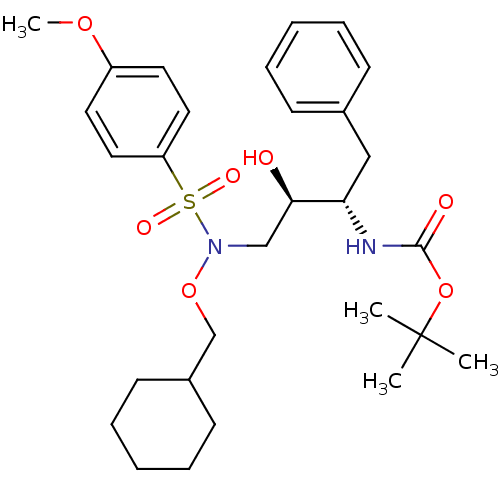 (N-alkoxysulfonamide analog 6 | N-alkoxysulfonamide...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | -45.2 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9161 (N-alkoxysulfonamide analog 1 | N-alkoxysulfonamide...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | -44.8 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9167 (N-alkoxysulfonamide analog 7 | N-alkoxysulfonamide...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | -43.7 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50072123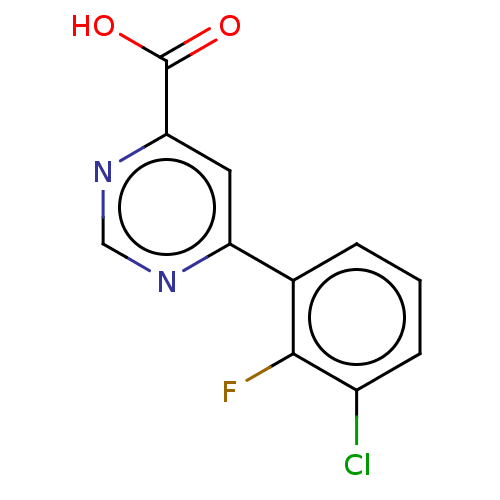 (CHEMBL3407904) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd Curated by ChEMBL | Assay Description Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis | J Med Chem 58: 1159-83 (2015) Article DOI: 10.1021/jm501350y BindingDB Entry DOI: 10.7270/Q2445P5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50072078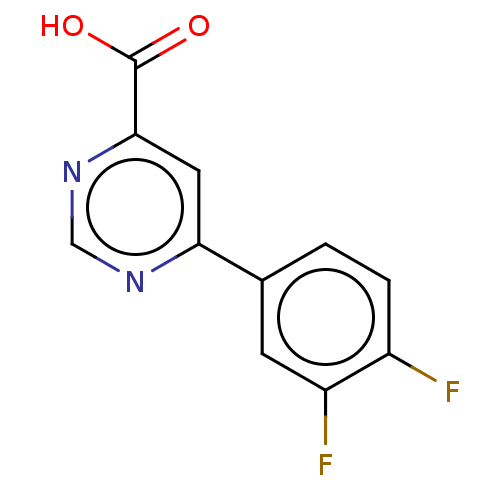 (CHEMBL3407905) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd Curated by ChEMBL | Assay Description Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis | J Med Chem 58: 1159-83 (2015) Article DOI: 10.1021/jm501350y BindingDB Entry DOI: 10.7270/Q2445P5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50072122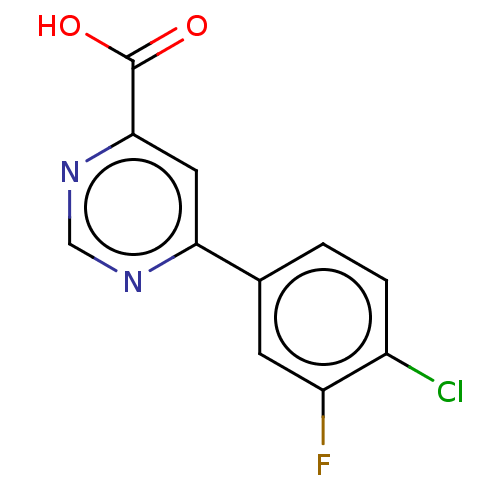 (CHEMBL3407903) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd Curated by ChEMBL | Assay Description Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis | J Med Chem 58: 1159-83 (2015) Article DOI: 10.1021/jm501350y BindingDB Entry DOI: 10.7270/Q2445P5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50072120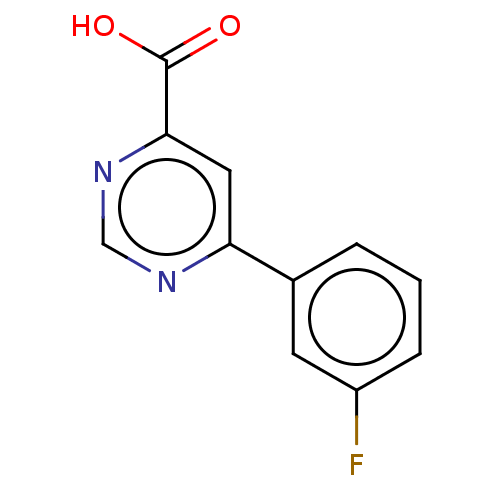 (CHEMBL3407901) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd Curated by ChEMBL | Assay Description Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis | J Med Chem 58: 1159-83 (2015) Article DOI: 10.1021/jm501350y BindingDB Entry DOI: 10.7270/Q2445P5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CXCR4-mediated chemotaxis in SDF1-stimulated human U937 cells treated 15 mins before SDF1 challenge measured after 2 hrs by luminescenc... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50418490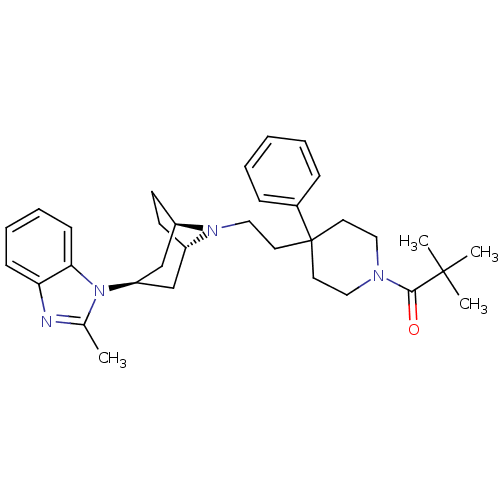 (CHEMBL1784385) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of 125I-MIP-1beta from human CCR5 receptor after 4 hrs by scintillation counting | J Med Chem 54: 3756-67 (2011) Article DOI: 10.1021/jm200279v BindingDB Entry DOI: 10.7270/Q26111K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331659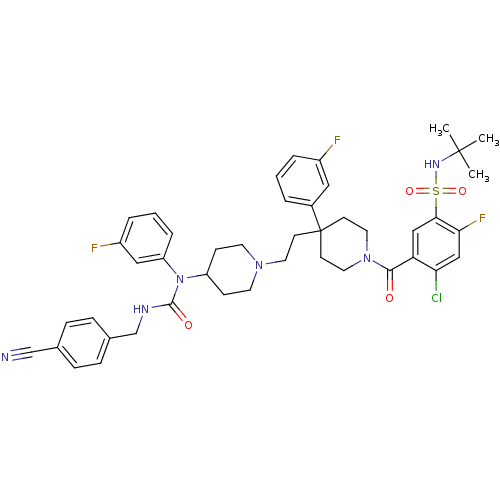 (CHEMBL1288663 | N-tert-butyl-4-chloro-5-(4-(2-(4-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50072082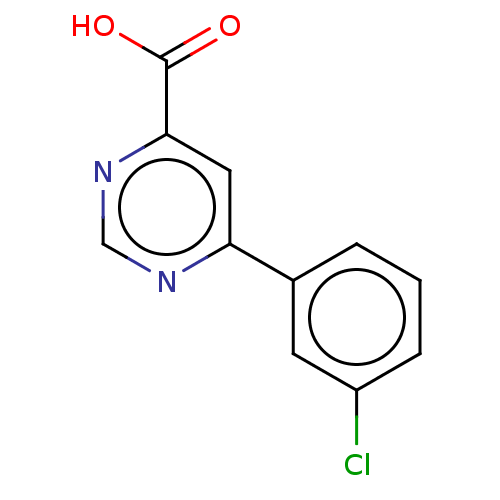 (CHEMBL3407865) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd Curated by ChEMBL | Assay Description Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis | J Med Chem 58: 1159-83 (2015) Article DOI: 10.1021/jm501350y BindingDB Entry DOI: 10.7270/Q2445P5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50072081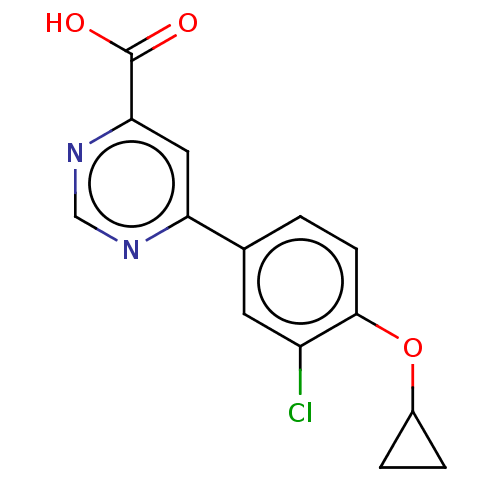 (CHEMBL3407922) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd Curated by ChEMBL | Assay Description Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis | J Med Chem 58: 1159-83 (2015) Article DOI: 10.1021/jm501350y BindingDB Entry DOI: 10.7270/Q2445P5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assay | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50072077 (CHEMBL3407866) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd Curated by ChEMBL | Assay Description Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis | J Med Chem 58: 1159-83 (2015) Article DOI: 10.1021/jm501350y BindingDB Entry DOI: 10.7270/Q2445P5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50072079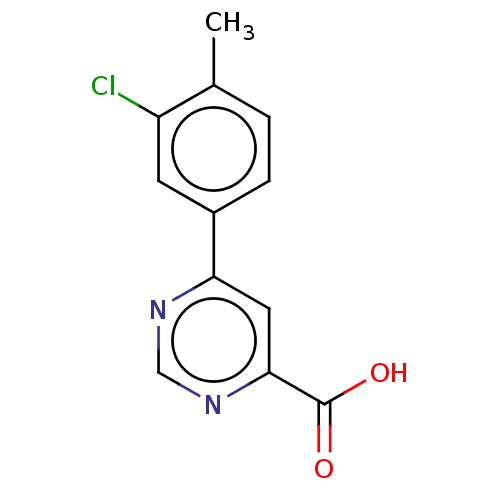 (CHEMBL3407913) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd Curated by ChEMBL | Assay Description Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis | J Med Chem 58: 1159-83 (2015) Article DOI: 10.1021/jm501350y BindingDB Entry DOI: 10.7270/Q2445P5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in HEK293 cells assessed as inhibition of SDF1-induced response treated 30 mins before agonist challenge... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50072131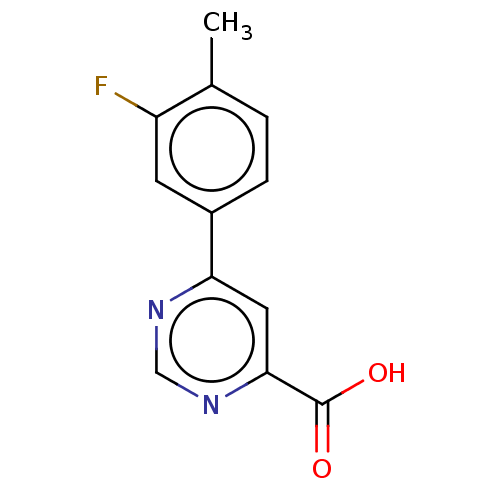 (CHEMBL3407914) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd Curated by ChEMBL | Assay Description Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis | J Med Chem 58: 1159-83 (2015) Article DOI: 10.1021/jm501350y BindingDB Entry DOI: 10.7270/Q2445P5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50257737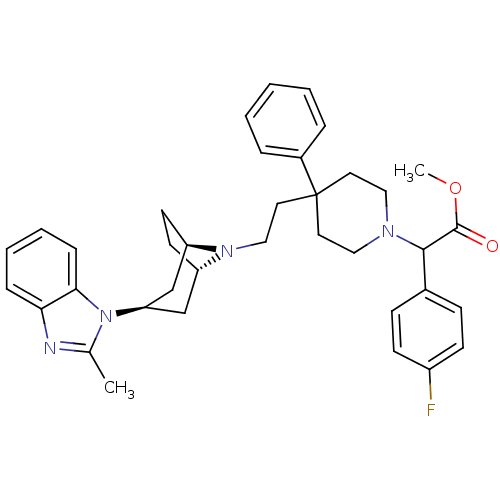 (CHEMBL444497 | methyl 2-(4-fluorophenyl)-2-(4-(2-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]MIP-1beta from CCR5 (unknown origin) expressed in CHO cell membrane | Bioorg Med Chem Lett 19: 1610-3 (2009) Article DOI: 10.1016/j.bmcl.2009.02.014 BindingDB Entry DOI: 10.7270/Q2VQ32JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50072121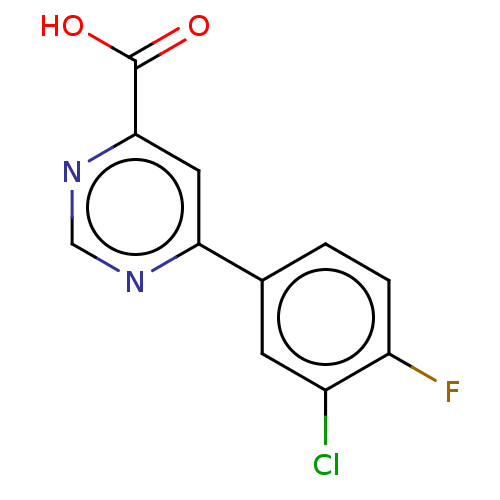 (CHEMBL3407902) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd Curated by ChEMBL | Assay Description Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis | J Med Chem 58: 1159-83 (2015) Article DOI: 10.1021/jm501350y BindingDB Entry DOI: 10.7270/Q2445P5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50412735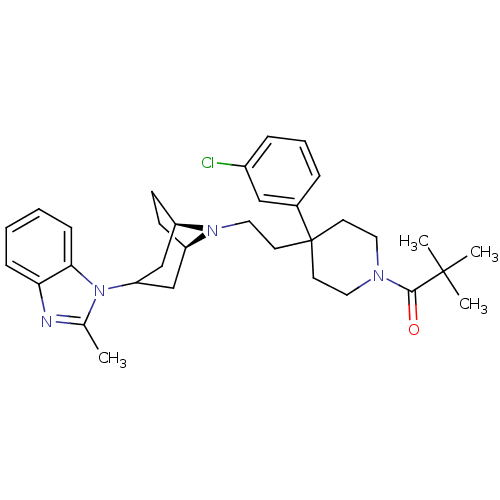 (CHEMBL454251) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]MIP-1beta from human CCR5 expressed in CHO cells | J Med Chem 51: 6538-46 (2008) Article DOI: 10.1021/jm800598a BindingDB Entry DOI: 10.7270/Q2WS8VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.07 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in HEK293 cells assessed as inhibition of SDF1-induced response treated before agonist challenge measure... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50072136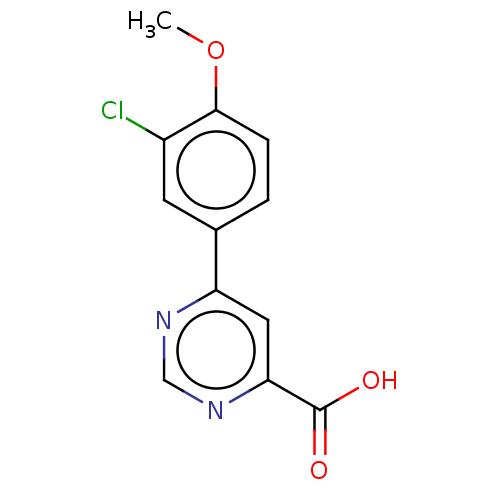 (CHEMBL3407920) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd Curated by ChEMBL | Assay Description Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis | J Med Chem 58: 1159-83 (2015) Article DOI: 10.1021/jm501350y BindingDB Entry DOI: 10.7270/Q2445P5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50072078 (CHEMBL3407905) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd Curated by ChEMBL | Assay Description Inhibition of mouse KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis | J Med Chem 58: 1159-83 (2015) Article DOI: 10.1021/jm501350y BindingDB Entry DOI: 10.7270/Q2445P5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50072143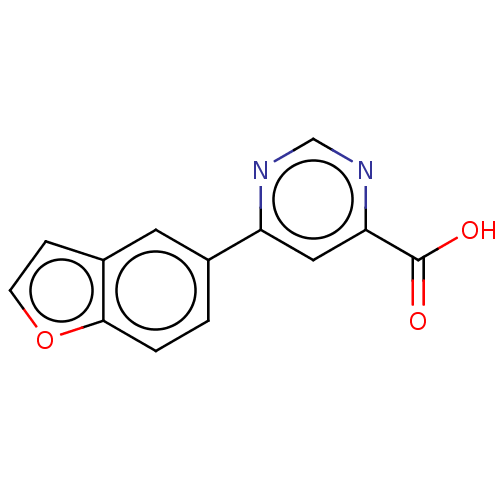 (CHEMBL3407924) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd Curated by ChEMBL | Assay Description Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis | J Med Chem 58: 1159-83 (2015) Article DOI: 10.1021/jm501350y BindingDB Entry DOI: 10.7270/Q2445P5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331659 (CHEMBL1288663 | N-tert-butyl-4-chloro-5-(4-(2-(4-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.71 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assay in presence of 45 mg/ml human ... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.97 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assay in presence of alpha-acid glyc... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.99 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assay in presence of 1 mg/ml alpha-a... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Rattus norvegicus) | BDBM50072078 (CHEMBL3407905) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd Curated by ChEMBL | Assay Description Inhibition of rat KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis | J Med Chem 58: 1159-83 (2015) Article DOI: 10.1021/jm501350y BindingDB Entry DOI: 10.7270/Q2445P5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50072175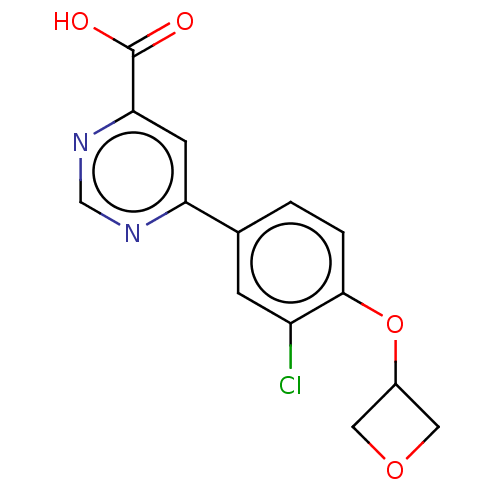 (CHEMBL3407926) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd Curated by ChEMBL | Assay Description Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis | J Med Chem 58: 1159-83 (2015) Article DOI: 10.1021/jm501350y BindingDB Entry DOI: 10.7270/Q2445P5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 360 total ) | Next | Last >> |