

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50147818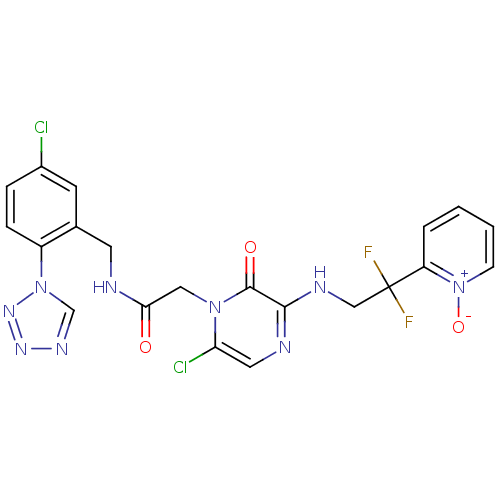 ((2-[6-CHLORO-3-{[2,2-DIFLUORO-2-(1-OXIDOPYRIDIN-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147824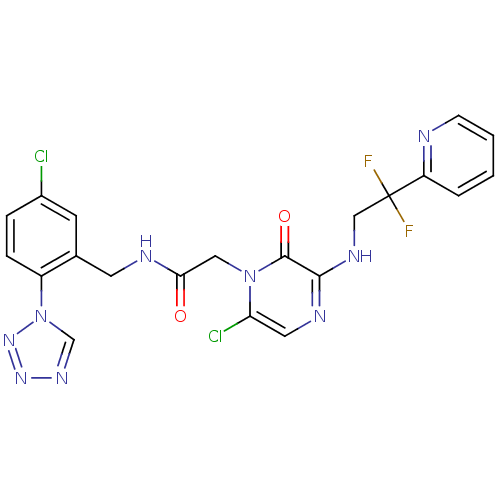 (2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147793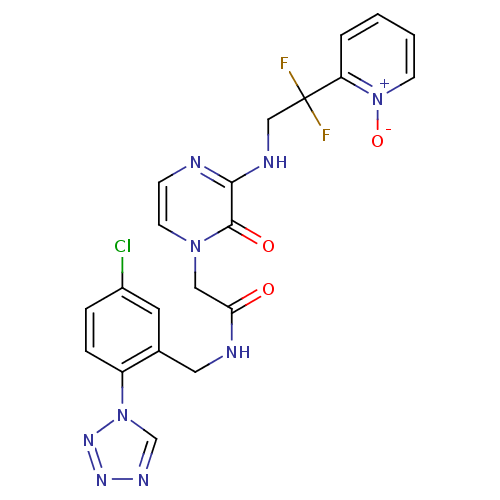 (CHEMBL323583 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147788 (CHEMBL103874 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50143784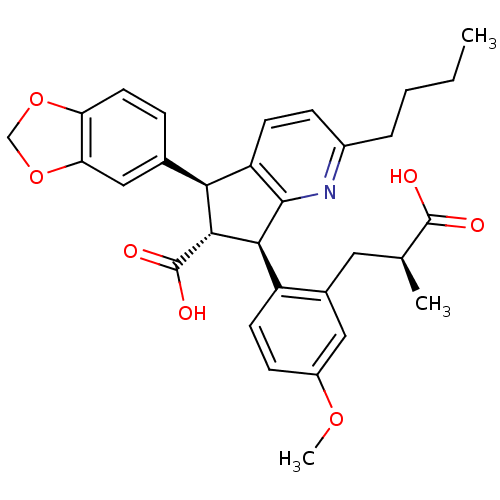 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-butyl-7-[2-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1262-70 (1999) BindingDB Entry DOI: 10.7270/Q2Q52N5Q | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147809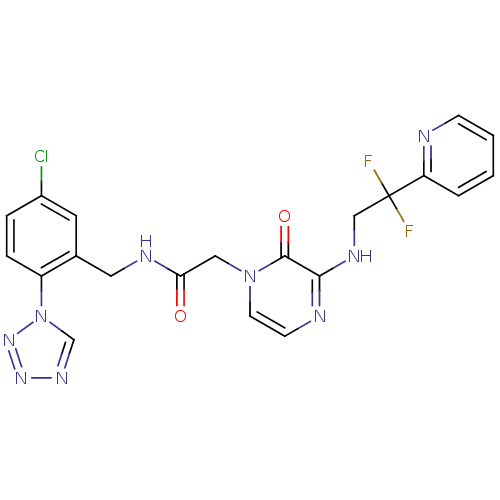 (CHEMBL103342 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50356880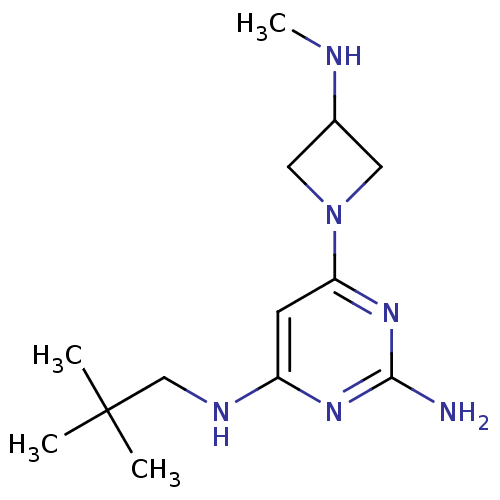 (CHEMBL1915536) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... | Bioorg Med Chem Lett 21: 6596-602 (2011) Article DOI: 10.1016/j.bmcl.2011.07.125 BindingDB Entry DOI: 10.7270/Q20C4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50172826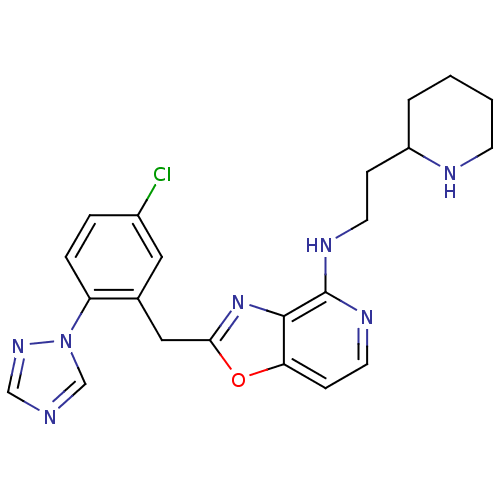 (CHEMBL426101 | [2-(5-Chloro-2-[1,2,4]triazol-1-yl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | Bioorg Med Chem Lett 15: 4411-6 (2005) Article DOI: 10.1016/j.bmcl.2005.07.022 BindingDB Entry DOI: 10.7270/Q22Z153P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50172842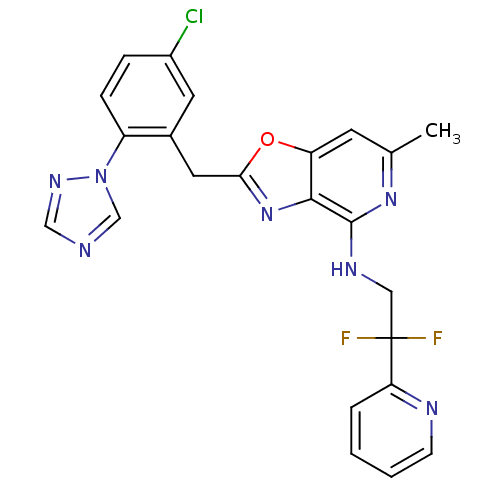 (CHEMBL198820 | [2-(5-Chloro-2-[1,2,4]triazol-1-yl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | Bioorg Med Chem Lett 15: 4411-6 (2005) Article DOI: 10.1016/j.bmcl.2005.07.022 BindingDB Entry DOI: 10.7270/Q22Z153P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123490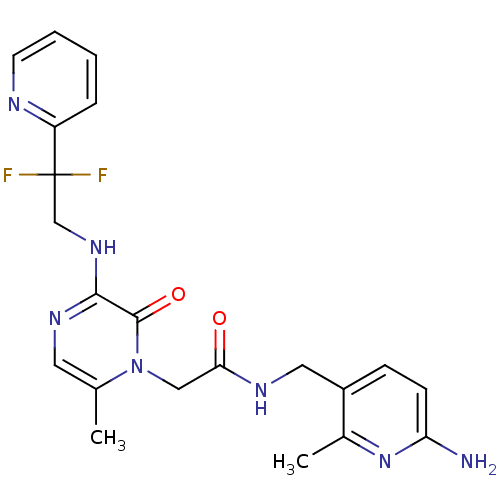 (CHEMBL143418 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin (IIa) | J Med Chem 46: 461-73 (2003) Article DOI: 10.1021/jm020311f BindingDB Entry DOI: 10.7270/Q2W958J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147812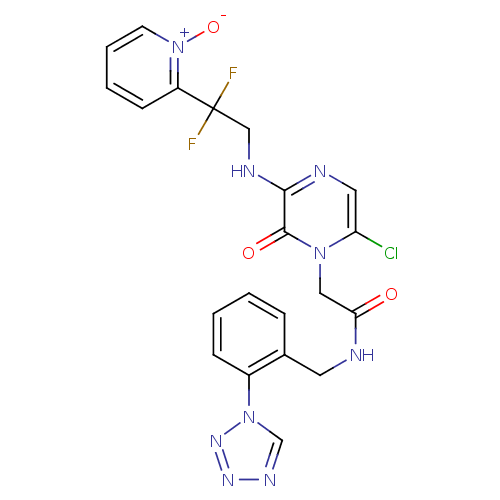 (2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254931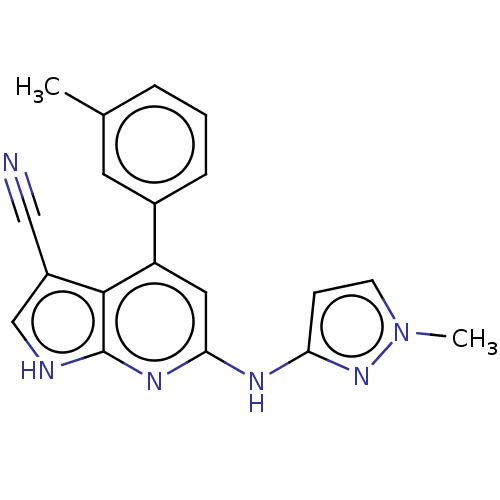 (US9499542, 14 | US9675594, 14) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50172829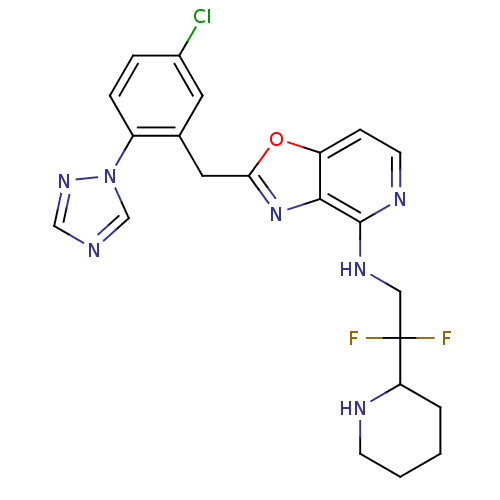 (CHEMBL198735 | [2-(5-Chloro-2-[1,2,4]triazol-1-yl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | Bioorg Med Chem Lett 15: 4411-6 (2005) Article DOI: 10.1016/j.bmcl.2005.07.022 BindingDB Entry DOI: 10.7270/Q22Z153P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50172841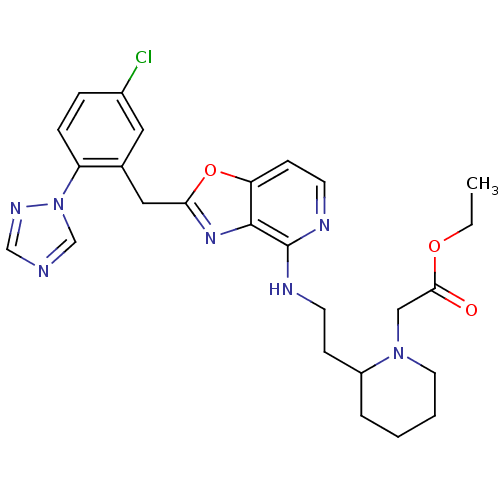 ((2-{2-[2-(5-Chloro-2-[1,2,4]triazol-1-yl-benzyl)-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | Bioorg Med Chem Lett 15: 4411-6 (2005) Article DOI: 10.1016/j.bmcl.2005.07.022 BindingDB Entry DOI: 10.7270/Q22Z153P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50172839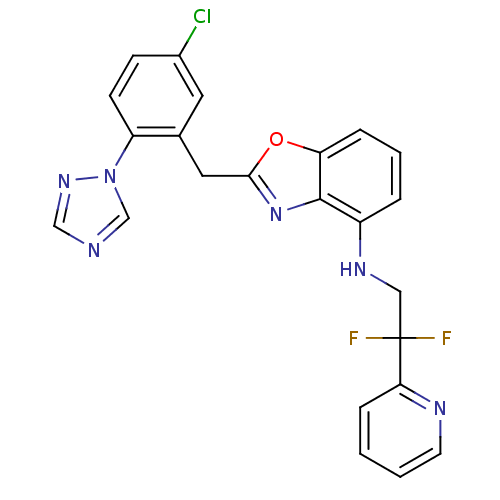 (CHEMBL197668 | [2-(5-Chloro-2-[1,2,4]triazol-1-yl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | Bioorg Med Chem Lett 15: 4411-6 (2005) Article DOI: 10.1016/j.bmcl.2005.07.022 BindingDB Entry DOI: 10.7270/Q22Z153P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50365855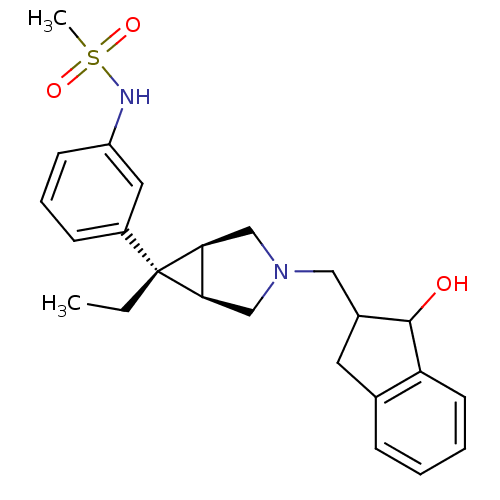 (CHEMBL1957843) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor by GTP-gamma S binding assay | Bioorg Med Chem Lett 22: 2200-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.099 BindingDB Entry DOI: 10.7270/Q2M90958 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50088653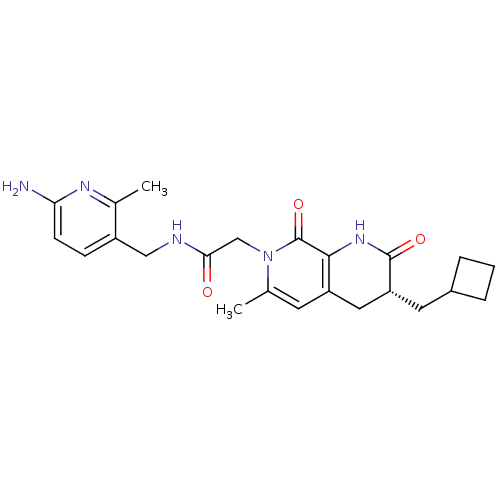 (CHEMBL274152 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147801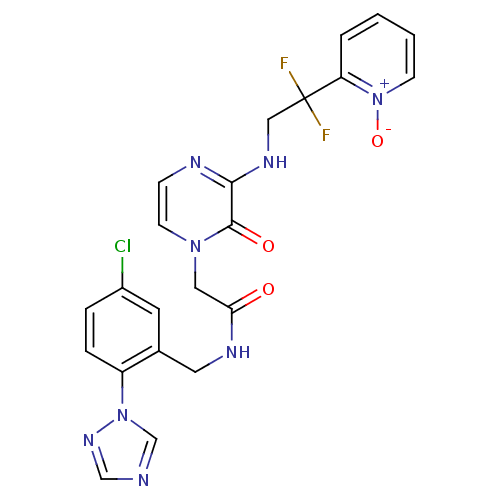 (CHEMBL102122 | N-(5-Chloro-2-[1,2,4]triazol-1-yl-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147821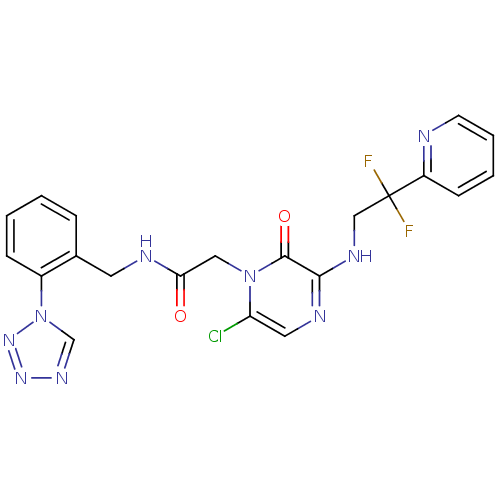 (2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50143784 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-butyl-7-[2-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1262-70 (1999) BindingDB Entry DOI: 10.7270/Q2Q52N5Q | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50118528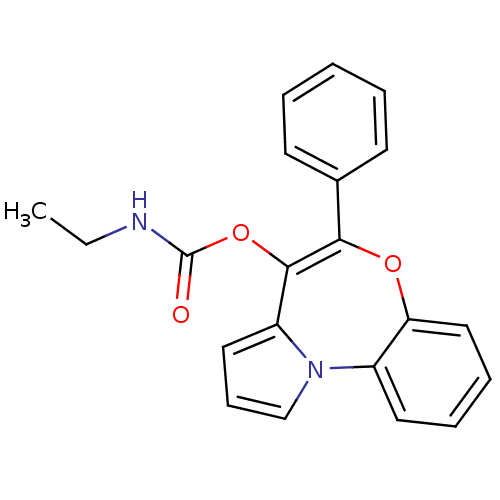 (CHEMBL135514 | Ethyl-carbamic acid 5-phenyl-6-oxa-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]-Ro-5-4864 binding to mitochondrial rat testis Peripheral type benzodiazepine receptor (PBR) | J Med Chem 45: 4276-81 (2002) BindingDB Entry DOI: 10.7270/Q20Z72MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123504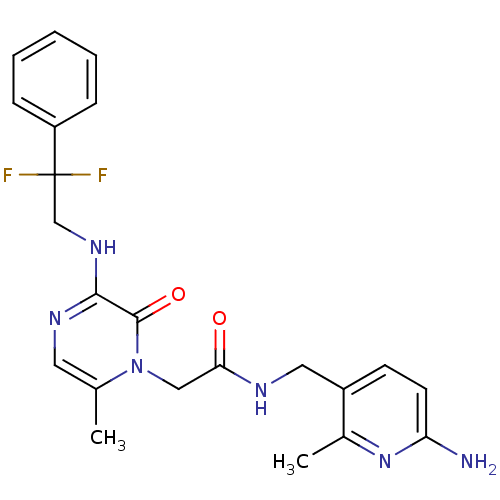 (CHEMBL142546 | N-((6-amino-2-methylpyridin-3-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin (IIa) | J Med Chem 46: 461-73 (2003) Article DOI: 10.1021/jm020311f BindingDB Entry DOI: 10.7270/Q2W958J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50365855 (CHEMBL1957843) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor | Bioorg Med Chem Lett 22: 2200-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.099 BindingDB Entry DOI: 10.7270/Q2M90958 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254931 (US9499542, 14 | US9675594, 14) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrat... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50113783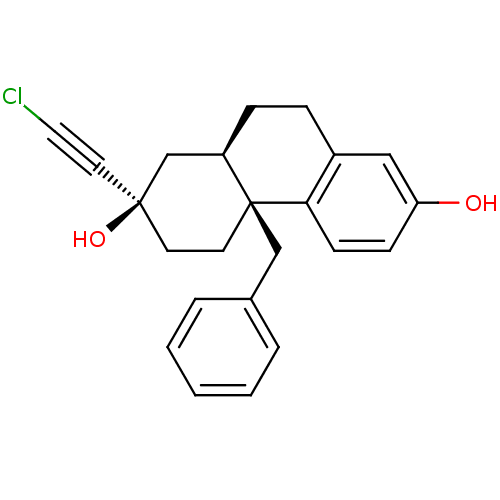 ((2R,4aS,10aR)-4a-benzyl-2-(chloroethynyl)-1,2,3,4,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description Displacement of [3H]DEX from human glucocorticoid receptor | J Med Chem 53: 3065-74 (2010) Article DOI: 10.1021/jm901452y BindingDB Entry DOI: 10.7270/Q2VQ33NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50113782 ((10R,13S,17S)-17-hydroxy-13-methyl-10-(4-methylben...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description Displacement of [3H]DEX from human glucocorticoid receptor | J Med Chem 53: 3065-74 (2010) Article DOI: 10.1021/jm901452y BindingDB Entry DOI: 10.7270/Q2VQ33NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50582714 (CHEMBL5075978) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human LRRK2 WT incubated for 2 hrs by TR-FRET based Lanthascreen kinase activity assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00720 BindingDB Entry DOI: 10.7270/Q2MP5754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50118528 (CHEMBL135514 | Ethyl-carbamic acid 5-phenyl-6-oxa-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]-PK11195 binding to Peripheral type benzodiazepine receptor (PBR) in rat cortex homogenate by 50% | J Med Chem 45: 4276-81 (2002) BindingDB Entry DOI: 10.7270/Q20Z72MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147822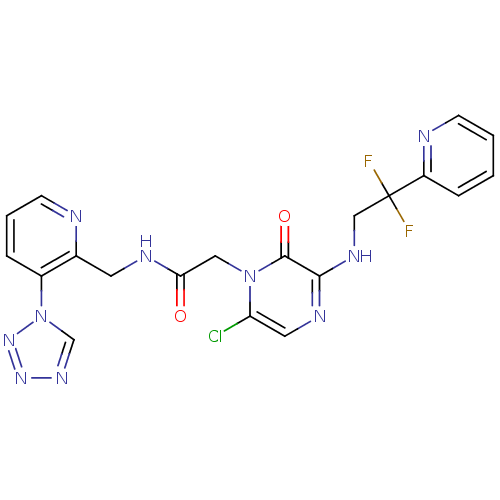 (2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50172831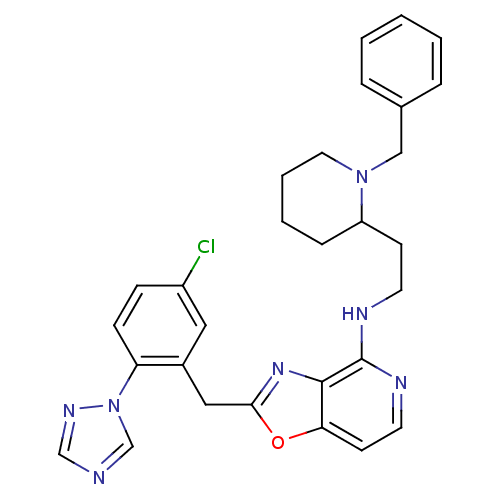 (CHEMBL198978 | [2-(5-Chloro-2-[1,2,4]triazol-1-yl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | Bioorg Med Chem Lett 15: 4411-6 (2005) Article DOI: 10.1016/j.bmcl.2005.07.022 BindingDB Entry DOI: 10.7270/Q22Z153P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147810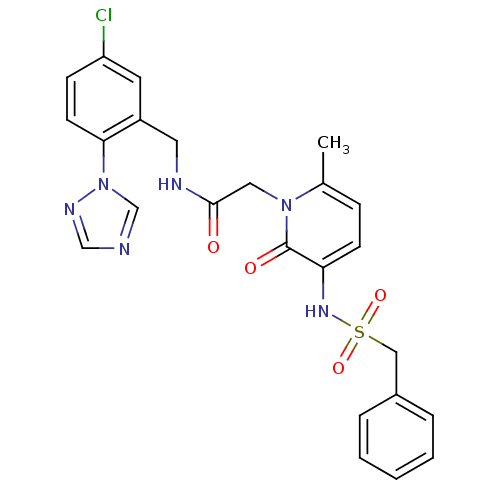 (CHEMBL100854 | N-(5-Chloro-2-[1,2,4]triazol-1-yl-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50172845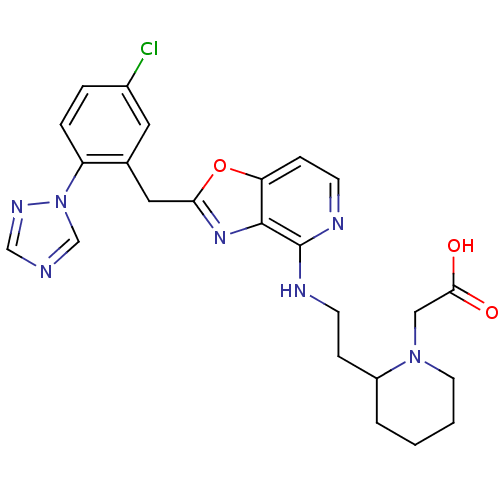 ((2-{2-[2-(5-Chloro-2-[1,2,4]triazol-1-yl-benzyl)-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | Bioorg Med Chem Lett 15: 4411-6 (2005) Article DOI: 10.1016/j.bmcl.2005.07.022 BindingDB Entry DOI: 10.7270/Q22Z153P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50113780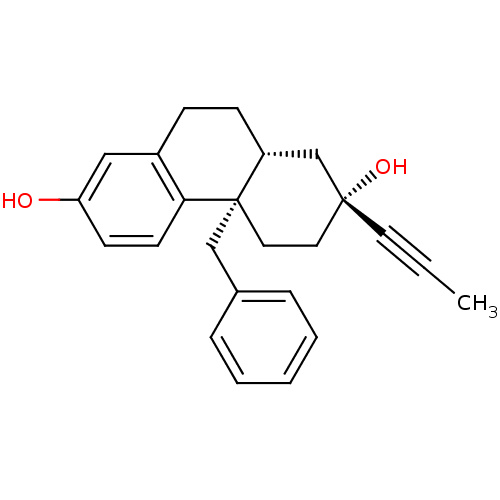 ((2R,4aS,10aR)-4a-benzyl-2-(prop-1-ynyl)-1,2,3,4,4a...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description Displacement of [3H]DEX from human glucocorticoid receptor | J Med Chem 53: 3065-74 (2010) Article DOI: 10.1021/jm901452y BindingDB Entry DOI: 10.7270/Q2VQ33NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50508336 (CHEMBL4530500) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from mouse MOR expressed in CHO cell membranes by competitive radioligand binding assay | J Med Chem 62: 561-574 (2019) Article DOI: 10.1021/acs.jmedchem.8b01158 BindingDB Entry DOI: 10.7270/Q2CN776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50118537 (CHEMBL135391 | Ethyl-carbamic acid 7-chloro-5-phen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]-PK11195 binding to Peripheral type benzodiazepine receptor (PBR) in rat cortex homogenate by 50% | J Med Chem 45: 4276-81 (2002) BindingDB Entry DOI: 10.7270/Q20Z72MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50582715 (CHEMBL5091909) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human LRRK2 WT incubated for 2 hrs by TR-FRET based Lanthascreen kinase activity assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00720 BindingDB Entry DOI: 10.7270/Q2MP5754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50118528 (CHEMBL135514 | Ethyl-carbamic acid 5-phenyl-6-oxa-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]-PK11195 binding to mitochondrial rat testis Peripheral type benzodiazepine receptor (PBR) | J Med Chem 45: 4276-81 (2002) BindingDB Entry DOI: 10.7270/Q20Z72MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147826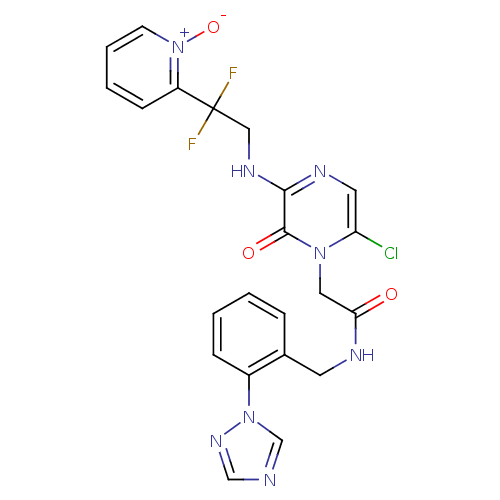 (2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50088660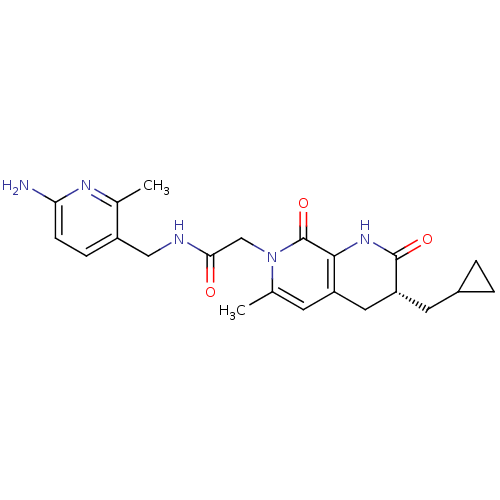 (CHEMBL10785 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50118539 (CHEMBL136036 | Diethyl-carbamic acid 5-thiophen-2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]-PK11195 binding to Peripheral type benzodiazepine receptor (PBR) in rat cortex homogenate by 50% | J Med Chem 45: 4276-81 (2002) BindingDB Entry DOI: 10.7270/Q20Z72MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50060938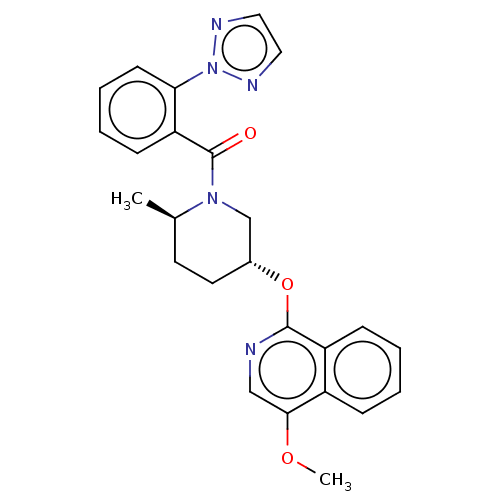 (CHEMBL3394847) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human OX2R by radioligand displacement assay | Bioorg Med Chem Lett 25: 444-50 (2015) Article DOI: 10.1016/j.bmcl.2014.12.056 BindingDB Entry DOI: 10.7270/Q2KD20MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50370389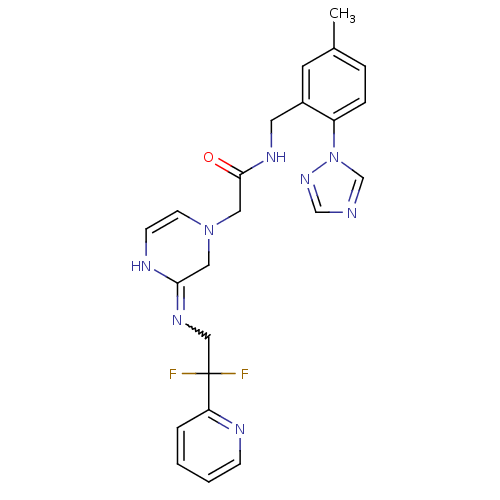 (CHEMBL1201843) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088657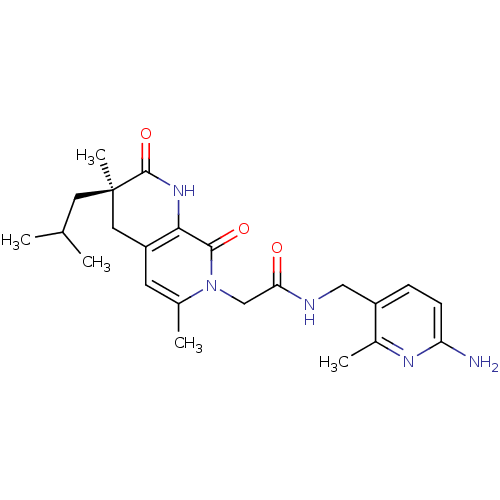 (CHEMBL11181 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description Displacement of [3H]DEX from human glucocorticoid receptor | J Med Chem 53: 3065-74 (2010) Article DOI: 10.1021/jm901452y BindingDB Entry DOI: 10.7270/Q2VQ33NH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50172832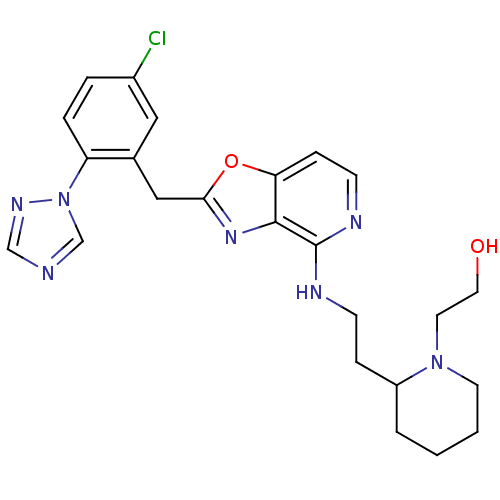 (2-(2-{2-[2-(5-Chloro-2-[1,2,4]triazol-1-yl-benzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | Bioorg Med Chem Lett 15: 4411-6 (2005) Article DOI: 10.1016/j.bmcl.2005.07.022 BindingDB Entry DOI: 10.7270/Q22Z153P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50052754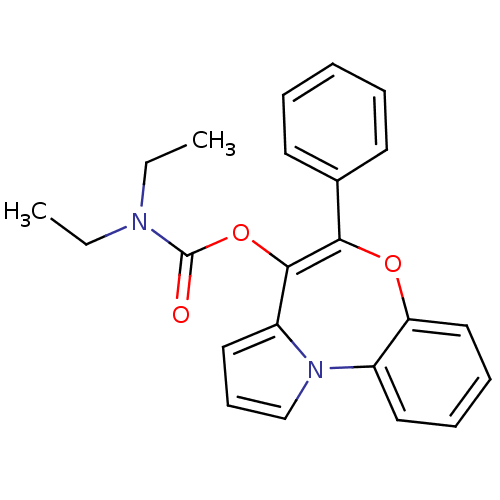 (CHEMBL115740 | Diethyl-carbamic acid 5-phenyl-6-ox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Binding affinity against peripheral-type benzodiazepine receptor (PBR) from rat cortex homogenate using [3H]-PK 11195 as radioligand | J Med Chem 39: 3435-50 (1996) Article DOI: 10.1021/jm960251b BindingDB Entry DOI: 10.7270/Q23B60SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123496 (CHEMBL143138 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin (IIa) | J Med Chem 46: 461-73 (2003) Article DOI: 10.1021/jm020311f BindingDB Entry DOI: 10.7270/Q2W958J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50150298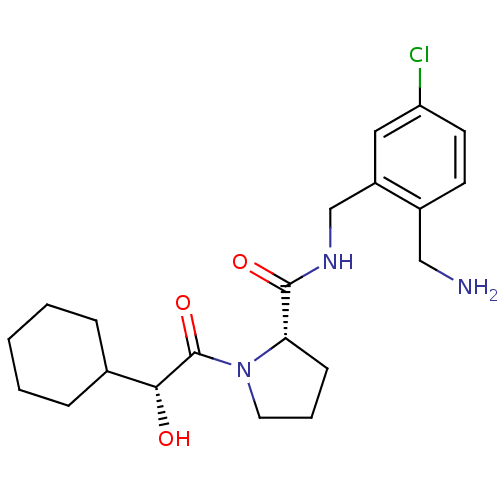 ((S)-1-((R)-2-Cyclohexyl-2-hydroxy-acetyl)-pyrrolid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition constant against human Thrombin | Bioorg Med Chem Lett 14: 4161-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.030 BindingDB Entry DOI: 10.7270/Q2833RH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50337483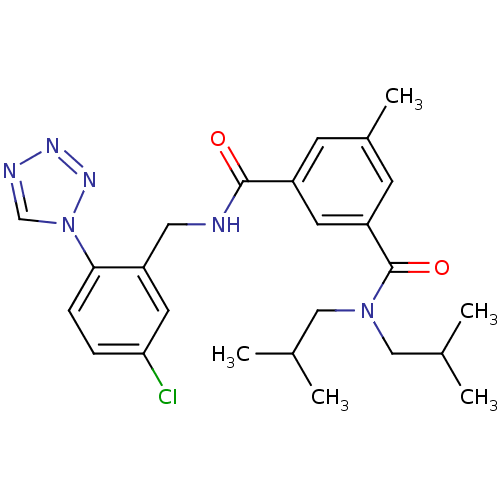 (CHEMBL1682777 | N1-(5-chloro-2-(1H-tetrazol-1-yl)b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 21: 1536-40 (2011) Article DOI: 10.1016/j.bmcl.2010.12.105 BindingDB Entry DOI: 10.7270/Q2V1252F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50337479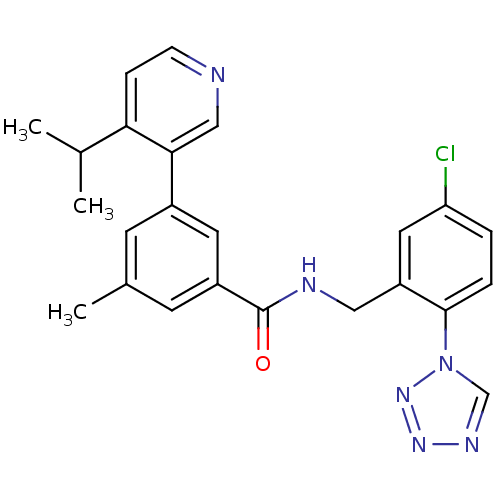 (CHEMBL1682781 | N-(5-chloro-2-(1H-tetrazol-1-yl)be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 21: 1536-40 (2011) Article DOI: 10.1016/j.bmcl.2010.12.105 BindingDB Entry DOI: 10.7270/Q2V1252F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 6289 total ) | Next | Last >> |