 Found 2183 hits with Last Name = 'williams' and Initial = 'c'
Found 2183 hits with Last Name = 'williams' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594821
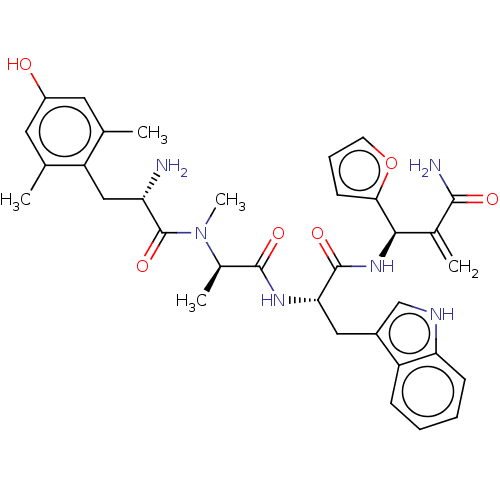
(CHEMBL5175179)Show SMILES C[C@@H](N(C)C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccco1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594822
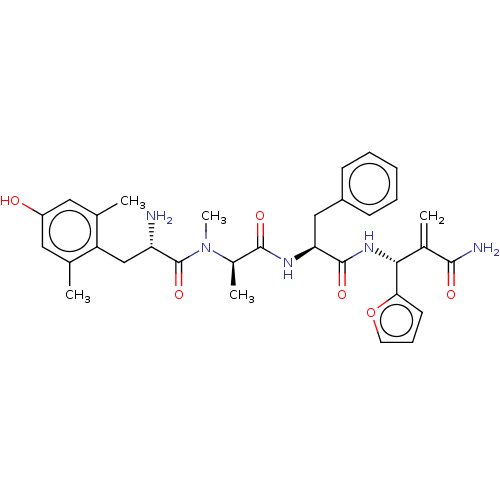
(CHEMBL5182849)Show SMILES C[C@@H](N(C)C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](C(=C)C(N)=O)c1ccco1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594820
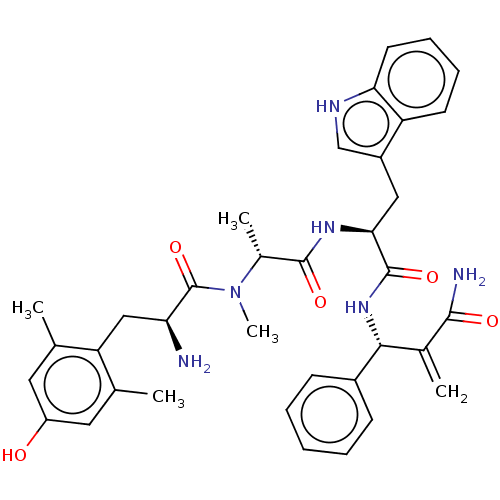
(CHEMBL5176887)Show SMILES C[C@@H](N(C)C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccccc1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(Homo sapiens (Human)) | BDBM50071733
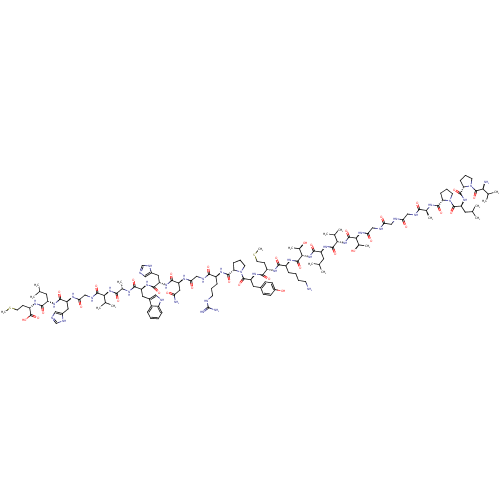
(CHEMBL413196 | Compound GRP)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)C(C)C)[C@@H](C)O)C(C)C)[C@@H](C)O)C(C)C)C(O)=O Show InChI InChI=1S/C130H203N37O32S2/c1-65(2)47-86(114(183)154-85(129(198)199)39-46-201-18)155-115(184)89(52-77-56-136-63-145-77)149-101(175)62-144-122(191)104(69(9)10)162-109(178)72(14)147-113(182)88(51-76-55-139-81-28-20-19-27-80(76)81)156-116(185)90(53-78-57-137-64-146-78)157-117(186)91(54-97(132)171)150-100(174)61-143-110(179)82(30-23-41-138-130(134)135)152-120(189)95-32-25-43-166(95)127(196)93(50-75-34-36-79(170)37-35-75)159-112(181)84(38-45-200-17)151-111(180)83(29-21-22-40-131)153-124(193)107(74(16)169)164-118(187)87(48-66(3)4)158-123(192)105(70(11)12)163-125(194)106(73(15)168)161-102(176)60-141-98(172)58-140-99(173)59-142-108(177)71(13)148-119(188)94-31-24-42-165(94)126(195)92(49-67(5)6)160-121(190)96-33-26-44-167(96)128(197)103(133)68(7)8/h19-20,27-28,34-37,55-57,63-74,82-96,103-107,139,168-170H,21-26,29-33,38-54,58-62,131,133H2,1-18H3,(H2,132,171)(H,136,145)(H,137,146)(H,140,173)(H,141,172)(H,142,177)(H,143,179)(H,144,191)(H,147,182)(H,148,188)(H,149,175)(H,150,174)(H,151,180)(H,152,189)(H,153,193)(H,154,183)(H,155,184)(H,156,185)(H,157,186)(H,158,192)(H,159,181)(H,160,190)(H,161,176)(H,162,178)(H,163,194)(H,164,187)(H,198,199)(H4,134,135,138)/t71-,72-,73+,74+,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,103-,104-,105-,106-,107-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Bombesin BB2 receptor in the presence of [125I]-[Tyr] bombesin. |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594825
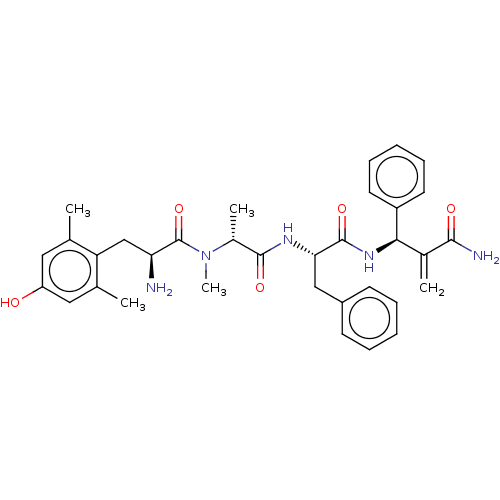
(CHEMBL5198856)Show SMILES C[C@@H](N(C)C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](C(=C)C(N)=O)c1ccccc1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0511 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061031
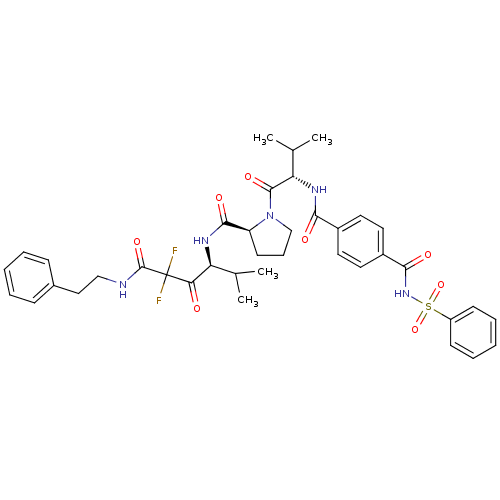
((S)-1-[(S)-2-(4-Benzenesulfonylaminocarbonyl-benzo...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C39H45F2N5O8S/c1-24(2)31(33(47)39(40,41)38(52)42-22-21-26-12-7-5-8-13-26)43-36(50)30-16-11-23-46(30)37(51)32(25(3)4)44-34(48)27-17-19-28(20-18-27)35(49)45-55(53,54)29-14-9-6-10-15-29/h5-10,12-15,17-20,24-25,30-32H,11,16,21-23H2,1-4H3,(H,42,52)(H,43,50)(H,44,48)(H,45,49)/t30-,31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50121132
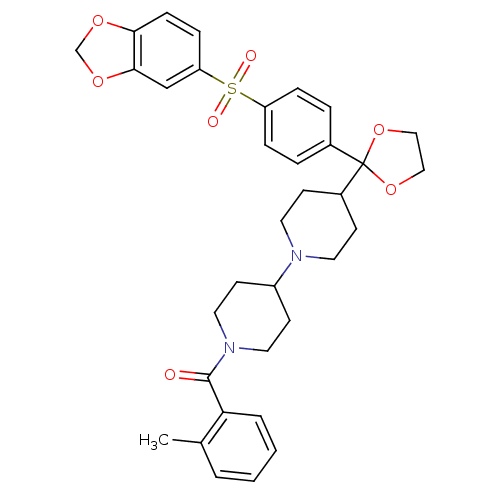
((4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1...)Show SMILES Cc1ccccc1C(=O)N1CCC(CC1)N1CCC(CC1)C1(OCCO1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C34H38N2O7S/c1-24-4-2-3-5-30(24)33(37)36-18-14-27(15-19-36)35-16-12-26(13-17-35)34(42-20-21-43-34)25-6-8-28(9-7-25)44(38,39)29-10-11-31-32(22-29)41-23-40-31/h2-11,22,26-27H,12-21,23H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 12: 3479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2VT1RGD |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071745
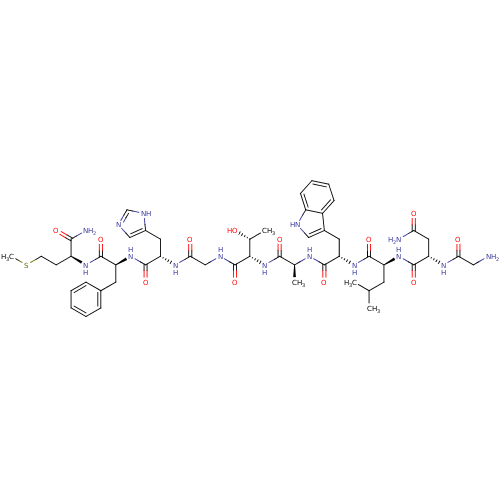
(CHEMBL403317 | Compound NMB | Gly-Asn-Leu-Trp-Ala-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)CN)[C@@H](C)O)C(N)=O Show InChI InChI=1S/C52H73N15O12S/c1-27(2)17-36(64-51(78)40(21-41(54)69)61-42(70)22-53)48(75)66-38(19-31-23-57-34-14-10-9-13-33(31)34)47(74)60-28(3)46(73)67-44(29(4)68)52(79)58-25-43(71)62-39(20-32-24-56-26-59-32)50(77)65-37(18-30-11-7-6-8-12-30)49(76)63-35(45(55)72)15-16-80-5/h6-14,23-24,26-29,35-40,44,57,68H,15-22,25,53H2,1-5H3,(H2,54,69)(H2,55,72)(H,56,59)(H,58,79)(H,60,74)(H,61,70)(H,62,71)(H,63,76)(H,64,78)(H,65,77)(H,66,75)(H,67,73)/t28-,29+,35-,36-,37-,38-,39-,40-,44-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50594846

(CHEMBL5209004)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50118528
(CHEMBL135514 | Ethyl-carbamic acid 5-phenyl-6-oxa-...)Show SMILES CCNC(=O)OC1=C(Oc2ccccc2-n2cccc12)c1ccccc1 |t:6| Show InChI InChI=1S/C21H18N2O3/c1-2-22-21(24)26-20-17-12-8-14-23(17)16-11-6-7-13-18(16)25-19(20)15-9-4-3-5-10-15/h3-14H,2H2,1H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro-5-4864 binding to mitochondrial rat testis Peripheral type benzodiazepine receptor (PBR) |
J Med Chem 45: 4276-81 (2002)
BindingDB Entry DOI: 10.7270/Q20Z72MT |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50113782

((10R,13S,17S)-17-hydroxy-13-methyl-10-(4-methylben...)Show SMILES CC#C[C@]1(O)CCC2C3CCC4=CC(=O)CC[C@]4(Cc4ccc(C)cc4)C3=CC[C@]12C |c:29,t:11| Show InChI InChI=1S/C29H34O2/c1-4-14-29(31)17-13-25-24-10-9-22-18-23(30)11-16-28(22,26(24)12-15-27(25,29)3)19-21-7-5-20(2)6-8-21/h5-8,12,18,24-25,31H,9-11,13,15-17,19H2,1-3H3/t24?,25?,27-,28+,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Displacement of [3H]DEX from human glucocorticoid receptor |
J Med Chem 53: 3065-74 (2010)
Article DOI: 10.1021/jm901452y
BindingDB Entry DOI: 10.7270/Q2VQ33NH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50113783
((2R,4aS,10aR)-4a-benzyl-2-(chloroethynyl)-1,2,3,4,...)Show SMILES Oc1ccc2c(CC[C@@H]3C[C@](O)(CC[C@@]23Cc2ccccc2)C#CCl)c1 Show InChI InChI=1S/C23H23ClO2/c24-13-12-22(26)10-11-23(15-17-4-2-1-3-5-17)19(16-22)7-6-18-14-20(25)8-9-21(18)23/h1-5,8-9,14,19,25-26H,6-7,10-11,15-16H2/t19-,22+,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Displacement of [3H]DEX from human glucocorticoid receptor |
J Med Chem 53: 3065-74 (2010)
Article DOI: 10.1021/jm901452y
BindingDB Entry DOI: 10.7270/Q2VQ33NH |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50118528

(CHEMBL135514 | Ethyl-carbamic acid 5-phenyl-6-oxa-...)Show SMILES CCNC(=O)OC1=C(Oc2ccccc2-n2cccc12)c1ccccc1 |t:6| Show InChI InChI=1S/C21H18N2O3/c1-2-22-21(24)26-20-17-12-8-14-23(17)16-11-6-7-13-18(16)25-19(20)15-9-4-3-5-10-15/h3-14H,2H2,1H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PK11195 binding to Peripheral type benzodiazepine receptor (PBR) in rat cortex homogenate by 50% |
J Med Chem 45: 4276-81 (2002)
BindingDB Entry DOI: 10.7270/Q20Z72MT |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(CALF) | BDBM50010483
(CHEMBL2181202)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C32H49N9O5/c1-19-15-22(42)16-20(2)23(19)18-24(34)29(44)40-26(12-8-14-38-32(36)37)30(45)41-27(17-21-9-4-3-5-10-21)31(46)39-25(28(35)43)11-6-7-13-33/h3-5,9-10,15-16,24-27,42H,6-8,11-14,17-18,33-34H2,1-2H3,(H2,35,43)(H,39,46)(H,40,44)(H,41,45)(H4,36,37,38)/t24-,25-,26+,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50016867
((S)-6-Amino-2-((S)-2-{(R)-2-[(S)-2-amino-3-(4-hydr...)Show SMILES NCCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C30H45N9O5/c31-15-5-4-9-23(26(33)41)37-29(44)25(18-19-7-2-1-3-8-19)39-28(43)24(10-6-16-36-30(34)35)38-27(42)22(32)17-20-11-13-21(40)14-12-20/h1-3,7-8,11-14,22-25,40H,4-6,9-10,15-18,31-32H2,(H2,33,41)(H,37,44)(H,38,42)(H,39,43)(H4,34,35,36)/t22-,23-,24+,25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071735
((S)-3-(1H-Indol-3-yl)-2-methyl-2-[3-(4-nitro-pheny...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)Nc1ccc(cc1)[N+]([O-])=O)C(=O)NCC1(CCCCC1)c1ccccn1 Show InChI InChI=1S/C31H34N6O4/c1-30(19-22-20-33-26-10-4-3-9-25(22)26,36-29(39)35-23-12-14-24(15-13-23)37(40)41)28(38)34-21-31(16-6-2-7-17-31)27-11-5-8-18-32-27/h3-5,8-15,18,20,33H,2,6-7,16-17,19,21H2,1H3,(H,34,38)(H2,35,36,39)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(Homo sapiens (Human)) | BDBM85484
(Bombesin)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCC(=O)N1)C(C)C)C(N)=O |wU:82.92,74.81,8.12,4.4,30.31,53.61,16.23,wD:93.101,39.52,62.69,102.104,34.35,(24.84,-22.59,;25.32,-21.13,;24.29,-19.98,;24.77,-18.52,;23.74,-17.37,;24.22,-15.91,;25.72,-15.59,;26.75,-16.74,;26.2,-14.13,;27.71,-13.81,;28.74,-14.96,;30.24,-14.64,;28.26,-16.42,;25.17,-12.98,;25.65,-11.52,;27.16,-11.2,;24.62,-10.37,;25.1,-8.91,;26.61,-8.59,;27.75,-9.62,;29.09,-8.86,;28.77,-7.35,;27.24,-7.19,;23.12,-10.69,;22.09,-9.54,;22.57,-8.08,;20.58,-9.86,;19.55,-8.72,;18.04,-9.03,;17.57,-10.5,;17.02,-7.89,;15.51,-8.2,;14.48,-7.06,;14.96,-5.59,;12.97,-7.38,;12.5,-8.84,;11.95,-6.23,;10.44,-6.55,;9.96,-8.01,;9.41,-5.4,;7.9,-5.72,;7.42,-7.18,;8.32,-8.42,;7.42,-9.66,;5.97,-9.19,;4.63,-9.96,;3.3,-9.19,;3.3,-7.65,;4.63,-6.88,;5.97,-7.65,;9.89,-3.94,;8.86,-2.79,;7.35,-3.11,;9.34,-1.33,;10.85,-1.01,;11.32,.45,;12.83,.77,;13.31,2.24,;13.86,-.37,;8.31,-.18,;8.79,1.28,;10.3,1.6,;7.76,2.43,;6.25,2.11,;5.22,3.26,;3.72,2.94,;5.7,4.72,;8.24,3.89,;9.75,4.21,;10.77,3.06,;10.22,5.67,;11.73,5.99,;12.21,7.46,;11.18,8.6,;13.72,7.77,;14.74,6.63,;16.25,6.94,;17.28,5.8,;16.73,8.41,;14.19,9.24,;13.17,10.38,;11.66,10.07,;13.64,11.85,;15.15,12.17,;15.63,13.63,;17.14,13.95,;17.61,15.41,;19.12,15.73,;19.6,17.19,;20.15,14.58,;12.62,12.99,;13.09,14.46,;14.6,14.78,;12.07,15.6,;10.56,15.29,;9.53,16.43,;8.02,16.11,;7,17.26,;7.55,14.65,;12.54,17.07,;11.52,18.21,;10.01,17.9,;11.99,19.68,;11.09,20.93,;12,22.17,;13.46,21.69,;14.71,22.59,;13.46,20.15,;17.49,-6.42,;19,-6.11,;16.47,-5.28,;22.23,-17.69,;21.75,-19.16,;21.2,-16.55,)| Show InChI InChI=1S/C71H110N24O18S/c1-34(2)24-47(92-62(105)43(14-11-22-79-71(76)77)89-64(107)45(15-18-52(72)96)90-63(106)44-17-20-55(99)85-44)61(104)81-31-56(100)87-51(28-54(74)98)69(112)91-46(16-19-53(73)97)65(108)94-49(26-38-29-80-41-13-10-9-12-40(38)41)66(109)84-37(7)60(103)95-58(36(5)6)70(113)82-32-57(101)86-50(27-39-30-78-33-83-39)68(111)93-48(25-35(3)4)67(110)88-42(59(75)102)21-23-114-8/h9-10,12-13,29-30,33-37,42-51,58,80H,11,14-28,31-32H2,1-8H3,(H2,72,96)(H2,73,97)(H2,74,98)(H2,75,102)(H,78,83)(H,81,104)(H,82,113)(H,84,109)(H,85,99)(H,86,101)(H,87,100)(H,88,110)(H,89,107)(H,90,106)(H,91,112)(H,92,105)(H,93,111)(H,94,108)(H,95,103)(H4,76,77,79)/t37-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,58-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Bombesin BB2 receptor in the presence of [125I]-[Tyr] bombesin. |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50113780
((2R,4aS,10aR)-4a-benzyl-2-(prop-1-ynyl)-1,2,3,4,4a...)Show SMILES CC#C[C@@]1(O)CC[C@]2(Cc3ccccc3)[C@H](CCc3cc(O)ccc23)C1 Show InChI InChI=1S/C24H26O2/c1-2-12-23(26)13-14-24(16-18-6-4-3-5-7-18)20(17-23)9-8-19-15-21(25)10-11-22(19)24/h3-7,10-11,15,20,25-26H,8-9,13-14,16-17H2,1H3/t20-,23-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Displacement of [3H]DEX from human glucocorticoid receptor |
J Med Chem 53: 3065-74 (2010)
Article DOI: 10.1021/jm901452y
BindingDB Entry DOI: 10.7270/Q2VQ33NH |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071739
((S)-3-(1H-Indol-3-yl)-N-[1-(5-methoxy-pyridin-2-yl...)Show SMILES COc1ccc(nc1)C1(CNC(=O)[C@](C)(Cc2c[nH]c3ccccc23)NC(=O)Nc2ccc(cc2)[N+]([O-])=O)CCCCC1 Show InChI InChI=1S/C32H36N6O5/c1-31(18-22-19-33-27-9-5-4-8-26(22)27,37-30(40)36-23-10-12-24(13-11-23)38(41)42)29(39)35-21-32(16-6-3-7-17-32)28-15-14-25(43-2)20-34-28/h4-5,8-15,19-20,33H,3,6-7,16-18,21H2,1-2H3,(H,35,39)(H2,36,37,40)/t31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50118537

(CHEMBL135391 | Ethyl-carbamic acid 7-chloro-5-phen...)Show SMILES CCNC(=O)OC1=C(Oc2c(Cl)cccc2-n2cccc12)c1ccccc1 |t:6| Show InChI InChI=1S/C21H17ClN2O3/c1-2-23-21(25)27-20-17-12-7-13-24(17)16-11-6-10-15(22)19(16)26-18(20)14-8-4-3-5-9-14/h3-13H,2H2,1H3,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PK11195 binding to Peripheral type benzodiazepine receptor (PBR) in rat cortex homogenate by 50% |
J Med Chem 45: 4276-81 (2002)
BindingDB Entry DOI: 10.7270/Q20Z72MT |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50118528

(CHEMBL135514 | Ethyl-carbamic acid 5-phenyl-6-oxa-...)Show SMILES CCNC(=O)OC1=C(Oc2ccccc2-n2cccc12)c1ccccc1 |t:6| Show InChI InChI=1S/C21H18N2O3/c1-2-22-21(24)26-20-17-12-8-14-23(17)16-11-6-7-13-18(16)25-19(20)15-9-4-3-5-10-15/h3-14H,2H2,1H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PK11195 binding to mitochondrial rat testis Peripheral type benzodiazepine receptor (PBR) |
J Med Chem 45: 4276-81 (2002)
BindingDB Entry DOI: 10.7270/Q20Z72MT |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50118539

(CHEMBL136036 | Diethyl-carbamic acid 5-thiophen-2-...)Show SMILES CCN(CC)C(=O)OC1=C(Oc2cccnc2-n2cccc12)c1cccs1 |t:8| Show InChI InChI=1S/C20H19N3O3S/c1-3-22(4-2)20(24)26-17-14-8-6-12-23(14)19-15(9-5-11-21-19)25-18(17)16-10-7-13-27-16/h5-13H,3-4H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PK11195 binding to Peripheral type benzodiazepine receptor (PBR) in rat cortex homogenate by 50% |
J Med Chem 45: 4276-81 (2002)
BindingDB Entry DOI: 10.7270/Q20Z72MT |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Displacement of [3H]DEX from human glucocorticoid receptor |
J Med Chem 53: 3065-74 (2010)
Article DOI: 10.1021/jm901452y
BindingDB Entry DOI: 10.7270/Q2VQ33NH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594830
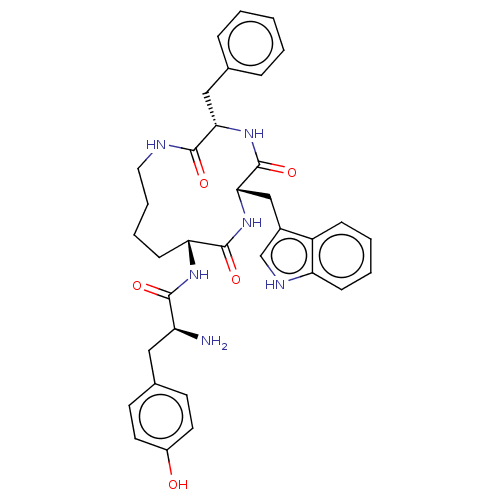
(CHEMBL5186493)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50143182
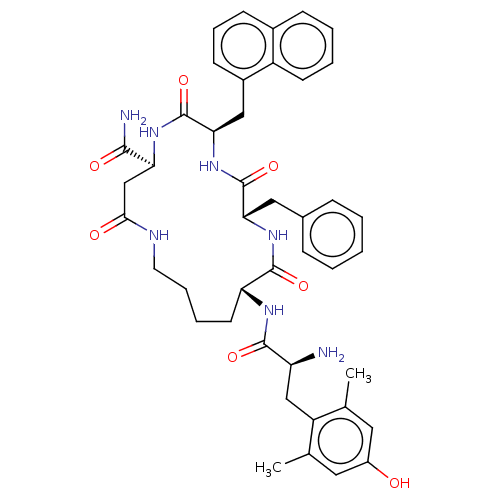
(CHEMBL3759167)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@@H](Cc2cccc3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C43H51N7O7/c1-25-19-30(51)20-26(2)32(25)23-33(44)40(54)47-34-17-8-9-18-46-38(52)24-35(39(45)53)48-43(57)37(22-29-15-10-14-28-13-6-7-16-31(28)29)50-42(56)36(49-41(34)55)21-27-11-4-3-5-12-27/h3-7,10-16,19-20,33-37,51H,8-9,17-18,21-24,44H2,1-2H3,(H2,45,53)(H,46,52)(H,47,54)(H,48,57)(H,49,55)(H,50,56)/t33-,34+,35-,36-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50052754
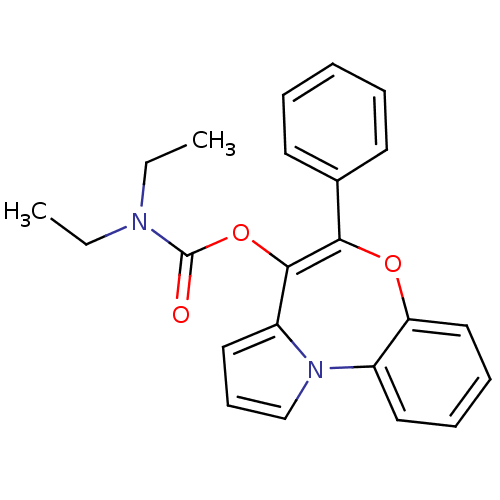
(CHEMBL115740 | Diethyl-carbamic acid 5-phenyl-6-ox...)Show SMILES CCN(CC)C(=O)OC1=C(Oc2ccccc2-n2cccc12)c1ccccc1 |t:8| Show InChI InChI=1S/C23H22N2O3/c1-3-24(4-2)23(26)28-22-19-14-10-16-25(19)18-13-8-9-15-20(18)27-21(22)17-11-6-5-7-12-17/h5-16H,3-4H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Binding affinity against peripheral-type benzodiazepine receptor (PBR) from rat cortex homogenate using [3H]-PK 11195 as radioligand |
J Med Chem 39: 3435-50 (1996)
Article DOI: 10.1021/jm960251b
BindingDB Entry DOI: 10.7270/Q23B60SK |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071750
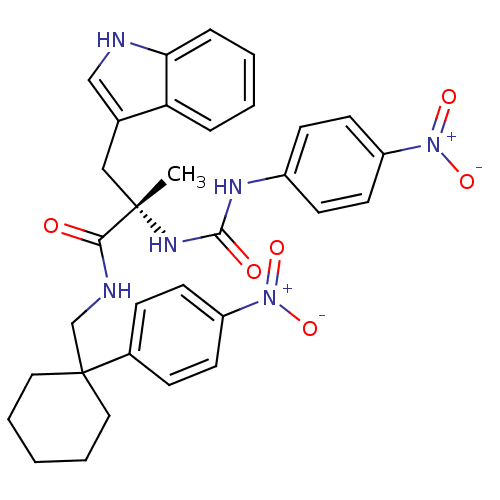
((S)-3-(1H-Indol-3-yl)-2-methyl-N-[1-(4-nitro-pheny...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)Nc1ccc(cc1)[N+]([O-])=O)C(=O)NCC1(CCCCC1)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C32H34N6O6/c1-31(19-22-20-33-28-8-4-3-7-27(22)28,36-30(40)35-24-11-15-26(16-12-24)38(43)44)29(39)34-21-32(17-5-2-6-18-32)23-9-13-25(14-10-23)37(41)42/h3-4,7-16,20,33H,2,5-6,17-19,21H2,1H3,(H,34,39)(H2,35,36,40)/t31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594819
(CHEMBL5208747)Show SMILES C[C@@H](N(C)C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccco1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50118541

(CHEMBL445583 | Cyclopropyl-carbamic acid 5-phenyl-...)Show SMILES O=C(NC1CC1)OC1=C(Oc2ccccc2-n2cccc12)c1ccccc1 |t:8| Show InChI InChI=1S/C22H18N2O3/c25-22(23-16-12-13-16)27-21-18-10-6-14-24(18)17-9-4-5-11-19(17)26-20(21)15-7-2-1-3-8-15/h1-11,14,16H,12-13H2,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PK11195 binding to mitochondrial rat testis Peripheral type benzodiazepine receptor (PBR) |
J Med Chem 45: 4276-81 (2002)
BindingDB Entry DOI: 10.7270/Q20Z72MT |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071746
((S)-2-[3-(4-Cyano-phenyl)-ureido]-3-(1H-indol-3-yl...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)Nc1ccc(cc1)C#N)C(=O)NCC1(CCCCC1)c1ccccn1 Show InChI InChI=1S/C32H34N6O2/c1-31(19-24-21-35-27-10-4-3-9-26(24)27,38-30(40)37-25-14-12-23(20-33)13-15-25)29(39)36-22-32(16-6-2-7-17-32)28-11-5-8-18-34-28/h3-5,8-15,18,21,35H,2,6-7,16-17,19,22H2,1H3,(H,36,39)(H2,37,38,40)/t31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061028
((S)-1-[(S)-2-(4-Methoxy-benzoylamino)-3-methyl-but...)Show SMILES COc1ccc(cc1)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)CNCc1ccccc1 Show InChI InChI=1S/C32H42F2N4O5/c1-20(2)26(28(39)32(33,34)19-35-18-22-10-7-6-8-11-22)36-30(41)25-12-9-17-38(25)31(42)27(21(3)4)37-29(40)23-13-15-24(43-5)16-14-23/h6-8,10-11,13-16,20-21,25-27,35H,9,12,17-19H2,1-5H3,(H,36,41)(H,37,40)/t25-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061047
(((S)-2,2-Difluoro-5-methyl-4-{[(S)-1-((S)-3-methyl...)Show SMILES CCCCOC(=O)NCC(F)(F)C(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)Oc1ccccc1)C(C)C)C(C)C Show InChI InChI=1S/C29H42F2N4O7/c1-6-7-16-41-27(39)32-17-29(30,31)24(36)22(18(2)3)33-25(37)21-14-11-15-35(21)26(38)23(19(4)5)34-28(40)42-20-12-9-8-10-13-20/h8-10,12-13,18-19,21-23H,6-7,11,14-17H2,1-5H3,(H,32,39)(H,33,37)(H,34,40)/t21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061043
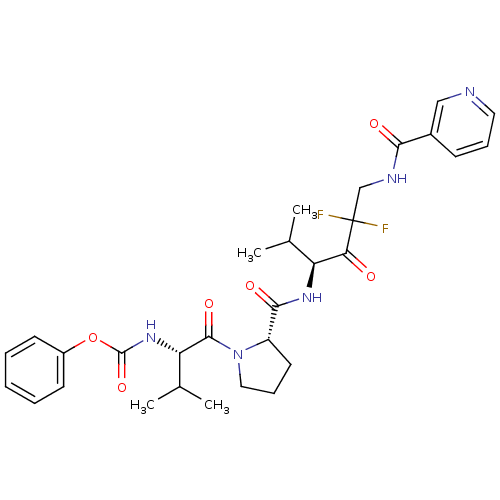
(CHEMBL106592 | [(S)-1-((S)-2-{(S)-3,3-Difluoro-1-i...)Show SMILES CC(C)[C@H](NC(=O)Oc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)CNC(=O)c1cccnc1 Show InChI InChI=1S/C30H37F2N5O6/c1-18(2)23(25(38)30(31,32)17-34-26(39)20-10-8-14-33-16-20)35-27(40)22-13-9-15-37(22)28(41)24(19(3)4)36-29(42)43-21-11-6-5-7-12-21/h5-8,10-12,14,16,18-19,22-24H,9,13,15,17H2,1-4H3,(H,34,39)(H,35,40)(H,36,42)/t22-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50052749
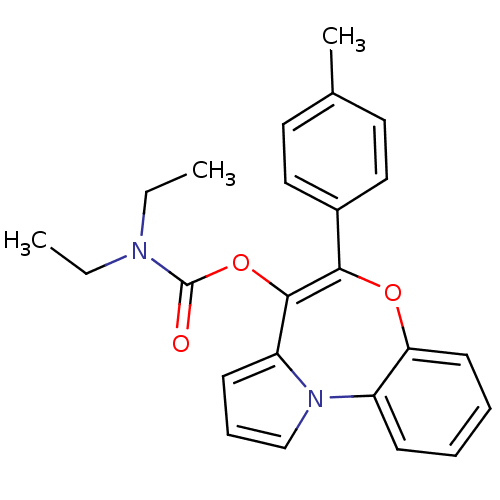
(CHEMBL325025 | Diethyl-carbamic acid 5-p-tolyl-6-o...)Show SMILES CCN(CC)C(=O)OC1=C(Oc2ccccc2-n2cccc12)c1ccc(C)cc1 |t:8| Show InChI InChI=1S/C24H24N2O3/c1-4-25(5-2)24(27)29-23-20-10-8-16-26(20)19-9-6-7-11-21(19)28-22(23)18-14-12-17(3)13-15-18/h6-16H,4-5H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Binding affinity against peripheral-type benzodiazepine receptor (PBR) from rat cortex homogenate using [3H]-PK 11195 as radioligand |
J Med Chem 39: 3435-50 (1996)
Article DOI: 10.1021/jm960251b
BindingDB Entry DOI: 10.7270/Q23B60SK |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061037
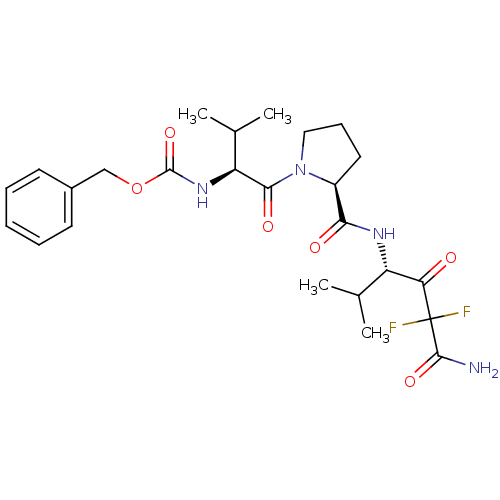
(CHEMBL302961 | {(S)-1-[(S)-2-((S)-3-Carbamoyl-3,3-...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)C(N)=O Show InChI InChI=1S/C25H34F2N4O6/c1-14(2)18(20(32)25(26,27)23(28)35)29-21(33)17-11-8-12-31(17)22(34)19(15(3)4)30-24(36)37-13-16-9-6-5-7-10-16/h5-7,9-10,14-15,17-19H,8,11-13H2,1-4H3,(H2,28,35)(H,29,33)(H,30,36)/t17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214370
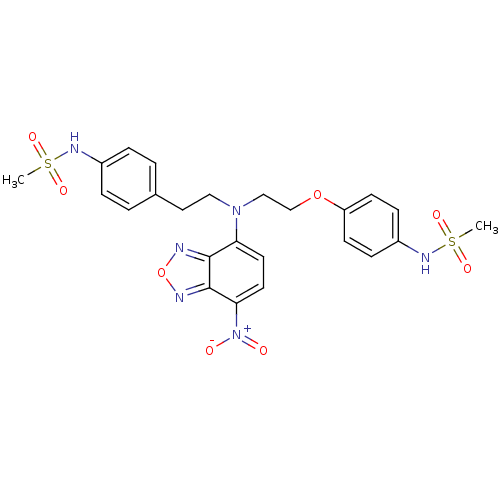
(CHEMBL227134 | N-(4-{2-[[2-(4-methanesulfonylamino...)Show SMILES CS(=O)(=O)Nc1ccc(CCN(CCOc2ccc(NS(C)(=O)=O)cc2)c2ccc([N+]([O-])=O)c3nonc23)cc1 Show InChI InChI=1S/C24H26N6O8S2/c1-39(33,34)27-18-5-3-17(4-6-18)13-14-29(21-11-12-22(30(31)32)24-23(21)25-38-26-24)15-16-37-20-9-7-19(8-10-20)28-40(2,35)36/h3-12,27-28H,13-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50121130
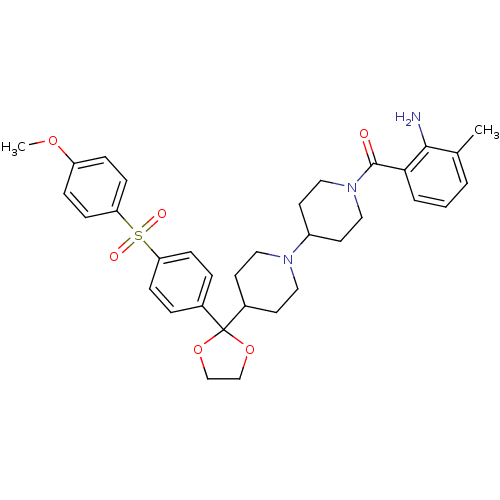
((2-Amino-3-methyl-phenyl)-(4-{2-[4-(4-methoxy-benz...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(cc1)C1(OCCO1)C1CCN(CC1)C1CCN(CC1)C(=O)c1cccc(C)c1N Show InChI InChI=1S/C34H41N3O6S/c1-24-4-3-5-31(32(24)35)33(38)37-20-16-27(17-21-37)36-18-14-26(15-19-36)34(42-22-23-43-34)25-6-10-29(11-7-25)44(39,40)30-12-8-28(41-2)9-13-30/h3-13,26-27H,14-23,35H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 12: 3479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2VT1RGD |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071748
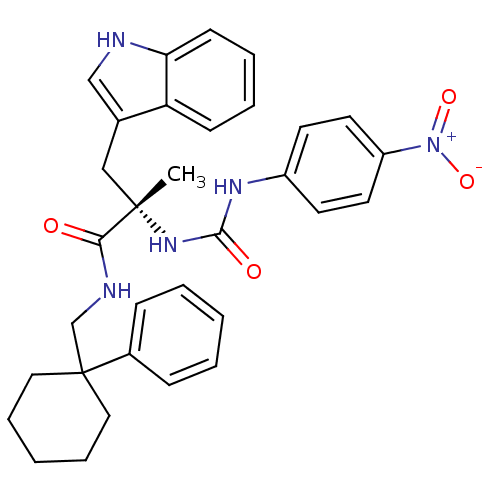
((S)-3-(1H-Indol-3-yl)-2-methyl-2-[3-(4-nitro-pheny...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)Nc1ccc(cc1)[N+]([O-])=O)C(=O)NCC1(CCCCC1)c1ccccc1 Show InChI InChI=1S/C32H35N5O4/c1-31(20-23-21-33-28-13-7-6-12-27(23)28,36-30(39)35-25-14-16-26(17-15-25)37(40)41)29(38)34-22-32(18-8-3-9-19-32)24-10-4-2-5-11-24/h2,4-7,10-17,21,33H,3,8-9,18-20,22H2,1H3,(H,34,38)(H2,35,36,39)/t31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50121134
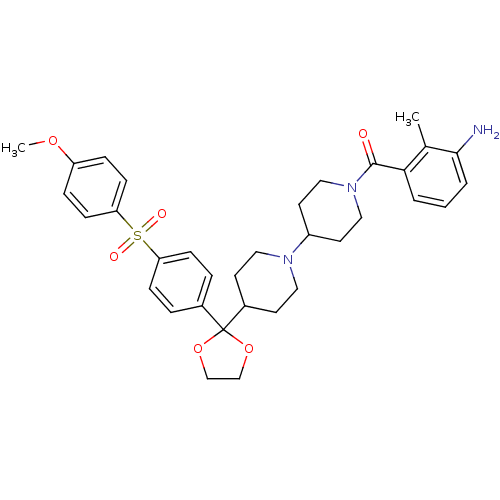
((3-Amino-2-methyl-phenyl)-(4-{2-[4-(4-methoxy-benz...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(cc1)C1(OCCO1)C1CCN(CC1)C1CCN(CC1)C(=O)c1cccc(N)c1C Show InChI InChI=1S/C34H41N3O6S/c1-24-31(4-3-5-32(24)35)33(38)37-20-16-27(17-21-37)36-18-14-26(15-19-36)34(42-22-23-43-34)25-6-10-29(11-7-25)44(39,40)30-12-8-28(41-2)9-13-30/h3-13,26-27H,14-23,35H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 12: 3479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2VT1RGD |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058357
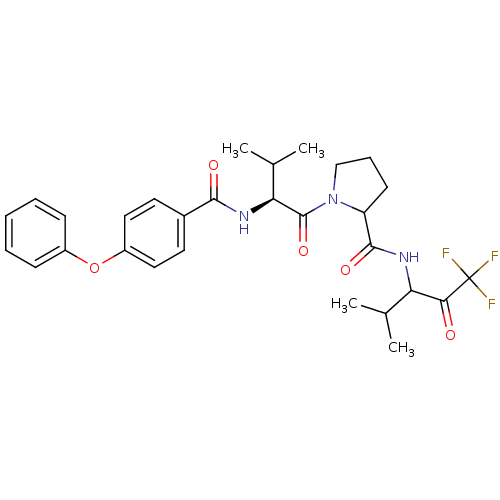
(1-[(S)-3-Methyl-2-(4-phenoxy-benzoylamino)-butyryl...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCCC1C(=O)NC(C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C29H34F3N3O5/c1-17(2)23(25(36)29(30,31)32)33-27(38)22-11-8-16-35(22)28(39)24(18(3)4)34-26(37)19-12-14-21(15-13-19)40-20-9-6-5-7-10-20/h5-7,9-10,12-15,17-18,22-24H,8,11,16H2,1-4H3,(H,33,38)(H,34,37)/t22?,23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human Neutrophil Elastase using acute lung injury model (ALIM) assay |
J Med Chem 40: 1876-85 (1997)
Article DOI: 10.1021/jm960819g
BindingDB Entry DOI: 10.7270/Q2X92BZJ |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058376
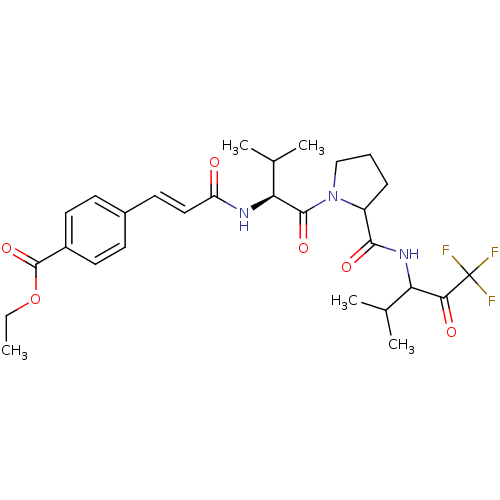
(4-((E)-2-{(S)-2-Methyl-1-[2-(3,3,3-trifluoro-1-iso...)Show SMILES CCOC(=O)c1ccc(\C=C\C(=O)N[C@@H](C(C)C)C(=O)N2CCCC2C(=O)NC(C(C)C)C(=O)C(F)(F)F)cc1 Show InChI InChI=1S/C28H36F3N3O6/c1-6-40-27(39)19-12-9-18(10-13-19)11-14-21(35)32-23(17(4)5)26(38)34-15-7-8-20(34)25(37)33-22(16(2)3)24(36)28(29,30)31/h9-14,16-17,20,22-23H,6-8,15H2,1-5H3,(H,32,35)(H,33,37)/b14-11+/t20?,22?,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human Neutrophil Elastase using acute lung injury model (ALIM) assay |
J Med Chem 40: 1876-85 (1997)
Article DOI: 10.1021/jm960819g
BindingDB Entry DOI: 10.7270/Q2X92BZJ |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50118534

(CHEMBL136926 | Dimethyl-carbamic acid 5-p-tolyl-6-...)Show SMILES CN(C)C(=O)OC1=C(Oc2cccnc2-n2cccc12)c1ccc(C)cc1 |t:6| Show InChI InChI=1S/C21H19N3O3/c1-14-8-10-15(11-9-14)18-19(27-21(25)23(2)3)16-6-5-13-24(16)20-17(26-18)7-4-12-22-20/h4-13H,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PK11195 binding to Peripheral type benzodiazepine receptor (PBR) in rat cortex homogenate by 50% |
J Med Chem 45: 4276-81 (2002)
BindingDB Entry DOI: 10.7270/Q20Z72MT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50121126

((2-Amino-4-fluoro-phenyl)-(4-{2-[4-(4-methoxy-benz...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(cc1)C1(OCCO1)C1CCN(CC1)C1CCN(CC1)C(=O)c1ccc(F)cc1N Show InChI InChI=1S/C33H38FN3O6S/c1-41-27-5-9-29(10-6-27)44(39,40)28-7-2-23(3-8-28)33(42-20-21-43-33)24-12-16-36(17-13-24)26-14-18-37(19-15-26)32(38)30-11-4-25(34)22-31(30)35/h2-11,22,24,26H,12-21,35H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 12: 3479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2VT1RGD |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071736
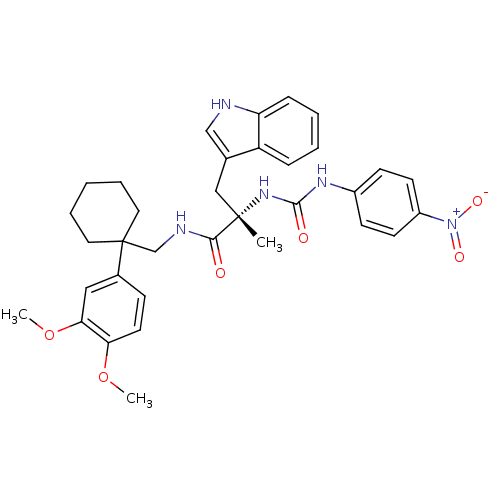
((S)-N-[1-(3,4-Dimethoxy-phenyl)-cyclohexylmethyl]-...)Show SMILES COc1ccc(cc1OC)C1(CNC(=O)[C@](C)(Cc2c[nH]c3ccccc23)NC(=O)Nc2ccc(cc2)[N+]([O-])=O)CCCCC1 Show InChI InChI=1S/C34H39N5O6/c1-33(20-23-21-35-28-10-6-5-9-27(23)28,38-32(41)37-25-12-14-26(15-13-25)39(42)43)31(40)36-22-34(17-7-4-8-18-34)24-11-16-29(44-2)30(19-24)45-3/h5-6,9-16,19,21,35H,4,7-8,17-18,20,22H2,1-3H3,(H,36,40)(H2,37,38,41)/t33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50143183

(CHEMBL3759179)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C43H51N7O7/c1-25-18-31(51)19-26(2)32(25)23-33(44)40(54)47-34-14-8-9-17-46-38(52)24-35(39(45)53)48-42(56)37(22-28-15-16-29-12-6-7-13-30(29)20-28)50-43(57)36(49-41(34)55)21-27-10-4-3-5-11-27/h3-7,10-13,15-16,18-20,33-37,51H,8-9,14,17,21-24,44H2,1-2H3,(H2,45,53)(H,46,52)(H,47,54)(H,48,56)(H,49,55)(H,50,57)/t33-,34+,35-,36-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594824

(CHEMBL5190232)Show SMILES C[C@@H](N(C)C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](C(=C)C(N)=O)c1ccco1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071743
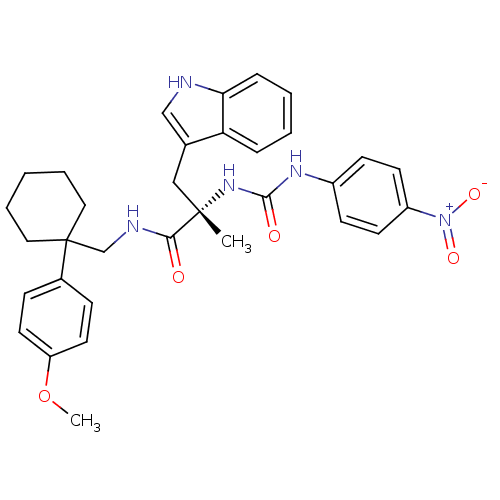
((S)-3-(1H-Indol-3-yl)-N-[1-(4-methoxy-phenyl)-cycl...)Show SMILES COc1ccc(cc1)C1(CNC(=O)[C@](C)(Cc2c[nH]c3ccccc23)NC(=O)Nc2ccc(cc2)[N+]([O-])=O)CCCCC1 Show InChI InChI=1S/C33H37N5O5/c1-32(20-23-21-34-29-9-5-4-8-28(23)29,37-31(40)36-25-12-14-26(15-13-25)38(41)42)30(39)35-22-33(18-6-3-7-19-33)24-10-16-27(43-2)17-11-24/h4-5,8-17,21,34H,3,6-7,18-20,22H2,1-2H3,(H,35,39)(H2,36,37,40)/t32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594836

(CHEMBL5199625)Show SMILES CCCCCCCCC(N)C(=O)N[C@@H](Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50118541

(CHEMBL445583 | Cyclopropyl-carbamic acid 5-phenyl-...)Show SMILES O=C(NC1CC1)OC1=C(Oc2ccccc2-n2cccc12)c1ccccc1 |t:8| Show InChI InChI=1S/C22H18N2O3/c25-22(23-16-12-13-16)27-21-18-10-6-14-24(18)17-9-4-5-11-19(17)26-20(21)15-7-2-1-3-8-15/h1-11,14,16H,12-13H2,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro-5-4864 binding to mitochondrial rat testis Peripheral type benzodiazepine receptor (PBR) |
J Med Chem 45: 4276-81 (2002)
BindingDB Entry DOI: 10.7270/Q20Z72MT |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594827

(CHEMBL5171745)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data