 Found 100 hits with Last Name = 'williams' and Initial = 'mj'
Found 100 hits with Last Name = 'williams' and Initial = 'mj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309834

((S)-1,3'-bipyrrolidin-1'-yl(4-((7-chloro-1H-indol-...)Show SMILES Clc1cccc2ccn(Cc3ccc(cc3)C(=O)N3CC[C@@H](C3)N3CCCC3)c12 |r| Show InChI InChI=1S/C24H26ClN3O/c25-22-5-3-4-19-10-14-27(23(19)22)16-18-6-8-20(9-7-18)24(29)28-15-11-21(17-28)26-12-1-2-13-26/h3-10,14,21H,1-2,11-13,15-17H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309848

((R)-1,3'-bipyrrolidin-1'-yl(4-((2-methyl-1H-benzo[...)Show SMILES Cc1nc2ccccc2n1Cc1ccc(cc1)C(=O)N1CC[C@H](C1)N1CCCC1 |r| Show InChI InChI=1S/C24H28N4O/c1-18-25-22-6-2-3-7-23(22)28(18)16-19-8-10-20(11-9-19)24(29)27-15-12-21(17-27)26-13-4-5-14-26/h2-3,6-11,21H,4-5,12-17H2,1H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309847
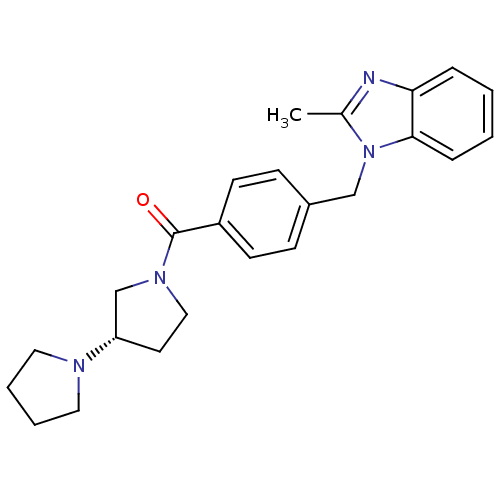
((S)-1,3'-bipyrrolidin-1'-yl(4-((2-methyl-1H-benzo[...)Show SMILES Cc1nc2ccccc2n1Cc1ccc(cc1)C(=O)N1CC[C@@H](C1)N1CCCC1 |r| Show InChI InChI=1S/C24H28N4O/c1-18-25-22-6-2-3-7-23(22)28(18)16-19-8-10-20(11-9-19)24(29)27-15-12-21(17-27)26-13-4-5-14-26/h2-3,6-11,21H,4-5,12-17H2,1H3/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309837
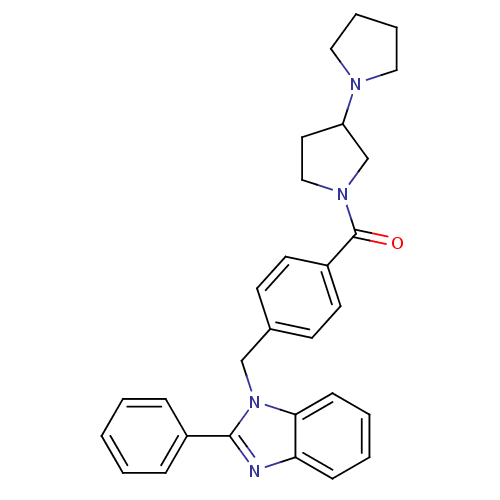
(1,3'-bipyrrolidin-1'-yl(4-((2-phenyl-1H-benzo[d]im...)Show SMILES O=C(N1CCC(C1)N1CCCC1)c1ccc(Cn2c(nc3ccccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C29H30N4O/c34-29(32-19-16-25(21-32)31-17-6-7-18-31)24-14-12-22(13-15-24)20-33-27-11-5-4-10-26(27)30-28(33)23-8-2-1-3-9-23/h1-5,8-15,25H,6-7,16-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309845
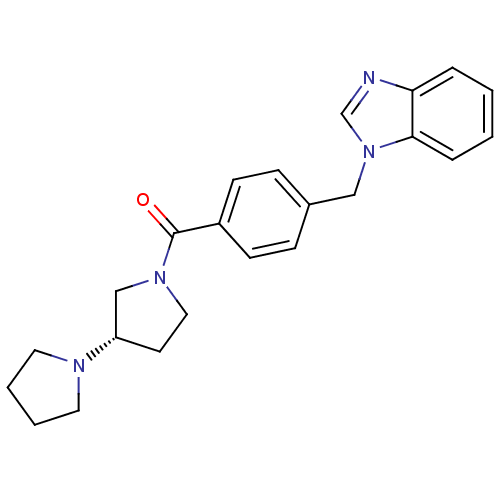
((S)-1,3'-bipyrrolidin-1'-yl(4-((1H-benzo[d]imidazo...)Show SMILES O=C(N1CC[C@@H](C1)N1CCCC1)c1ccc(Cn2cnc3ccccc23)cc1 |r| Show InChI InChI=1S/C23H26N4O/c28-23(26-14-11-20(16-26)25-12-3-4-13-25)19-9-7-18(8-10-19)15-27-17-24-21-5-1-2-6-22(21)27/h1-2,5-10,17,20H,3-4,11-16H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309851
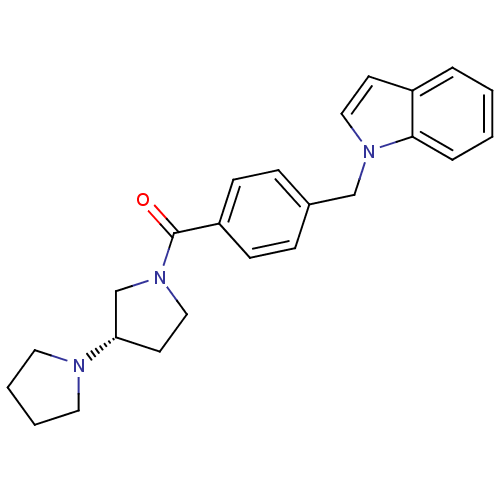
((S)-1,3'-bipyrrolidin-1'-yl(4-((1H-indol-1-yl)meth...)Show SMILES O=C(N1CC[C@@H](C1)N1CCCC1)c1ccc(Cn2ccc3ccccc23)cc1 |r| Show InChI InChI=1S/C24H27N3O/c28-24(27-16-12-22(18-27)25-13-3-4-14-25)21-9-7-19(8-10-21)17-26-15-11-20-5-1-2-6-23(20)26/h1-2,5-11,15,22H,3-4,12-14,16-18H2/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309843
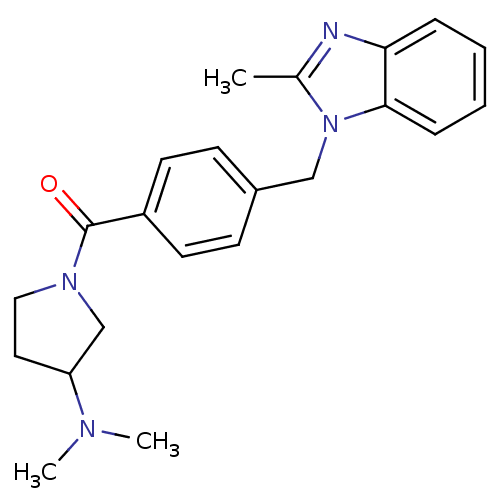
((3-(dimethylamino)pyrrolidin-1-yl)(4-((2-methyl-1H...)Show SMILES CN(C)C1CCN(C1)C(=O)c1ccc(Cn2c(C)nc3ccccc23)cc1 Show InChI InChI=1S/C22H26N4O/c1-16-23-20-6-4-5-7-21(20)26(16)14-17-8-10-18(11-9-17)22(27)25-13-12-19(15-25)24(2)3/h4-11,19H,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50309834

((S)-1,3'-bipyrrolidin-1'-yl(4-((7-chloro-1H-indol-...)Show SMILES Clc1cccc2ccn(Cc3ccc(cc3)C(=O)N3CC[C@@H](C3)N3CCCC3)c12 |r| Show InChI InChI=1S/C24H26ClN3O/c25-22-5-3-4-19-10-14-27(23(19)22)16-18-6-8-20(9-7-18)24(29)28-15-11-21(17-28)26-12-1-2-13-26/h3-10,14,21H,1-2,11-13,15-17H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to rat histamine H3 receptor |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309844
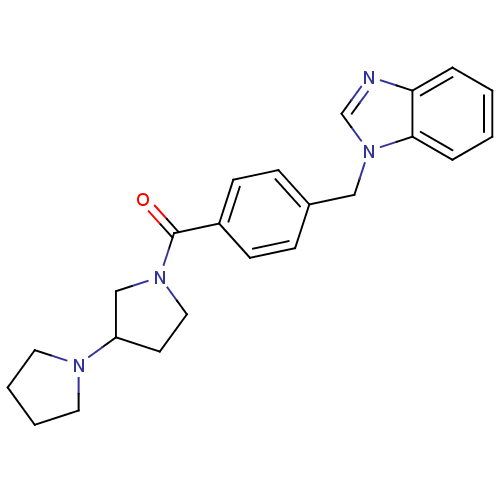
(1,3'-bipyrrolidin-1'-yl(4-((1H-benzo[d]imidazol-1-...)Show SMILES O=C(N1CCC(C1)N1CCCC1)c1ccc(Cn2cnc3ccccc23)cc1 Show InChI InChI=1S/C23H26N4O/c28-23(26-14-11-20(16-26)25-12-3-4-13-25)19-9-7-18(8-10-19)15-27-17-24-21-5-1-2-6-22(21)27/h1-2,5-10,17,20H,3-4,11-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309846
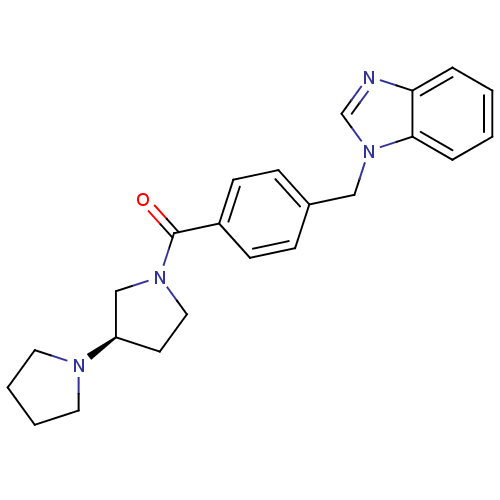
((R)-1,3'-bipyrrolidin-1'-yl(4-((1H-benzo[d]imidazo...)Show SMILES O=C(N1CC[C@H](C1)N1CCCC1)c1ccc(Cn2cnc3ccccc23)cc1 |r| Show InChI InChI=1S/C23H26N4O/c28-23(26-14-11-20(16-26)25-12-3-4-13-25)19-9-7-18(8-10-19)15-27-17-24-21-5-1-2-6-22(21)27/h1-2,5-10,17,20H,3-4,11-16H2/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309839

((S)-(3-(dimethylamino)pyrrolidin-1-yl)(4-((2-pheny...)Show SMILES CN(C)[C@H]1CCN(C1)C(=O)c1ccc(Cn2c(nc3ccccc23)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C27H28N4O/c1-29(2)23-16-17-30(19-23)27(32)22-14-12-20(13-15-22)18-31-25-11-7-6-10-24(25)28-26(31)21-8-4-3-5-9-21/h3-15,23H,16-19H2,1-2H3/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309836
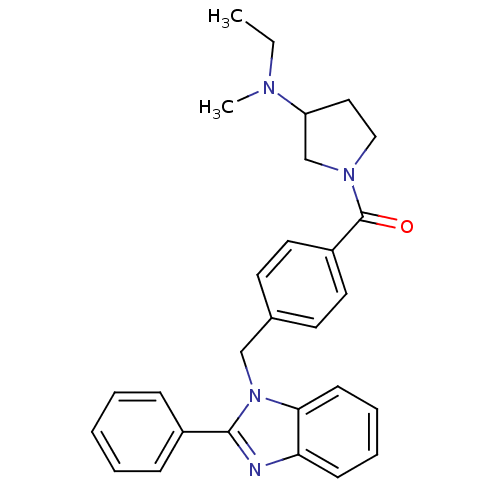
((3-(ethyl(methyl)amino)pyrrolidin-1-yl)(4-((2-phen...)Show SMILES CCN(C)C1CCN(C1)C(=O)c1ccc(Cn2c(nc3ccccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C28H30N4O/c1-3-30(2)24-17-18-31(20-24)28(33)23-15-13-21(14-16-23)19-32-26-12-8-7-11-25(26)29-27(32)22-9-5-4-6-10-22/h4-16,24H,3,17-20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309835
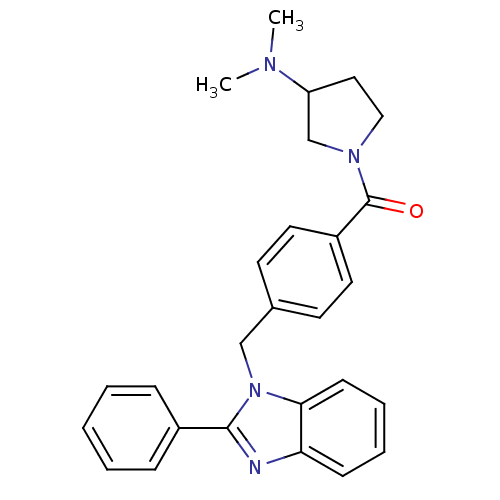
((3-(dimethylamino)pyrrolidin-1-yl)(4-((2-phenyl-1H...)Show SMILES CN(C)C1CCN(C1)C(=O)c1ccc(Cn2c(nc3ccccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C27H28N4O/c1-29(2)23-16-17-30(19-23)27(32)22-14-12-20(13-15-22)18-31-25-11-7-6-10-24(25)28-26(31)21-8-4-3-5-9-21/h3-15,23H,16-19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309853
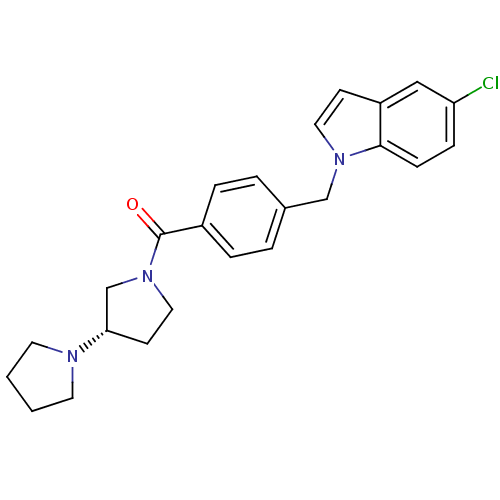
((S)-1,3'-bipyrrolidin-1'-yl(4-((5-chloro-1H-indol-...)Show SMILES Clc1ccc2n(Cc3ccc(cc3)C(=O)N3CC[C@@H](C3)N3CCCC3)ccc2c1 |r| Show InChI InChI=1S/C24H26ClN3O/c25-21-7-8-23-20(15-21)9-13-27(23)16-18-3-5-19(6-4-18)24(29)28-14-10-22(17-28)26-11-1-2-12-26/h3-9,13,15,22H,1-2,10-12,14,16-17H2/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309852
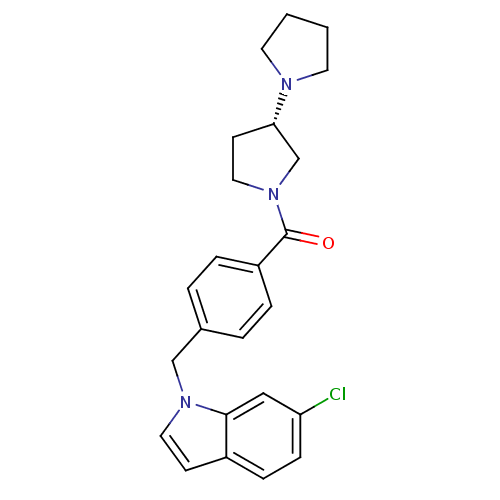
((S)-1,3'-bipyrrolidin-1'-yl(4-((6-chloro-1H-indol-...)Show SMILES Clc1ccc2ccn(Cc3ccc(cc3)C(=O)N3CC[C@@H](C3)N3CCCC3)c2c1 |r| Show InChI InChI=1S/C24H26ClN3O/c25-21-8-7-19-9-13-27(23(19)15-21)16-18-3-5-20(6-4-18)24(29)28-14-10-22(17-28)26-11-1-2-12-26/h3-9,13,15,22H,1-2,10-12,14,16-17H2/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309841
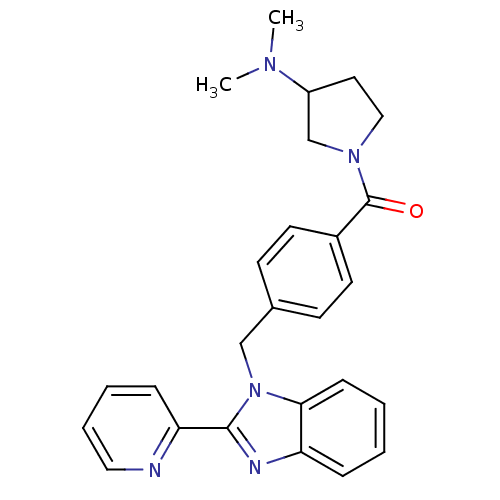
((3-(dimethylamino)pyrrolidin-1-yl)(4-((2-(pyridin-...)Show SMILES CN(C)C1CCN(C1)C(=O)c1ccc(Cn2c(nc3ccccc23)-c2ccccn2)cc1 Show InChI InChI=1S/C26H27N5O/c1-29(2)21-14-16-30(18-21)26(32)20-12-10-19(11-13-20)17-31-24-9-4-3-7-22(24)28-25(31)23-8-5-6-15-27-23/h3-13,15,21H,14,16-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 235 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309842
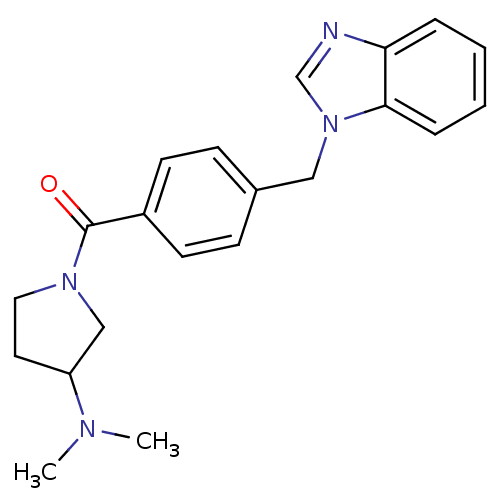
((4-((1H-benzo[d]imidazol-1-yl)methyl)phenyl)(3-(di...)Show SMILES CN(C)C1CCN(C1)C(=O)c1ccc(Cn2cnc3ccccc23)cc1 Show InChI InChI=1S/C21H24N4O/c1-23(2)18-11-12-24(14-18)21(26)17-9-7-16(8-10-17)13-25-15-22-19-5-3-4-6-20(19)25/h3-10,15,18H,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309849
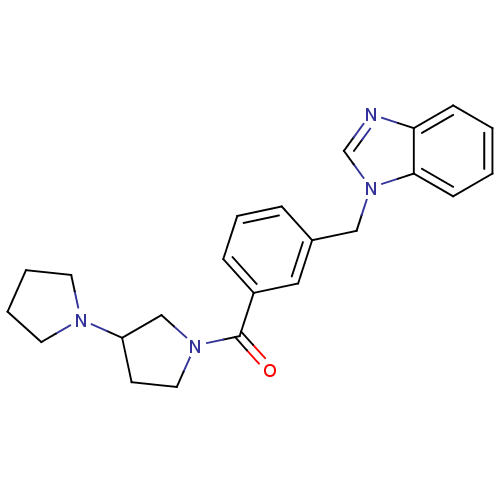
(1,3'-bipyrrolidin-1'-yl(3-((1H-benzo[d]imidazol-1-...)Show SMILES O=C(N1CCC(C1)N1CCCC1)c1cccc(Cn2cnc3ccccc23)c1 Show InChI InChI=1S/C23H26N4O/c28-23(26-13-10-20(16-26)25-11-3-4-12-25)19-7-5-6-18(14-19)15-27-17-24-21-8-1-2-9-22(21)27/h1-2,5-9,14,17,20H,3-4,10-13,15-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309840
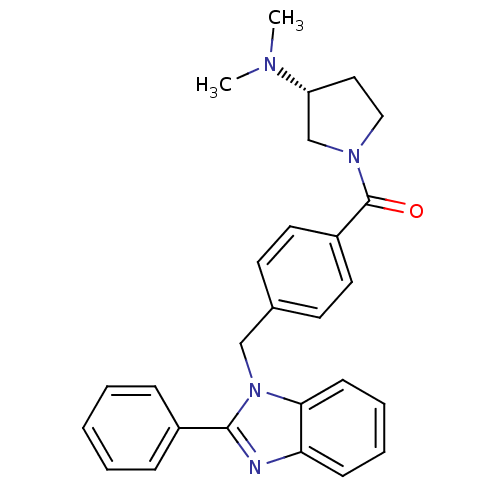
((R)-(3-(dimethylamino)pyrrolidin-1-yl)(4-((2-pheny...)Show SMILES CN(C)[C@@H]1CCN(C1)C(=O)c1ccc(Cn2c(nc3ccccc23)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C27H28N4O/c1-29(2)23-16-17-30(19-23)27(32)22-14-12-20(13-15-22)18-31-25-11-7-6-10-24(25)28-26(31)21-8-4-3-5-9-21/h3-15,23H,16-19H2,1-2H3/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309838
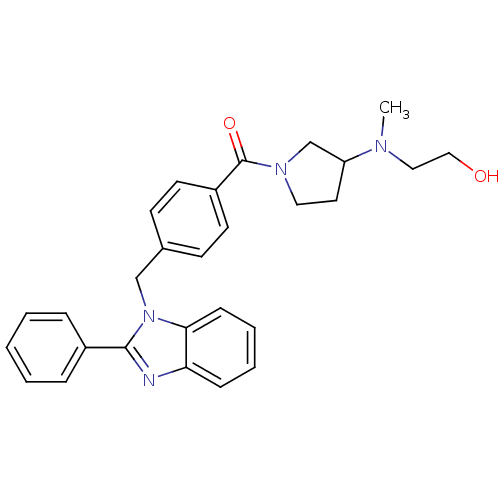
((3-((2-hydroxyethyl)(methyl)amino)pyrrolidin-1-yl)...)Show SMILES CN(CCO)C1CCN(C1)C(=O)c1ccc(Cn2c(nc3ccccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C28H30N4O2/c1-30(17-18-33)24-15-16-31(20-24)28(34)23-13-11-21(12-14-23)19-32-26-10-6-5-9-25(26)29-27(32)22-7-3-2-4-8-22/h2-14,24,33H,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50309850
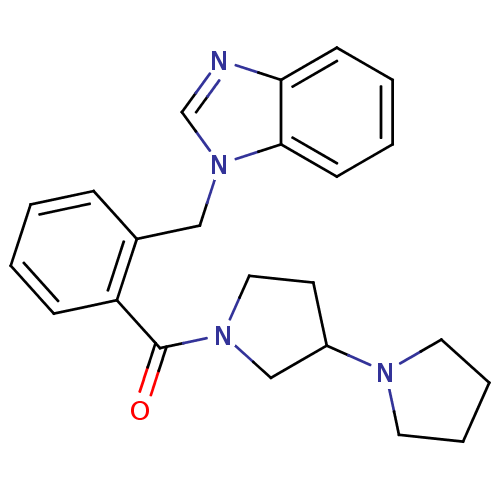
(1,3'-bipyrrolidin-1'-yl(2-((1H-benzo[d]imidazol-1-...)Show SMILES O=C(N1CCC(C1)N1CCCC1)c1ccccc1Cn1cnc2ccccc12 Show InChI InChI=1S/C23H26N4O/c28-23(26-14-11-19(16-26)25-12-5-6-13-25)20-8-2-1-7-18(20)15-27-17-24-21-9-3-4-10-22(21)27/h1-4,7-10,17,19H,5-6,11-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 1237-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.122
BindingDB Entry DOI: 10.7270/Q2028RNZ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50329198
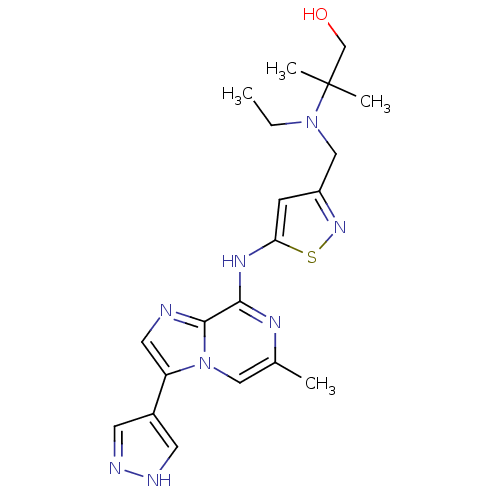
(2-(ethyl((5-(6-methyl-3-(1H-pyrazol-4-yl)imidazo[1...)Show SMILES CCN(Cc1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1)C(C)(C)CO Show InChI InChI=1S/C20H26N8OS/c1-5-27(20(3,4)12-29)11-15-6-17(30-26-15)25-18-19-21-9-16(14-7-22-23-8-14)28(19)10-13(2)24-18/h6-10,29H,5,11-12H2,1-4H3,(H,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IRK4 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM50329197
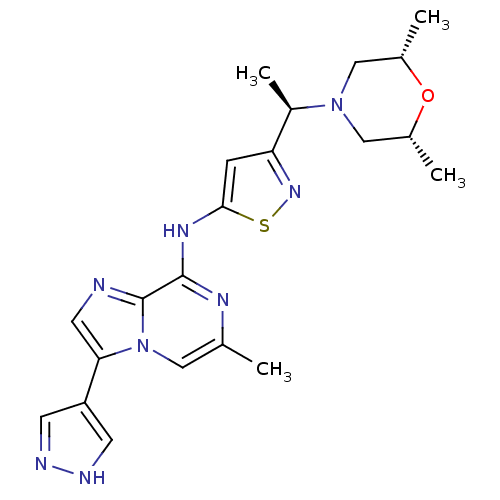
(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PLK3 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Testis-specific serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TSSK2 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IKK-beta |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform delta
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CSNK1d |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of cMET |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type IV
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Camk4 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50329198

(2-(ethyl((5-(6-methyl-3-(1H-pyrazol-4-yl)imidazo[1...)Show SMILES CCN(Cc1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1)C(C)(C)CO Show InChI InChI=1S/C20H26N8OS/c1-5-27(20(3,4)12-29)11-15-6-17(30-26-15)25-18-19-21-9-16(14-7-22-23-8-14)28(19)10-13(2)24-18/h6-10,29H,5,11-12H2,1-4H3,(H,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50306888

(4,6-dimethoxy-3-methyl-1H-pyrazolo[3,4-b]quinoline...)Show InChI InChI=1S/C13H13N3O2/c1-7-11-12(18-3)9-6-8(17-2)4-5-10(9)14-13(11)16-15-7/h4-6H,1-3H3,(H,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50306874
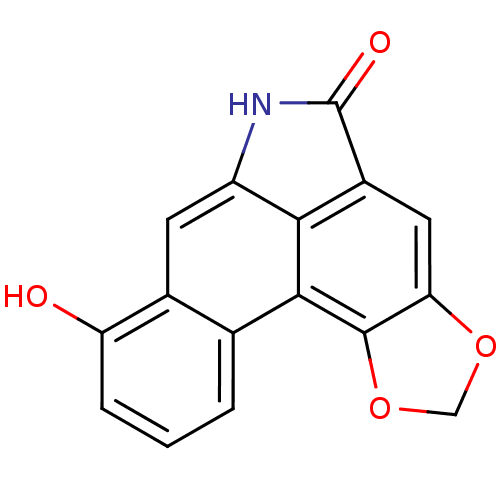
(8-Hydroxy-6H-benzo[f][1,3]dioxolo[4',5':4,5]benzo[...)Show InChI InChI=1S/C16H9NO4/c18-11-3-1-2-7-8(11)4-10-13-9(16(19)17-10)5-12-15(14(7)13)21-6-20-12/h1-5,18H,6H2,(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50329201
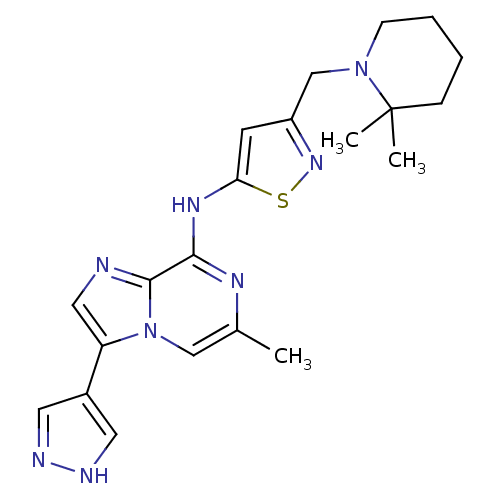
(3-((2,2-dimethylpiperidin-1-yl)methyl)-N-(6-methyl...)Show SMILES Cc1cn2c(cnc2c(Nc2cc(CN3CCCCC3(C)C)ns2)n1)-c1cn[nH]c1 Show InChI InChI=1S/C21H26N8S/c1-14-12-29-17(15-9-23-24-10-15)11-22-20(29)19(25-14)26-18-8-16(27-30-18)13-28-7-5-4-6-21(28,2)3/h8-12H,4-7,13H2,1-3H3,(H,23,24)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50329196
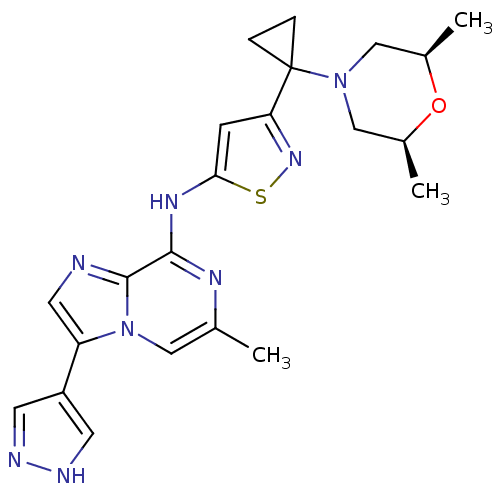
(3-(1-((2S,6R)-2,6-dimethylmorpholino)cyclopropyl)-...)Show SMILES C[C@H]1CN(C[C@@H](C)O1)C1(CC1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C22H26N8OS/c1-13-10-30-17(16-7-24-25-8-16)9-23-21(30)20(26-13)27-19-6-18(28-32-19)22(4-5-22)29-11-14(2)31-15(3)12-29/h6-10,14-15H,4-5,11-12H2,1-3H3,(H,24,25)(H,26,27)/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50329196

(3-(1-((2S,6R)-2,6-dimethylmorpholino)cyclopropyl)-...)Show SMILES C[C@H]1CN(C[C@@H](C)O1)C1(CC1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C22H26N8OS/c1-13-10-30-17(16-7-24-25-8-16)9-23-21(30)20(26-13)27-19-6-18(28-32-19)22(4-5-22)29-11-14(2)31-15(3)12-29/h6-10,14-15H,4-5,11-12H2,1-3H3,(H,24,25)(H,26,27)/t14-,15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50197834
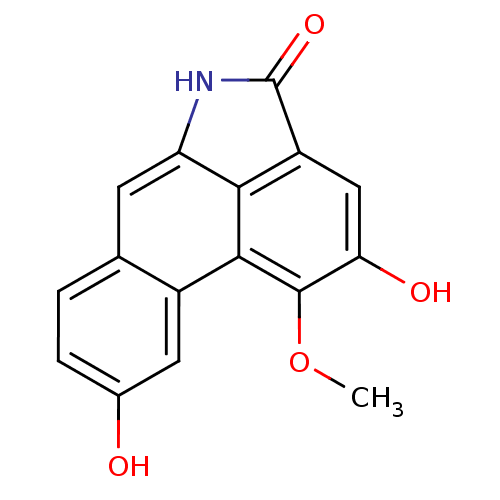
(2,9-dihydroxy-1-methoxydibenzo[cd,f]indol-4(5H)-on...)Show InChI InChI=1S/C16H11NO4/c1-21-15-12(19)6-10-13-11(17-16(10)20)4-7-2-3-8(18)5-9(7)14(13)15/h2-6,18-19H,1H3,(H,17,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50329198

(2-(ethyl((5-(6-methyl-3-(1H-pyrazol-4-yl)imidazo[1...)Show SMILES CCN(Cc1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1)C(C)(C)CO Show InChI InChI=1S/C20H26N8OS/c1-5-27(20(3,4)12-29)11-15-6-17(30-26-15)25-18-19-21-9-16(14-7-22-23-8-14)28(19)10-13(2)24-18/h6-10,29H,5,11-12H2,1-4H3,(H,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50197834

(2,9-dihydroxy-1-methoxydibenzo[cd,f]indol-4(5H)-on...)Show InChI InChI=1S/C16H11NO4/c1-21-15-12(19)6-10-13-11(17-16(10)20)4-7-2-3-8(18)5-9(7)14(13)15/h2-6,18-19H,1H3,(H,17,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDC2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50306888

(4,6-dimethoxy-3-methyl-1H-pyrazolo[3,4-b]quinoline...)Show InChI InChI=1S/C13H13N3O2/c1-7-11-12(18-3)9-6-8(17-2)4-5-10(9)14-13(11)16-15-7/h4-6H,1-3H3,(H,14,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDC2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50306874

(8-Hydroxy-6H-benzo[f][1,3]dioxolo[4',5':4,5]benzo[...)Show InChI InChI=1S/C16H9NO4/c18-11-3-1-2-7-8(11)4-10-13-9(16(19)17-10)5-12-15(14(7)13)21-6-20-12/h1-5,18H,6H2,(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 214 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDC2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50329200
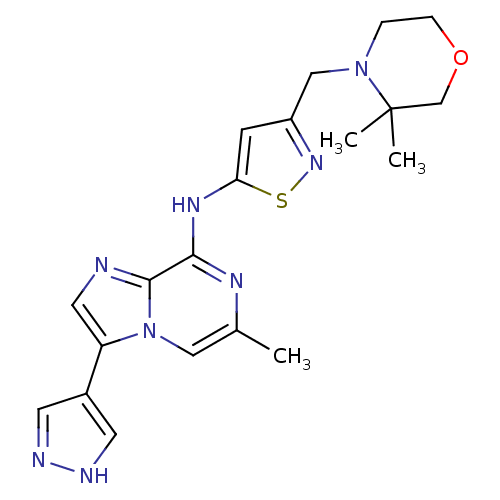
(3-((3,3-dimethylmorpholino)methyl)-N-(6-methyl-3-(...)Show SMILES Cc1cn2c(cnc2c(Nc2cc(CN3CCOCC3(C)C)ns2)n1)-c1cn[nH]c1 Show InChI InChI=1S/C20H24N8OS/c1-13-10-28-16(14-7-22-23-8-14)9-21-19(28)18(24-13)25-17-6-15(26-30-17)11-27-4-5-29-12-20(27,2)3/h6-10H,4-5,11-12H2,1-3H3,(H,22,23)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 384 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data