 Found 25 hits with Last Name = 'wills' and Initial = 'vs'
Found 25 hits with Last Name = 'wills' and Initial = 'vs' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50151859
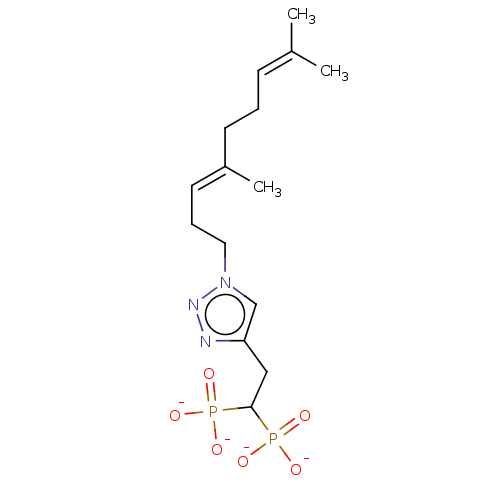
(CHEMBL3775876)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 Show InChI InChI=1S/C15H27N3O6P2.4Na/c1-12(2)6-4-7-13(3)8-5-9-18-11-14(16-17-18)10-15(25(19,20)21)26(22,23)24;;;;/h6,8,11,15H,4-5,7,9-10H2,1-3H3,(H2,19,20,21)(H2,22,23,24);;;;/q;4*+1/p-4/b13-8+;;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GGDPS (unknown origin) assessed as radiolabeled GGPP formation preincubated for 10 mins followed by addition of 10 uM FPP s... |
ACS Med Chem Lett 6: 1195-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00334
BindingDB Entry DOI: 10.7270/Q25B04DN |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50151859

(CHEMBL3775876)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 Show InChI InChI=1S/C15H27N3O6P2.4Na/c1-12(2)6-4-7-13(3)8-5-9-18-11-14(16-17-18)10-15(25(19,20)21)26(22,23)24;;;;/h6,8,11,15H,4-5,7,9-10H2,1-3H3,(H2,19,20,21)(H2,22,23,24);;;;/q;4*+1/p-4/b13-8+;;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GGDPS (unknown origin) using FPP as substrate pretreated for 10 mins followed by substrate addition after 30 mins in presen... |
Bioorg Med Chem 25: 2437-2444 (2017)
Article DOI: 10.1016/j.bmc.2017.02.066
BindingDB Entry DOI: 10.7270/Q27H1MV0 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25270
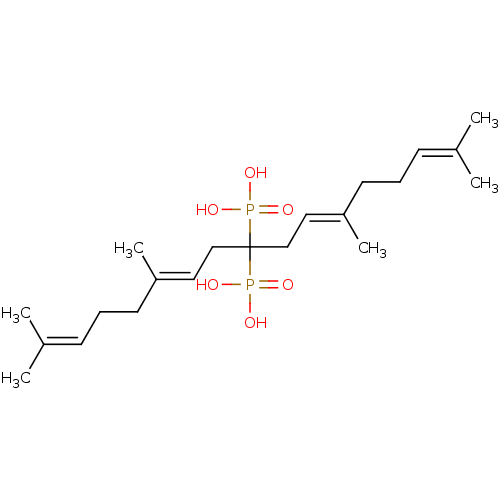
([(6E,11E)-2,6,12,16-tetramethyl-9-phosphonoheptade...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]C([#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])(P([#8])([#8])=O)P([#8])([#8])=O Show InChI InChI=1S/C21H38O6P2/c1-17(2)9-7-11-19(5)13-15-21(28(22,23)24,29(25,26)27)16-14-20(6)12-8-10-18(3)4/h9-10,13-14H,7-8,11-12,15-16H2,1-6H3,(H2,22,23,24)(H2,25,26,27)/b19-13+,20-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GGDPS (unknown origin) assessed as decrease in radiolabeld GGPP level using FPP and [14C]IPP as substrate treated with enzy... |
Bioorg Med Chem 22: 2791-8 (2014)
Article DOI: 10.1016/j.bmc.2014.03.014
BindingDB Entry DOI: 10.7270/Q2TD9ZWV |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50151852
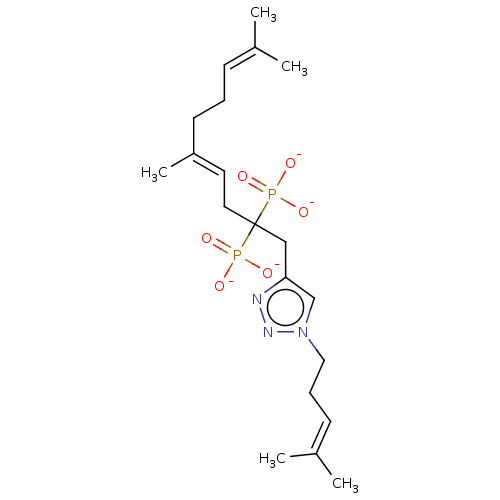
(CHEMBL3775694)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]/[#6]-[#6]\[#6](-[#6])=[#6]\[#6]C([#6]-c1cn(-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])nn1)(P([#8-])([#8-])=O)P([#8-])([#8-])=O Show InChI InChI=1S/C20H35N3O6P2.4Na/c1-16(2)8-6-10-18(5)11-12-20(30(24,25)26,31(27,28)29)14-19-15-23(22-21-19)13-7-9-17(3)4;;;;/h8-9,11,15H,6-7,10,12-14H2,1-5H3,(H2,24,25,26)(H2,27,28,29);;;;/q;4*+1/p-4/b18-11+;;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GGDPS (unknown origin) assessed as radiolabeled GGPP formation preincubated for 10 mins followed by addition of 10 uM FPP s... |
ACS Med Chem Lett 6: 1195-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00334
BindingDB Entry DOI: 10.7270/Q25B04DN |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50013157
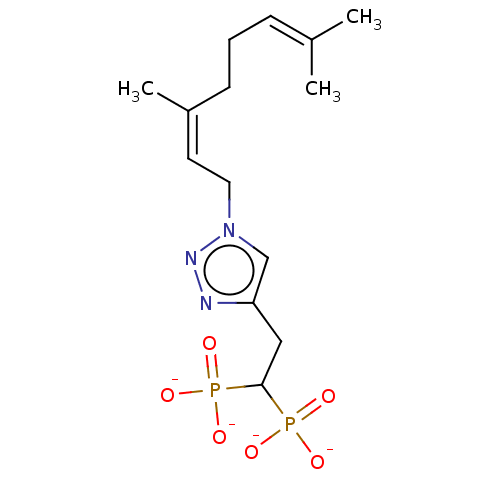
(CHEMBL3262352)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]/[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 Show InChI InChI=1S/C14H25N3O6P2.4Na/c1-11(2)5-4-6-12(3)7-8-17-10-13(15-16-17)9-14(24(18,19)20)25(21,22)23;;;;/h5,7,10,14H,4,6,8-9H2,1-3H3,(H2,18,19,20)(H2,21,22,23);;;;/q;4*+1/p-4/b12-7-;;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GGDPS (unknown origin) assessed as decrease in radiolabeld GGPP level using FPP and [14C]IPP as substrate treated with enzy... |
Bioorg Med Chem 22: 2791-8 (2014)
Article DOI: 10.1016/j.bmc.2014.03.014
BindingDB Entry DOI: 10.7270/Q2TD9ZWV |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50151855
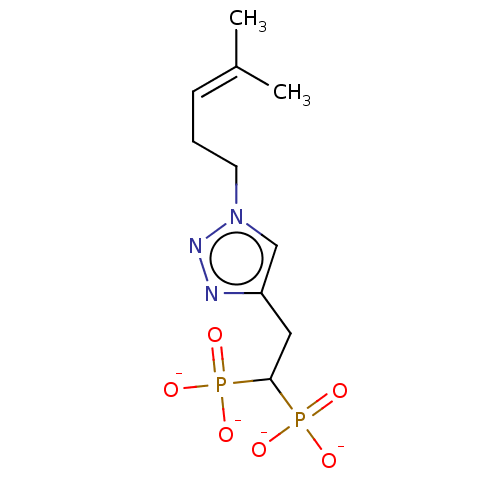
(CHEMBL3775345)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 Show InChI InChI=1S/C10H19N3O6P2.4Na/c1-8(2)4-3-5-13-7-9(11-12-13)6-10(20(14,15)16)21(17,18)19;;;;/h4,7,10H,3,5-6H2,1-2H3,(H2,14,15,16)(H2,17,18,19);;;;/q;4*+1/p-4 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GGDPS (unknown origin) assessed as radiolabeled GGPP formation preincubated for 10 mins followed by addition of 10 uM FPP s... |
ACS Med Chem Lett 6: 1195-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00334
BindingDB Entry DOI: 10.7270/Q25B04DN |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50237988
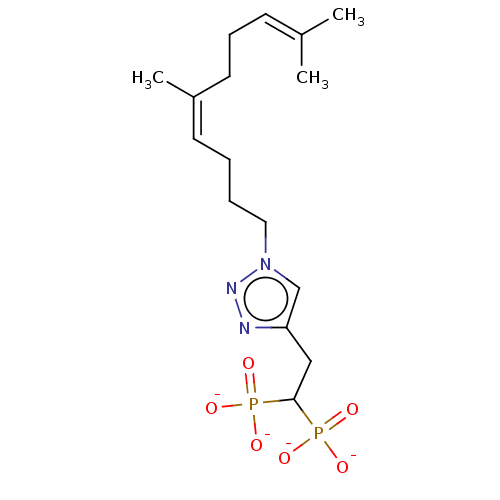
(CHEMBL4096072)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]/[#6]-[#6]\[#6](-[#6])=[#6]/[#6]-[#6]-[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 Show InChI InChI=1S/C16H29N3O6P2/c1-13(2)7-6-9-14(3)8-4-5-10-19-12-15(17-18-19)11-16(26(20,21)22)27(23,24)25/h7-8,12,16H,4-6,9-11H2,1-3H3,(H2,20,21,22)(H2,23,24,25)/p-4/b14-8- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GGDPS (unknown origin) using FPP as substrate pretreated for 10 mins followed by substrate addition after 30 mins in presen... |
Bioorg Med Chem 25: 2437-2444 (2017)
Article DOI: 10.1016/j.bmc.2017.02.066
BindingDB Entry DOI: 10.7270/Q27H1MV0 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50237986

(CHEMBL4078024)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]-[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 Show InChI InChI=1S/C16H29N3O6P2/c1-13(2)7-6-9-14(3)8-4-5-10-19-12-15(17-18-19)11-16(26(20,21)22)27(23,24)25/h7-8,12,16H,4-6,9-11H2,1-3H3,(H2,20,21,22)(H2,23,24,25)/p-4/b14-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GGDPS (unknown origin) using FPP as substrate pretreated for 10 mins followed by substrate addition after 30 mins in presen... |
Bioorg Med Chem 25: 2437-2444 (2017)
Article DOI: 10.1016/j.bmc.2017.02.066
BindingDB Entry DOI: 10.7270/Q27H1MV0 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50237987
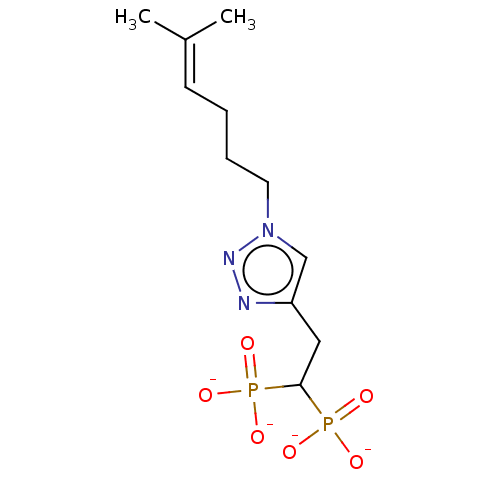
(CHEMBL4069787)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6]-[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 Show InChI InChI=1S/C11H21N3O6P2/c1-9(2)5-3-4-6-14-8-10(12-13-14)7-11(21(15,16)17)22(18,19)20/h5,8,11H,3-4,6-7H2,1-2H3,(H2,15,16,17)(H2,18,19,20)/p-4 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GGDPS (unknown origin) using FPP as substrate pretreated for 10 mins followed by substrate addition after 30 mins in presen... |
Bioorg Med Chem 25: 2437-2444 (2017)
Article DOI: 10.1016/j.bmc.2017.02.066
BindingDB Entry DOI: 10.7270/Q27H1MV0 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50151855

(CHEMBL3775345)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 Show InChI InChI=1S/C10H19N3O6P2.4Na/c1-8(2)4-3-5-13-7-9(11-12-13)6-10(20(14,15)16)21(17,18)19;;;;/h4,7,10H,3,5-6H2,1-2H3,(H2,14,15,16)(H2,17,18,19);;;;/q;4*+1/p-4 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FDPS (unknown origin) assessed as radiolabeled FPP formation preincubated for 10 mins followed by addition of 10 uM GPP sub... |
ACS Med Chem Lett 6: 1195-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00334
BindingDB Entry DOI: 10.7270/Q25B04DN |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50151852

(CHEMBL3775694)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]/[#6]-[#6]\[#6](-[#6])=[#6]\[#6]C([#6]-c1cn(-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])nn1)(P([#8-])([#8-])=O)P([#8-])([#8-])=O Show InChI InChI=1S/C20H35N3O6P2.4Na/c1-16(2)8-6-10-18(5)11-12-20(30(24,25)26,31(27,28)29)14-19-15-23(22-21-19)13-7-9-17(3)4;;;;/h8-9,11,15H,6-7,10,12-14H2,1-5H3,(H2,24,25,26)(H2,27,28,29);;;;/q;4*+1/p-4/b18-11+;;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FDPS (unknown origin) assessed as radiolabeled FPP formation preincubated for 10 mins followed by addition of 10 uM GPP sub... |
ACS Med Chem Lett 6: 1195-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00334
BindingDB Entry DOI: 10.7270/Q25B04DN |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50013159
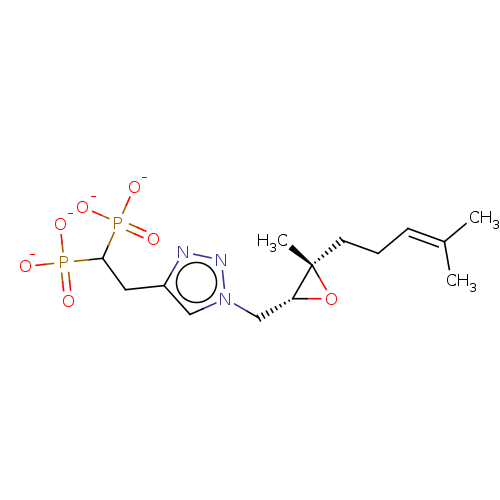
(CHEMBL3262354)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@]1([#6])[#8]-[#6@@H]1-[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 |r| Show InChI InChI=1S/C14H25N3O7P2.4Na/c1-10(2)5-4-6-14(3)12(24-14)9-17-8-11(15-16-17)7-13(25(18,19)20)26(21,22)23;;;;/h5,8,12-13H,4,6-7,9H2,1-3H3,(H2,18,19,20)(H2,21,22,23);;;;/q;4*+1/p-4/t12-,14+;;;;/m1..../s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GGDPS (unknown origin) assessed as decrease in radiolabeld GGPP level using FPP and [14C]IPP as substrate treated with enzy... |
Bioorg Med Chem 22: 2791-8 (2014)
Article DOI: 10.1016/j.bmc.2014.03.014
BindingDB Entry DOI: 10.7270/Q2TD9ZWV |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50013156
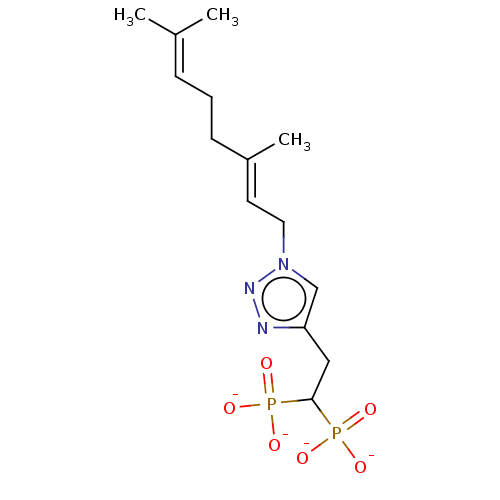
(CHEMBL3262351)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 Show InChI InChI=1S/C14H25N3O6P2.4Na/c1-11(2)5-4-6-12(3)7-8-17-10-13(15-16-17)9-14(24(18,19)20)25(21,22)23;;;;/h5,7,10,14H,4,6,8-9H2,1-3H3,(H2,18,19,20)(H2,21,22,23);;;;/q;4*+1/p-4/b12-7+;;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GGDPS (unknown origin) assessed as decrease in radiolabeld GGPP level using FPP and [14C]IPP as substrate treated with enzy... |
Bioorg Med Chem 22: 2791-8 (2014)
Article DOI: 10.1016/j.bmc.2014.03.014
BindingDB Entry DOI: 10.7270/Q2TD9ZWV |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50013156

(CHEMBL3262351)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 Show InChI InChI=1S/C14H25N3O6P2.4Na/c1-11(2)5-4-6-12(3)7-8-17-10-13(15-16-17)9-14(24(18,19)20)25(21,22)23;;;;/h5,7,10,14H,4,6,8-9H2,1-3H3,(H2,18,19,20)(H2,21,22,23);;;;/q;4*+1/p-4/b12-7+;;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GGDPS (unknown origin) assessed as decrease in radiolabeld GGPP level using FPP and [14C]IPP as substrate treated with enzy... |
Bioorg Med Chem 22: 2791-8 (2014)
Article DOI: 10.1016/j.bmc.2014.03.014
BindingDB Entry DOI: 10.7270/Q2TD9ZWV |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50013156

(CHEMBL3262351)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 Show InChI InChI=1S/C14H25N3O6P2.4Na/c1-11(2)5-4-6-12(3)7-8-17-10-13(15-16-17)9-14(24(18,19)20)25(21,22)23;;;;/h5,7,10,14H,4,6,8-9H2,1-3H3,(H2,18,19,20)(H2,21,22,23);;;;/q;4*+1/p-4/b12-7+;;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GGDPS (unknown origin) assessed as radiolabeled GGPP formation preincubated for 10 mins followed by addition of 10 uM FPP s... |
ACS Med Chem Lett 6: 1195-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00334
BindingDB Entry DOI: 10.7270/Q25B04DN |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50013158
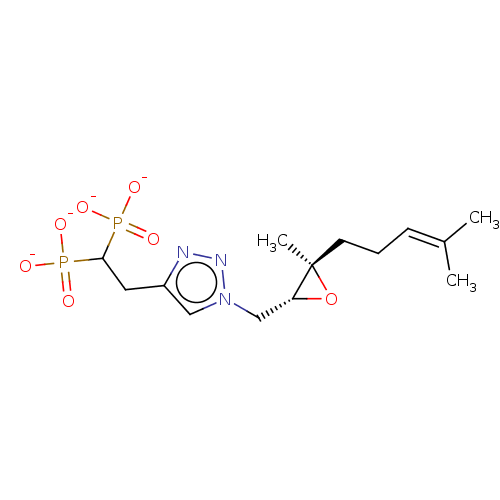
(CHEMBL3262353)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@@]1([#6])[#8]-[#6@@H]1-[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 |r| Show InChI InChI=1S/C14H25N3O7P2.4Na/c1-10(2)5-4-6-14(3)12(24-14)9-17-8-11(15-16-17)7-13(25(18,19)20)26(21,22)23;;;;/h5,8,12-13H,4,6-7,9H2,1-3H3,(H2,18,19,20)(H2,21,22,23);;;;/q;4*+1/p-4/t12-,14-;;;;/m1..../s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GGDPS (unknown origin) assessed as decrease in radiolabeld GGPP level using FPP and [14C]IPP as substrate treated with enzy... |
Bioorg Med Chem 22: 2791-8 (2014)
Article DOI: 10.1016/j.bmc.2014.03.014
BindingDB Entry DOI: 10.7270/Q2TD9ZWV |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50151859

(CHEMBL3775876)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 Show InChI InChI=1S/C15H27N3O6P2.4Na/c1-12(2)6-4-7-13(3)8-5-9-18-11-14(16-17-18)10-15(25(19,20)21)26(22,23)24;;;;/h6,8,11,15H,4-5,7,9-10H2,1-3H3,(H2,19,20,21)(H2,22,23,24);;;;/q;4*+1/p-4/b13-8+;;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FDPS (unknown origin) assessed as radiolabeled FPP formation preincubated for 10 mins followed by addition of 10 uM GPP sub... |
ACS Med Chem Lett 6: 1195-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00334
BindingDB Entry DOI: 10.7270/Q25B04DN |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50237988

(CHEMBL4096072)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]/[#6]-[#6]\[#6](-[#6])=[#6]/[#6]-[#6]-[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 Show InChI InChI=1S/C16H29N3O6P2/c1-13(2)7-6-9-14(3)8-4-5-10-19-12-15(17-18-19)11-16(26(20,21)22)27(23,24)25/h7-8,12,16H,4-6,9-11H2,1-3H3,(H2,20,21,22)(H2,23,24,25)/p-4/b14-8- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FDPS (unknown origin) using GPP as substrate pretreated for 10 mins followed by substrate addition after 30 mins in presenc... |
Bioorg Med Chem 25: 2437-2444 (2017)
Article DOI: 10.1016/j.bmc.2017.02.066
BindingDB Entry DOI: 10.7270/Q27H1MV0 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50013158

(CHEMBL3262353)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@@]1([#6])[#8]-[#6@@H]1-[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 |r| Show InChI InChI=1S/C14H25N3O7P2.4Na/c1-10(2)5-4-6-14(3)12(24-14)9-17-8-11(15-16-17)7-13(25(18,19)20)26(21,22)23;;;;/h5,8,12-13H,4,6-7,9H2,1-3H3,(H2,18,19,20)(H2,21,22,23);;;;/q;4*+1/p-4/t12-,14-;;;;/m1..../s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FDPS (unknown origin) assessed as decrease in radiolabeld GGPP level using GPP and [14C]IPP as substrate treated with enzym... |
Bioorg Med Chem 22: 2791-8 (2014)
Article DOI: 10.1016/j.bmc.2014.03.014
BindingDB Entry DOI: 10.7270/Q2TD9ZWV |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50237986

(CHEMBL4078024)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]-[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 Show InChI InChI=1S/C16H29N3O6P2/c1-13(2)7-6-9-14(3)8-4-5-10-19-12-15(17-18-19)11-16(26(20,21)22)27(23,24)25/h7-8,12,16H,4-6,9-11H2,1-3H3,(H2,20,21,22)(H2,23,24,25)/p-4/b14-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FDPS (unknown origin) using GPP as substrate pretreated for 10 mins followed by substrate addition after 30 mins in presenc... |
Bioorg Med Chem 25: 2437-2444 (2017)
Article DOI: 10.1016/j.bmc.2017.02.066
BindingDB Entry DOI: 10.7270/Q27H1MV0 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50013159

(CHEMBL3262354)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@]1([#6])[#8]-[#6@@H]1-[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 |r| Show InChI InChI=1S/C14H25N3O7P2.4Na/c1-10(2)5-4-6-14(3)12(24-14)9-17-8-11(15-16-17)7-13(25(18,19)20)26(21,22)23;;;;/h5,8,12-13H,4,6-7,9H2,1-3H3,(H2,18,19,20)(H2,21,22,23);;;;/q;4*+1/p-4/t12-,14+;;;;/m1..../s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FDPS (unknown origin) assessed as decrease in radiolabeld GGPP level using GPP and [14C]IPP as substrate treated with enzym... |
Bioorg Med Chem 22: 2791-8 (2014)
Article DOI: 10.1016/j.bmc.2014.03.014
BindingDB Entry DOI: 10.7270/Q2TD9ZWV |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50013156

(CHEMBL3262351)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 Show InChI InChI=1S/C14H25N3O6P2.4Na/c1-11(2)5-4-6-12(3)7-8-17-10-13(15-16-17)9-14(24(18,19)20)25(21,22)23;;;;/h5,7,10,14H,4,6,8-9H2,1-3H3,(H2,18,19,20)(H2,21,22,23);;;;/q;4*+1/p-4/b12-7+;;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FDPS (unknown origin) assessed as decrease in radiolabeld GGPP level using GPP and [14C]IPP as substrate treated with enzym... |
Bioorg Med Chem 22: 2791-8 (2014)
Article DOI: 10.1016/j.bmc.2014.03.014
BindingDB Entry DOI: 10.7270/Q2TD9ZWV |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50013156

(CHEMBL3262351)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 Show InChI InChI=1S/C14H25N3O6P2.4Na/c1-11(2)5-4-6-12(3)7-8-17-10-13(15-16-17)9-14(24(18,19)20)25(21,22)23;;;;/h5,7,10,14H,4,6,8-9H2,1-3H3,(H2,18,19,20)(H2,21,22,23);;;;/q;4*+1/p-4/b12-7+;;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FDPS (unknown origin) assessed as decrease in radiolabeld GGPP level using GPP and [14C]IPP as substrate treated with enzym... |
Bioorg Med Chem 22: 2791-8 (2014)
Article DOI: 10.1016/j.bmc.2014.03.014
BindingDB Entry DOI: 10.7270/Q2TD9ZWV |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50013157

(CHEMBL3262352)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]/[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 Show InChI InChI=1S/C14H25N3O6P2.4Na/c1-11(2)5-4-6-12(3)7-8-17-10-13(15-16-17)9-14(24(18,19)20)25(21,22)23;;;;/h5,7,10,14H,4,6,8-9H2,1-3H3,(H2,18,19,20)(H2,21,22,23);;;;/q;4*+1/p-4/b12-7-;;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FDPS (unknown origin) assessed as decrease in radiolabeld GGPP level using GPP and [14C]IPP as substrate treated with enzym... |
Bioorg Med Chem 22: 2791-8 (2014)
Article DOI: 10.1016/j.bmc.2014.03.014
BindingDB Entry DOI: 10.7270/Q2TD9ZWV |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50237987

(CHEMBL4069787)Show SMILES [Na+].[Na+].[Na+].[Na+].[#6]\[#6](-[#6])=[#6]\[#6]-[#6]-[#6]-n1cc(-[#6]-[#6](P([#8-])([#8-])=O)P([#8-])([#8-])=O)nn1 Show InChI InChI=1S/C11H21N3O6P2/c1-9(2)5-3-4-6-14-8-10(12-13-14)7-11(21(15,16)17)22(18,19)20/h5,8,11H,3-4,6-7H2,1-2H3,(H2,15,16,17)(H2,18,19,20)/p-4 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FDPS (unknown origin) using GPP as substrate pretreated for 10 mins followed by substrate addition after 30 mins in presenc... |
Bioorg Med Chem 25: 2437-2444 (2017)
Article DOI: 10.1016/j.bmc.2017.02.066
BindingDB Entry DOI: 10.7270/Q27H1MV0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data