 Found 60 hits with Last Name = 'winkler' and Initial = 'i'
Found 60 hits with Last Name = 'winkler' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434389
(CHEMBL2387288)Show InChI InChI=1S/C21H28N4OS/c1-16-4-2-11-25(16)19-10-12-24(15-19)18-8-6-17(7-9-18)23-21(26)22-14-20-5-3-13-27-20/h3,5-9,13,16,19H,2,4,10-12,14-15H2,1H3,(H2,22,23,26) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434405
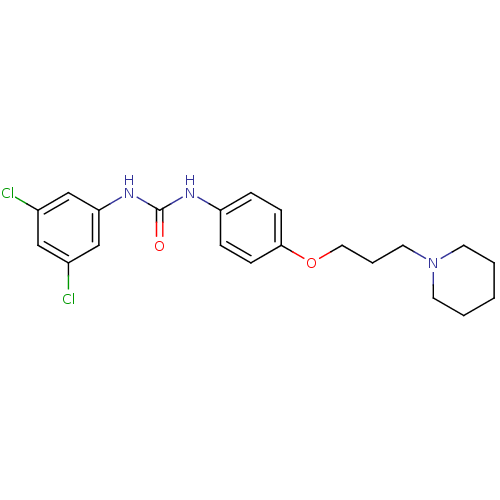
(CHEMBL2387306)Show SMILES Clc1cc(Cl)cc(NC(=O)Nc2ccc(OCCCN3CCCCC3)cc2)c1 Show InChI InChI=1S/C21H25Cl2N3O2/c22-16-13-17(23)15-19(14-16)25-21(27)24-18-5-7-20(8-6-18)28-12-4-11-26-9-2-1-3-10-26/h5-8,13-15H,1-4,9-12H2,(H2,24,25,27) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434382
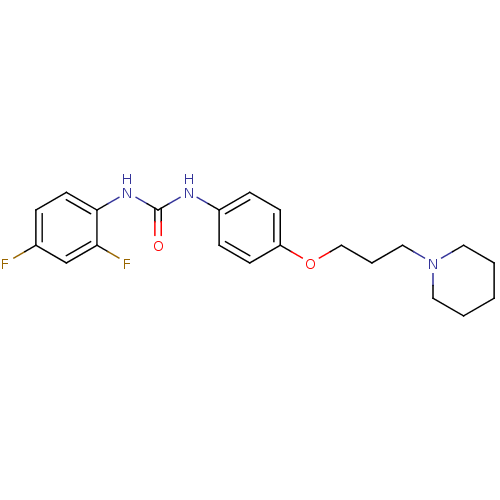
(CHEMBL2387309)Show SMILES Fc1ccc(NC(=O)Nc2ccc(OCCCN3CCCCC3)cc2)c(F)c1 Show InChI InChI=1S/C21H25F2N3O2/c22-16-5-10-20(19(23)15-16)25-21(27)24-17-6-8-18(9-7-17)28-14-4-13-26-11-2-1-3-12-26/h5-10,15H,1-4,11-14H2,(H2,24,25,27) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434401
(CHEMBL2387314)Show InChI InChI=1S/C22H34N4O/c1-17-6-5-14-26(17)21-13-15-25(16-21)20-11-9-19(10-12-20)24-22(27)23-18-7-3-2-4-8-18/h9-12,17-18,21H,2-8,13-16H2,1H3,(H2,23,24,27) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434400
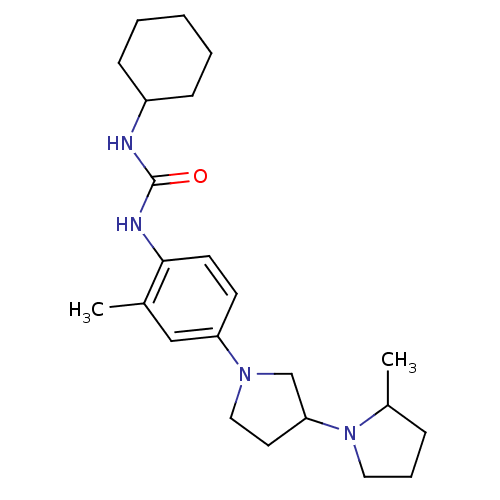
(CHEMBL2387315)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)NC2CCCCC2)c(C)c1 Show InChI InChI=1S/C23H36N4O/c1-17-15-20(26-14-12-21(16-26)27-13-6-7-18(27)2)10-11-22(17)25-23(28)24-19-8-4-3-5-9-19/h10-11,15,18-19,21H,3-9,12-14,16H2,1-2H3,(H2,24,25,28) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434392
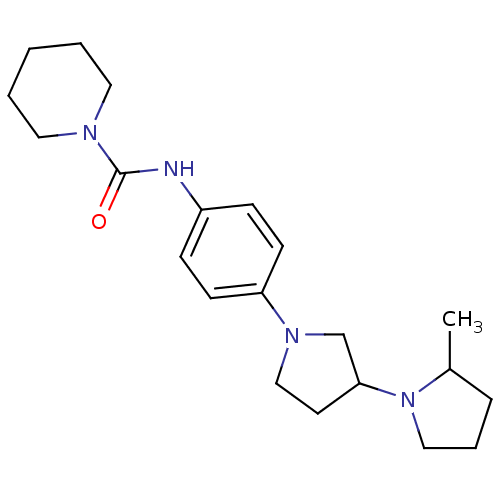
(CHEMBL2387285)Show InChI InChI=1S/C21H32N4O/c1-17-6-5-14-25(17)20-11-15-24(16-20)19-9-7-18(8-10-19)22-21(26)23-12-3-2-4-13-23/h7-10,17,20H,2-6,11-16H2,1H3,(H,22,26) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434387
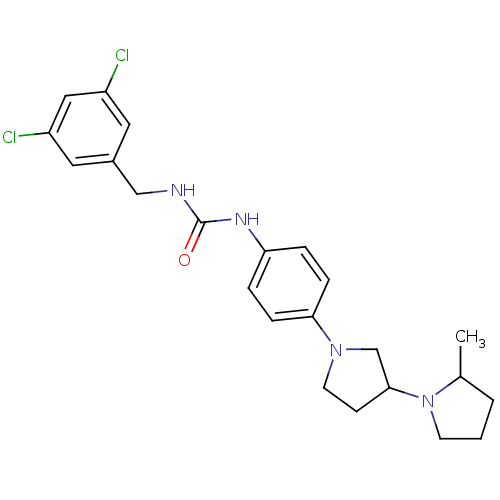
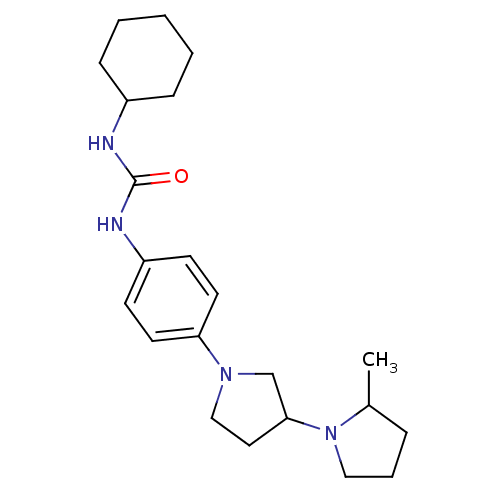
(CHEMBL2387290)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)NCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C23H28Cl2N4O/c1-16-3-2-9-29(16)22-8-10-28(15-22)21-6-4-20(5-7-21)27-23(30)26-14-17-11-18(24)13-19(25)12-17/h4-7,11-13,16,22H,2-3,8-10,14-15H2,1H3,(H2,26,27,30) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434388
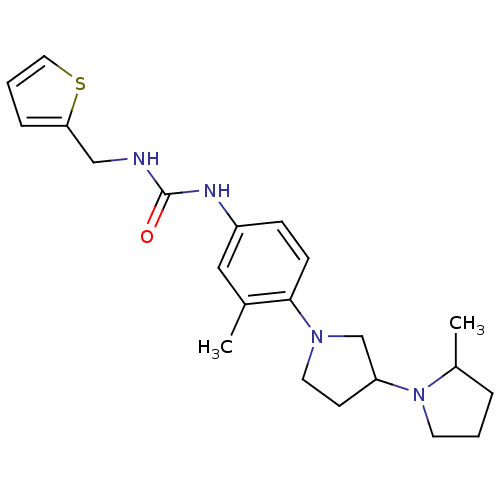
(CHEMBL2387289)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)NCc2cccs2)cc1C Show InChI InChI=1S/C22H30N4OS/c1-16-13-18(24-22(27)23-14-20-6-4-12-28-20)7-8-21(16)25-11-9-19(15-25)26-10-3-5-17(26)2/h4,6-8,12-13,17,19H,3,5,9-11,14-15H2,1-2H3,(H2,23,24,27) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434397
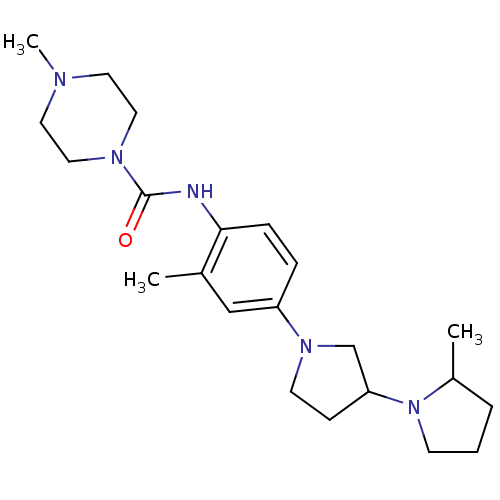
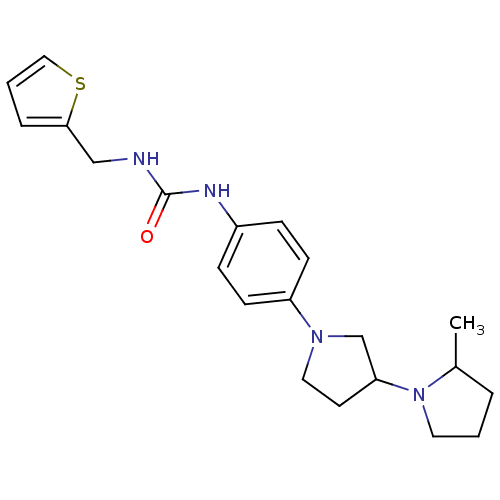
(CHEMBL2387318)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)N2CCN(C)CC2)c(C)c1 Show InChI InChI=1S/C22H35N5O/c1-17-15-19(26-10-8-20(16-26)27-9-4-5-18(27)2)6-7-21(17)23-22(28)25-13-11-24(3)12-14-25/h6-7,15,18,20H,4-5,8-14,16H2,1-3H3,(H,23,28) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434393
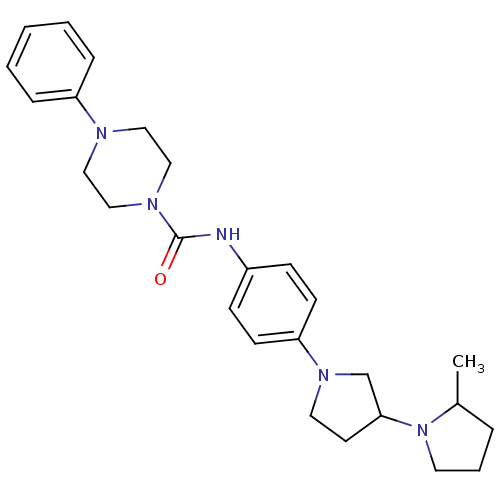
(CHEMBL2387284)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)N2CCN(CC2)c2ccccc2)cc1 Show InChI InChI=1S/C26H35N5O/c1-21-6-5-14-31(21)25-13-15-30(20-25)24-11-9-22(10-12-24)27-26(32)29-18-16-28(17-19-29)23-7-3-2-4-8-23/h2-4,7-12,21,25H,5-6,13-20H2,1H3,(H,27,32) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434399
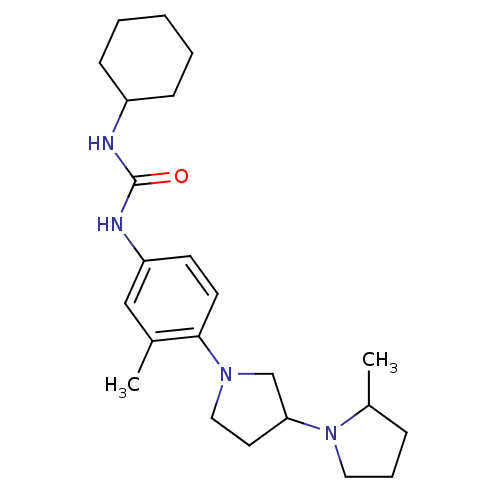
(CHEMBL2387316)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)NC2CCCCC2)cc1C Show InChI InChI=1S/C23H36N4O/c1-17-15-20(25-23(28)24-19-8-4-3-5-9-19)10-11-22(17)26-14-12-21(16-26)27-13-6-7-18(27)2/h10-11,15,18-19,21H,3-9,12-14,16H2,1-2H3,(H2,24,25,28) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434396
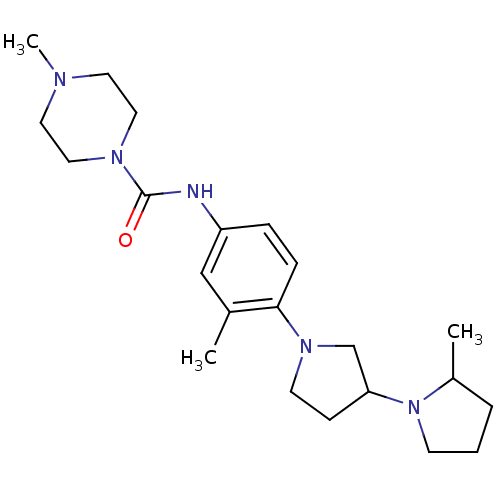
(CHEMBL2387319)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)N2CCN(C)CC2)cc1C Show InChI InChI=1S/C22H35N5O/c1-17-15-19(23-22(28)25-13-11-24(3)12-14-25)6-7-21(17)26-10-8-20(16-26)27-9-4-5-18(27)2/h6-7,15,18,20H,4-5,8-14,16H2,1-3H3,(H,23,28) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434381
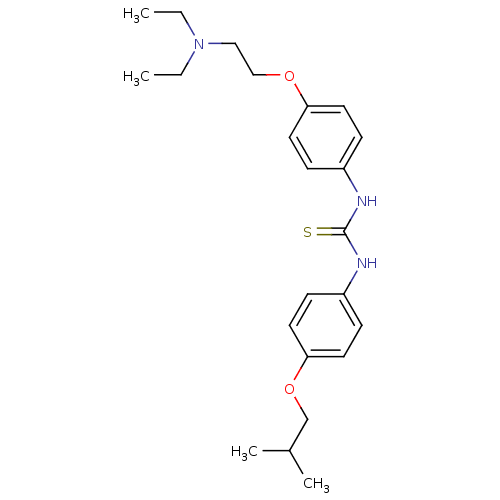
(CHEMBL2387310)Show SMILES CCN(CC)CCOc1ccc(NC(=S)Nc2ccc(OCC(C)C)cc2)cc1 Show InChI InChI=1S/C23H33N3O2S/c1-5-26(6-2)15-16-27-21-11-7-19(8-12-21)24-23(29)25-20-9-13-22(14-10-20)28-17-18(3)4/h7-14,18H,5-6,15-17H2,1-4H3,(H2,24,25,29) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50435303
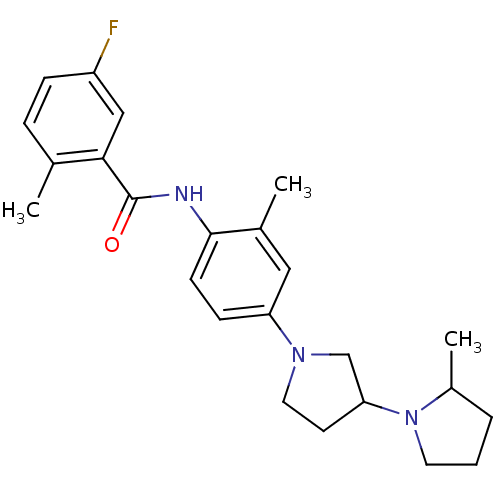
(CHEMBL2391167)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)c2cc(F)ccc2C)c(C)c1 Show InChI InChI=1S/C24H30FN3O/c1-16-6-7-19(25)14-22(16)24(29)26-23-9-8-20(13-17(23)2)27-12-10-21(15-27)28-11-4-5-18(28)3/h6-9,13-14,18,21H,4-5,10-12,15H2,1-3H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat histamine H3 receptor |
Bioorg Med Chem Lett 23: 3421-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.081
BindingDB Entry DOI: 10.7270/Q2HH6MGF |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434383
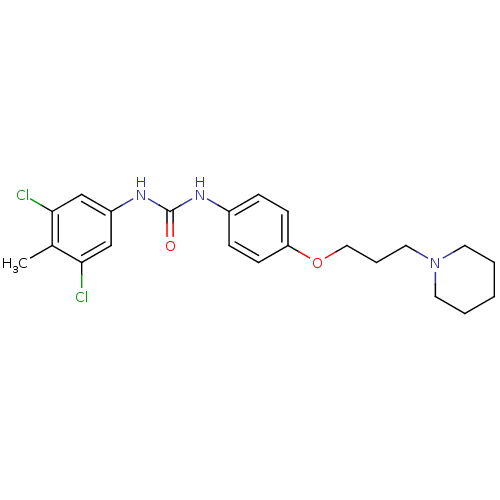
(CHEMBL2387307)Show SMILES Cc1c(Cl)cc(NC(=O)Nc2ccc(OCCCN3CCCCC3)cc2)cc1Cl Show InChI InChI=1S/C22H27Cl2N3O2/c1-16-20(23)14-18(15-21(16)24)26-22(28)25-17-6-8-19(9-7-17)29-13-5-12-27-10-3-2-4-11-27/h6-9,14-15H,2-5,10-13H2,1H3,(H2,25,26,28) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434404
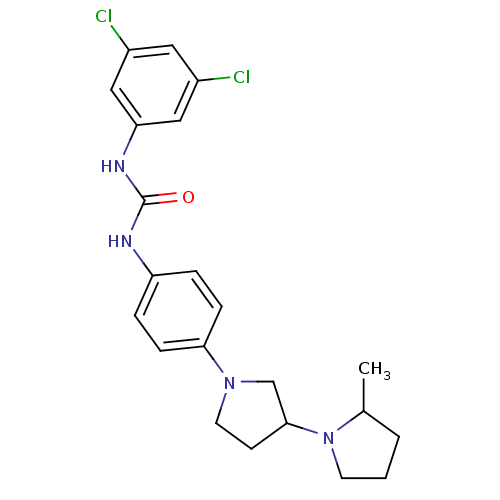
(CHEMBL2387311)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)Nc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C22H26Cl2N4O/c1-15-3-2-9-28(15)21-8-10-27(14-21)20-6-4-18(5-7-20)25-22(29)26-19-12-16(23)11-17(24)13-19/h4-7,11-13,15,21H,2-3,8-10,14H2,1H3,(H2,25,26,29) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434403
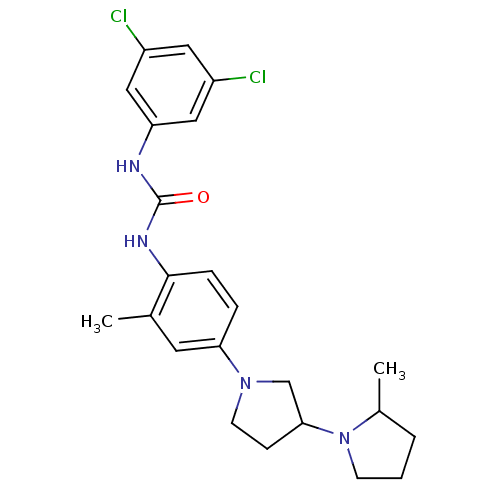
(CHEMBL2387312)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)Nc2cc(Cl)cc(Cl)c2)c(C)c1 Show InChI InChI=1S/C23H28Cl2N4O/c1-15-10-20(28-9-7-21(14-28)29-8-3-4-16(29)2)5-6-22(15)27-23(30)26-19-12-17(24)11-18(25)13-19/h5-6,10-13,16,21H,3-4,7-9,14H2,1-2H3,(H2,26,27,30) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434394

(CHEMBL2387283)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)N2CCN(CC2)C(C)=O)cc1C Show InChI InChI=1S/C23H35N5O2/c1-17-15-20(24-23(30)26-13-11-25(12-14-26)19(3)29)6-7-22(17)27-10-8-21(16-27)28-9-4-5-18(28)2/h6-7,15,18,21H,4-5,8-14,16H2,1-3H3,(H,24,30) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434398
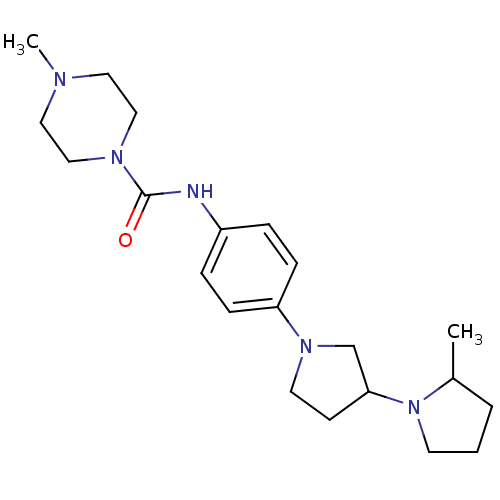
(CHEMBL2387317)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)N2CCN(C)CC2)cc1 Show InChI InChI=1S/C21H33N5O/c1-17-4-3-10-26(17)20-9-11-25(16-20)19-7-5-18(6-8-19)22-21(27)24-14-12-23(2)13-15-24/h5-8,17,20H,3-4,9-16H2,1-2H3,(H,22,27) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434386
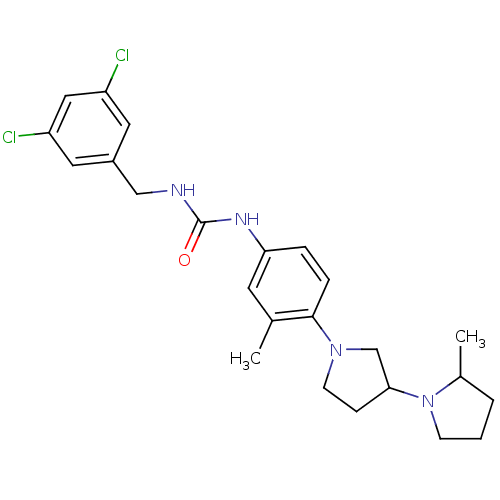
(CHEMBL2387291)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)NCc2cc(Cl)cc(Cl)c2)cc1C Show InChI InChI=1S/C24H30Cl2N4O/c1-16-10-21(28-24(31)27-14-18-11-19(25)13-20(26)12-18)5-6-23(16)29-9-7-22(15-29)30-8-3-4-17(30)2/h5-6,10-13,17,22H,3-4,7-9,14-15H2,1-2H3,(H2,27,28,31) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50434382

(CHEMBL2387309)Show SMILES Fc1ccc(NC(=O)Nc2ccc(OCCCN3CCCCC3)cc2)c(F)c1 Show InChI InChI=1S/C21H25F2N3O2/c22-16-5-10-20(19(23)15-16)25-21(27)24-17-6-8-18(9-7-17)28-14-4-13-26-11-2-1-3-12-26/h5-10,15H,1-4,11-14H2,(H2,24,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rat histamine H3 receptor transfected in CHO cells after 1 hr by scintillation counting analy... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434395
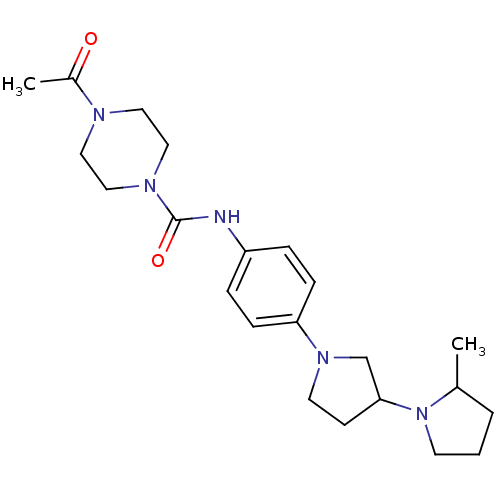
(CHEMBL2387282)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)N2CCN(CC2)C(C)=O)cc1 Show InChI InChI=1S/C22H33N5O2/c1-17-4-3-10-27(17)21-9-11-26(16-21)20-7-5-19(6-8-20)23-22(29)25-14-12-24(13-15-25)18(2)28/h5-8,17,21H,3-4,9-16H2,1-2H3,(H,23,29) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434391
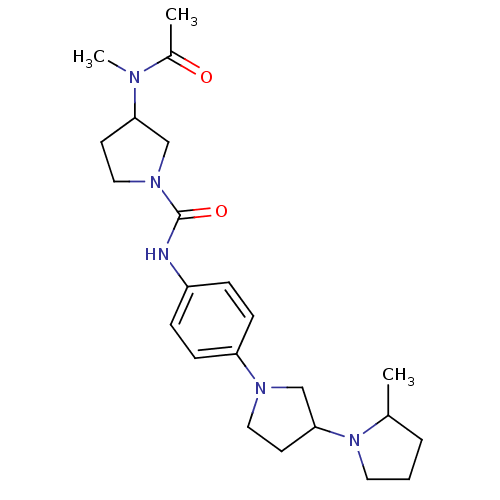
(CHEMBL2387286)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)N2CCC(C2)N(C)C(C)=O)cc1 Show InChI InChI=1S/C23H35N5O2/c1-17-5-4-12-28(17)22-11-13-26(16-22)20-8-6-19(7-9-20)24-23(30)27-14-10-21(15-27)25(3)18(2)29/h6-9,17,21-22H,4-5,10-16H2,1-3H3,(H,24,30) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50434381

(CHEMBL2387310)Show SMILES CCN(CC)CCOc1ccc(NC(=S)Nc2ccc(OCC(C)C)cc2)cc1 Show InChI InChI=1S/C23H33N3O2S/c1-5-26(6-2)15-16-27-21-11-7-19(8-12-21)24-23(29)25-20-9-13-22(14-10-20)28-17-18(3)4/h7-14,18H,5-6,15-17H2,1-4H3,(H2,24,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rat histamine H3 receptor transfected in CHO cells after 1 hr by scintillation counting analy... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434402

(CHEMBL2387313)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)Nc2cc(Cl)cc(Cl)c2)cc1C Show InChI InChI=1S/C23H28Cl2N4O/c1-15-10-19(26-23(30)27-20-12-17(24)11-18(25)13-20)5-6-22(15)28-9-7-21(14-28)29-8-3-4-16(29)2/h5-6,10-13,16,21H,3-4,7-9,14H2,1-2H3,(H2,26,27,30) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434385

(CHEMBL2387292)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)Nc2ccc(Oc3ccccc3)cc2)cc1 Show InChI InChI=1S/C28H32N4O2/c1-21-6-5-18-32(21)25-17-19-31(20-25)24-13-9-22(10-14-24)29-28(33)30-23-11-15-27(16-12-23)34-26-7-3-2-4-8-26/h2-4,7-16,21,25H,5-6,17-20H2,1H3,(H2,29,30,33) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50435304

(CHEMBL2391166)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)c2ccccc2F)c(C)c1 Show InChI InChI=1S/C23H28FN3O/c1-16-14-18(26-13-11-19(15-26)27-12-5-6-17(27)2)9-10-22(16)25-23(28)20-7-3-4-8-21(20)24/h3-4,7-10,14,17,19H,5-6,11-13,15H2,1-2H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat histamine H3 receptor |
Bioorg Med Chem Lett 23: 3421-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.081
BindingDB Entry DOI: 10.7270/Q2HH6MGF |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434390

(CHEMBL2387287)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)N2CCC(C2)N(C)C(C)=O)cc1C Show InChI InChI=1S/C24H37N5O2/c1-17-14-20(25-24(31)28-13-9-21(15-28)26(4)19(3)30)7-8-23(17)27-12-10-22(16-27)29-11-5-6-18(29)2/h7-8,14,18,21-22H,5-6,9-13,15-16H2,1-4H3,(H,25,31) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50434384

(CHEMBL2387293)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)Nc2ccc(Oc3ccccc3)cc2)cc1C Show InChI InChI=1S/C29H34N4O2/c1-21-19-24(12-15-28(21)32-18-16-25(20-32)33-17-6-7-22(33)2)31-29(34)30-23-10-13-27(14-11-23)35-26-8-4-3-5-9-26/h3-5,8-15,19,22,25H,6-7,16-18,20H2,1-2H3,(H2,30,31,34) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50434383

(CHEMBL2387307)Show SMILES Cc1c(Cl)cc(NC(=O)Nc2ccc(OCCCN3CCCCC3)cc2)cc1Cl Show InChI InChI=1S/C22H27Cl2N3O2/c1-16-20(23)14-18(15-21(16)24)26-22(28)25-17-6-8-19(9-7-17)29-13-5-12-27-10-3-2-4-11-27/h6-9,14-15H,2-5,10-13H2,1H3,(H2,25,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from cloned rat histamine H3 receptor transfected in CHO cells after 1 hr by scintillation counting analy... |
Bioorg Med Chem Lett 23: 3416-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.080
BindingDB Entry DOI: 10.7270/Q2RB75Z5 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50435303

(CHEMBL2391167)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)c2cc(F)ccc2C)c(C)c1 Show InChI InChI=1S/C24H30FN3O/c1-16-6-7-19(25)14-22(16)24(29)26-23-9-8-20(13-17(23)2)27-12-10-21(15-27)28-11-4-5-18(28)3/h6-9,13-14,18,21H,4-5,10-12,15H2,1-3H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H1 receptor |
Bioorg Med Chem Lett 23: 3421-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.081
BindingDB Entry DOI: 10.7270/Q2HH6MGF |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50435304

(CHEMBL2391166)Show SMILES CC1CCCN1C1CCN(C1)c1ccc(NC(=O)c2ccccc2F)c(C)c1 Show InChI InChI=1S/C23H28FN3O/c1-16-14-18(26-13-11-19(15-26)27-12-5-6-17(27)2)9-10-22(16)25-23(28)20-7-3-4-8-21(20)24/h3-4,7-10,14,17,19H,5-6,11-13,15H2,1-2H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H1 receptor |
Bioorg Med Chem Lett 23: 3421-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.081
BindingDB Entry DOI: 10.7270/Q2HH6MGF |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50374671

(CHEMBL272660)Show SMILES NC(=O)CN1CCCNC(=O)CCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:27.28,wD:16.16,49.51,38.40,(28.48,-20.35,;27.16,-19.57,;27.17,-18.03,;25.81,-20.32,;25.8,-21.86,;23.64,-21.92,;23.15,-20.32,;24.48,-19.55,;24.48,-18.01,;23.15,-17.24,;23.15,-15.7,;21.81,-18.01,;21.81,-19.55,;20.48,-20.32,;19.15,-19.55,;20.48,-21.86,;19.15,-22.63,;17.81,-21.86,;16.48,-22.63,;15.15,-21.85,;13.82,-22.62,;13.81,-24.16,;15.16,-24.93,;16.48,-24.16,;19.15,-24.17,;17.81,-24.94,;20.48,-24.94,;20.48,-26.48,;19.15,-27.25,;19.15,-28.79,;17.81,-29.56,;17.8,-31.09,;19.14,-31.87,;20.48,-31.09,;20.47,-29.55,;21.81,-27.25,;21.81,-28.79,;23.15,-26.48,;24.48,-27.25,;24.48,-28.79,;25.81,-29.56,;25.81,-31.1,;27.15,-31.87,;27.15,-33.41,;28.48,-34.18,;25.81,-34.18,;25.81,-26.48,;27.15,-27.25,;25.81,-24.94,;27.15,-24.17,;28.48,-24.94,;29.82,-24.17,;29.98,-22.63,;31.49,-22.31,;32.24,-23.66,;33.75,-23.99,;34.21,-25.46,;33.18,-26.59,;31.67,-26.26,;31.21,-24.8,;27.15,-22.63,;28.48,-21.86,)| Show InChI InChI=1S/C44H55N11O7/c45-37(56)27-55-22-10-21-48-38(57)18-19-39(58)51-34(23-28-11-3-1-4-12-28)41(60)53-35(24-29-13-5-2-6-14-29)42(61)52-33(17-9-20-49-44(46)47)40(59)54-36(43(55)62)25-30-26-50-32-16-8-7-15-31(30)32/h1-8,11-16,26,33-36,50H,9-10,17-25,27H2,(H2,45,56)(H,48,57)(H,51,58)(H,52,61)(H,53,60)(H,54,59)(H4,46,47,49)/t33-,34-,35+,36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from MC4R |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50374672

(CHEMBL271586)Show SMILES NC(=O)CN1CCCNC(=O)CCCCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:29.30,wD:51.53,18.18,40.42,(30.64,-.68,;29.3,.09,;29.31,1.63,;27.97,-.68,;26.64,.1,;26.64,1.64,;27.98,2.4,;27.98,3.94,;25.3,3.95,;23.96,4.72,;23.96,6.26,;22.63,3.95,;21.3,4.72,;19.96,3.95,;19.96,2.41,;18.63,1.64,;17.29,2.41,;18.63,.1,;17.29,-.67,;15.96,.1,;14.63,-.67,;13.3,.11,;11.96,-.66,;11.96,-2.2,;13.31,-2.97,;14.63,-2.2,;17.29,-2.21,;15.96,-2.98,;18.63,-2.98,;18.63,-4.52,;17.29,-5.29,;17.29,-6.83,;15.95,-7.59,;15.95,-9.13,;17.29,-9.9,;18.63,-9.12,;18.62,-7.59,;19.96,-5.29,;19.96,-6.83,;21.3,-4.52,;22.63,-5.29,;22.63,-6.83,;23.96,-7.6,;23.96,-9.14,;25.3,-9.91,;25.3,-11.45,;26.63,-12.22,;23.96,-12.22,;23.96,-4.52,;25.3,-5.29,;23.96,-2.98,;25.3,-2.21,;26.63,-2.98,;28.17,-2.98,;29.08,-1.72,;30.54,-2.19,;30.53,-3.74,;31.67,-4.77,;31.35,-6.28,;29.88,-6.75,;28.74,-5.71,;29.07,-4.21,;25.3,-.67,;23.97,.1,)| Show InChI InChI=1S/C46H59N11O7/c47-39(58)29-57-24-12-23-50-40(59)20-9-10-21-41(60)53-36(25-30-13-3-1-4-14-30)43(62)55-37(26-31-15-5-2-6-16-31)44(63)54-35(19-11-22-51-46(48)49)42(61)56-38(45(57)64)27-32-28-52-34-18-8-7-17-33(32)34/h1-8,13-18,28,35-38,52H,9-12,19-27,29H2,(H2,47,58)(H,50,59)(H,53,60)(H,54,63)(H,55,62)(H,56,61)(H4,48,49,51)/t35-,36-,37+,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from MC4R |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50374673

(CHEMBL408398)Show SMILES NC(=O)CN1CCNC(=O)CCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:26.27,wD:15.15,48.50,37.39,(3.62,2.67,;2.29,1.89,;.96,2.65,;2.3,.35,;.97,-.43,;.99,1.1,;-.35,1.87,;-.35,3.41,;-1.68,4.18,;-1.68,5.72,;-3.01,3.41,;-3.01,1.87,;-4.35,1.1,;-5.68,1.87,;-4.35,-.44,;-5.68,-1.21,;-7.01,-.44,;-8.35,-1.21,;-9.68,-.43,;-11.01,-1.19,;-11.01,-2.74,;-9.67,-3.51,;-8.34,-2.73,;-5.68,-2.75,;-7.01,-3.52,;-4.35,-3.52,;-4.35,-5.06,;-5.68,-5.83,;-5.68,-7.37,;-7.02,-8.13,;-7.02,-9.67,;-5.69,-10.44,;-4.35,-9.66,;-4.36,-8.12,;-3.01,-5.83,;-3.01,-7.37,;-1.68,-5.06,;-.35,-5.83,;-.35,-7.37,;.99,-8.14,;.99,-9.68,;2.32,-10.45,;2.32,-11.99,;3.65,-12.76,;.99,-12.76,;.99,-5.06,;2.32,-5.83,;.99,-3.52,;2.32,-2.75,;3.65,-3.52,;4.99,-2.75,;5.16,-1.2,;6.66,-.89,;7.42,-2.23,;8.92,-2.56,;9.39,-4.03,;8.35,-5.17,;6.85,-4.84,;6.38,-3.37,;2.32,-1.21,;3.65,-.44,)| Show InChI InChI=1S/C43H53N11O7/c44-36(55)26-54-21-20-47-37(56)17-18-38(57)50-33(22-27-10-3-1-4-11-27)40(59)52-34(23-28-12-5-2-6-13-28)41(60)51-32(16-9-19-48-43(45)46)39(58)53-35(42(54)61)24-29-25-49-31-15-8-7-14-30(29)31/h1-8,10-15,25,32-35,49H,9,16-24,26H2,(H2,44,55)(H,47,56)(H,50,57)(H,51,60)(H,52,59)(H,53,58)(H4,45,46,48)/t32-,33-,34+,35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from MC4R |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50374670

(CHEMBL270923)Show SMILES NC(=O)CN1CCNC(=O)c2ccccc2C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:30.32,wD:52.55,19.20,41.44,(6.67,-17.07,;5.13,-17.07,;4.36,-15.74,;4.37,-18.41,;2.83,-18.41,;2.05,-17.08,;.51,-17.09,;-.25,-18.42,;-1.79,-18.43,;-2.56,-19.76,;-2.57,-17.1,;-1.81,-15.76,;-2.59,-14.43,;-4.14,-14.45,;-4.89,-15.78,;-4.11,-17.1,;-4.87,-18.43,;-6.41,-18.44,;-4.1,-19.76,;-5.43,-20.54,;-6.76,-19.76,;-8.09,-20.54,;-9.42,-19.75,;-10.76,-20.53,;-10.76,-22.07,;-9.41,-22.83,;-8.09,-22.06,;-5.43,-22.08,;-6.76,-22.84,;-4.1,-22.84,;-4.1,-24.38,;-5.43,-25.16,;-5.43,-26.7,;-6.77,-27.46,;-6.77,-28.99,;-5.43,-29.77,;-4.1,-28.99,;-4.1,-27.45,;-2.76,-25.16,;-2.76,-26.7,;-1.42,-24.38,;-.09,-25.16,;-.09,-26.7,;1.24,-27.46,;1.24,-29,;2.58,-29.78,;2.58,-31.32,;3.91,-32.08,;1.24,-32.08,;1.24,-24.38,;2.58,-25.16,;1.24,-22.84,;2.58,-22.08,;3.91,-22.84,;5.24,-22.08,;5.41,-20.53,;6.91,-20.22,;7.67,-21.56,;9.18,-21.89,;9.64,-23.36,;8.6,-24.49,;7.1,-24.16,;6.63,-22.7,;2.06,-19.75,;.52,-19.75,)| Show InChI InChI=1S/C47H53N11O7/c48-40(59)28-58-23-22-51-41(60)33-17-7-8-18-34(33)42(61)55-37(24-29-12-3-1-4-13-29)45(64)56-38(25-30-14-5-2-6-15-30)44(63)54-36(20-11-21-52-47(49)50)43(62)57-39(46(58)65)26-31-27-53-35-19-10-9-16-32(31)35/h1-10,12-19,27,36-39,53H,11,20-26,28H2,(H2,48,59)(H,51,60)(H,54,63)(H,55,61)(H,56,64)(H,57,62)(H4,49,50,52)/t36-,37-,38+,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from MC4R |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50374673

(CHEMBL408398)Show SMILES NC(=O)CN1CCNC(=O)CCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:26.27,wD:15.15,48.50,37.39,(3.62,2.67,;2.29,1.89,;.96,2.65,;2.3,.35,;.97,-.43,;.99,1.1,;-.35,1.87,;-.35,3.41,;-1.68,4.18,;-1.68,5.72,;-3.01,3.41,;-3.01,1.87,;-4.35,1.1,;-5.68,1.87,;-4.35,-.44,;-5.68,-1.21,;-7.01,-.44,;-8.35,-1.21,;-9.68,-.43,;-11.01,-1.19,;-11.01,-2.74,;-9.67,-3.51,;-8.34,-2.73,;-5.68,-2.75,;-7.01,-3.52,;-4.35,-3.52,;-4.35,-5.06,;-5.68,-5.83,;-5.68,-7.37,;-7.02,-8.13,;-7.02,-9.67,;-5.69,-10.44,;-4.35,-9.66,;-4.36,-8.12,;-3.01,-5.83,;-3.01,-7.37,;-1.68,-5.06,;-.35,-5.83,;-.35,-7.37,;.99,-8.14,;.99,-9.68,;2.32,-10.45,;2.32,-11.99,;3.65,-12.76,;.99,-12.76,;.99,-5.06,;2.32,-5.83,;.99,-3.52,;2.32,-2.75,;3.65,-3.52,;4.99,-2.75,;5.16,-1.2,;6.66,-.89,;7.42,-2.23,;8.92,-2.56,;9.39,-4.03,;8.35,-5.17,;6.85,-4.84,;6.38,-3.37,;2.32,-1.21,;3.65,-.44,)| Show InChI InChI=1S/C43H53N11O7/c44-36(55)26-54-21-20-47-37(56)17-18-38(57)50-33(22-27-10-3-1-4-11-27)40(59)52-34(23-28-12-5-2-6-13-28)41(60)51-32(16-9-19-48-43(45)46)39(58)53-35(42(54)61)24-29-25-49-31-15-8-7-14-30(29)31/h1-8,10-15,25,32-35,49H,9,16-24,26H2,(H2,44,55)(H,47,56)(H,50,57)(H,51,60)(H,52,59)(H,53,58)(H4,45,46,48)/t32-,33-,34+,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 770 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC3R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Mus musculus) | BDBM50374673

(CHEMBL408398)Show SMILES NC(=O)CN1CCNC(=O)CCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:26.27,wD:15.15,48.50,37.39,(3.62,2.67,;2.29,1.89,;.96,2.65,;2.3,.35,;.97,-.43,;.99,1.1,;-.35,1.87,;-.35,3.41,;-1.68,4.18,;-1.68,5.72,;-3.01,3.41,;-3.01,1.87,;-4.35,1.1,;-5.68,1.87,;-4.35,-.44,;-5.68,-1.21,;-7.01,-.44,;-8.35,-1.21,;-9.68,-.43,;-11.01,-1.19,;-11.01,-2.74,;-9.67,-3.51,;-8.34,-2.73,;-5.68,-2.75,;-7.01,-3.52,;-4.35,-3.52,;-4.35,-5.06,;-5.68,-5.83,;-5.68,-7.37,;-7.02,-8.13,;-7.02,-9.67,;-5.69,-10.44,;-4.35,-9.66,;-4.36,-8.12,;-3.01,-5.83,;-3.01,-7.37,;-1.68,-5.06,;-.35,-5.83,;-.35,-7.37,;.99,-8.14,;.99,-9.68,;2.32,-10.45,;2.32,-11.99,;3.65,-12.76,;.99,-12.76,;.99,-5.06,;2.32,-5.83,;.99,-3.52,;2.32,-2.75,;3.65,-3.52,;4.99,-2.75,;5.16,-1.2,;6.66,-.89,;7.42,-2.23,;8.92,-2.56,;9.39,-4.03,;8.35,-5.17,;6.85,-4.84,;6.38,-3.37,;2.32,-1.21,;3.65,-.44,)| Show InChI InChI=1S/C43H53N11O7/c44-36(55)26-54-21-20-47-37(56)17-18-38(57)50-33(22-27-10-3-1-4-11-27)40(59)52-34(23-28-12-5-2-6-13-28)41(60)51-32(16-9-19-48-43(45)46)39(58)53-35(42(54)61)24-29-25-49-31-15-8-7-14-30(29)31/h1-8,10-15,25,32-35,49H,9,16-24,26H2,(H2,44,55)(H,47,56)(H,50,57)(H,51,60)(H,52,59)(H,53,58)(H4,45,46,48)/t32-,33-,34+,35-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 64 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC1R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Mus musculus) | BDBM50374672

(CHEMBL271586)Show SMILES NC(=O)CN1CCCNC(=O)CCCCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:29.30,wD:51.53,18.18,40.42,(30.64,-.68,;29.3,.09,;29.31,1.63,;27.97,-.68,;26.64,.1,;26.64,1.64,;27.98,2.4,;27.98,3.94,;25.3,3.95,;23.96,4.72,;23.96,6.26,;22.63,3.95,;21.3,4.72,;19.96,3.95,;19.96,2.41,;18.63,1.64,;17.29,2.41,;18.63,.1,;17.29,-.67,;15.96,.1,;14.63,-.67,;13.3,.11,;11.96,-.66,;11.96,-2.2,;13.31,-2.97,;14.63,-2.2,;17.29,-2.21,;15.96,-2.98,;18.63,-2.98,;18.63,-4.52,;17.29,-5.29,;17.29,-6.83,;15.95,-7.59,;15.95,-9.13,;17.29,-9.9,;18.63,-9.12,;18.62,-7.59,;19.96,-5.29,;19.96,-6.83,;21.3,-4.52,;22.63,-5.29,;22.63,-6.83,;23.96,-7.6,;23.96,-9.14,;25.3,-9.91,;25.3,-11.45,;26.63,-12.22,;23.96,-12.22,;23.96,-4.52,;25.3,-5.29,;23.96,-2.98,;25.3,-2.21,;26.63,-2.98,;28.17,-2.98,;29.08,-1.72,;30.54,-2.19,;30.53,-3.74,;31.67,-4.77,;31.35,-6.28,;29.88,-6.75,;28.74,-5.71,;29.07,-4.21,;25.3,-.67,;23.97,.1,)| Show InChI InChI=1S/C46H59N11O7/c47-39(58)29-57-24-12-23-50-40(59)20-9-10-21-41(60)53-36(25-30-13-3-1-4-14-30)43(62)55-37(26-31-15-5-2-6-16-31)44(63)54-35(19-11-22-51-46(48)49)42(61)56-38(45(57)64)27-32-28-52-34-18-8-7-17-33(32)34/h1-8,13-18,28,35-38,52H,9-12,19-27,29H2,(H2,47,58)(H,50,59)(H,53,60)(H,54,63)(H,55,62)(H,56,61)(H4,48,49,51)/t35-,36-,37+,38-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC1R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Mus musculus) | BDBM50374669

(CHEMBL406985)Show SMILES NC(=O)CN1CCNC(=O)CCCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:27.28,wD:16.16,49.51,38.40,(13.37,-36.69,;12.04,-35.93,;12.04,-34.39,;10.72,-36.69,;10.72,-38.22,;8.06,-38.19,;8.05,-36.66,;9.38,-35.89,;9.37,-34.36,;10.7,-33.58,;8.04,-33.6,;6.71,-34.37,;6.71,-35.91,;5.38,-36.68,;4.04,-35.91,;5.38,-38.22,;4.04,-38.99,;2.71,-38.22,;1.37,-38.99,;.04,-38.21,;-1.29,-38.98,;-1.29,-40.52,;.05,-41.29,;1.38,-40.52,;4.04,-40.53,;2.71,-41.3,;5.38,-41.3,;5.38,-42.84,;4.04,-43.61,;4.04,-45.15,;2.7,-45.91,;2.7,-47.45,;4.04,-48.22,;5.38,-47.44,;5.37,-45.91,;6.71,-43.61,;6.71,-45.15,;8.04,-42.84,;9.38,-43.61,;9.38,-45.15,;10.71,-45.92,;10.71,-47.46,;12.05,-48.23,;12.05,-49.77,;13.38,-50.54,;10.71,-50.54,;10.71,-42.84,;12.05,-43.61,;10.71,-41.3,;12.05,-40.53,;13.38,-41.3,;14.72,-40.53,;14.88,-38.98,;16.39,-38.67,;17.14,-40.02,;18.64,-40.35,;19.12,-41.81,;18.08,-42.95,;16.57,-42.62,;16.11,-41.15,;12.05,-38.99,;13.38,-38.22,)| Show InChI InChI=1S/C44H55N11O7/c45-37(56)27-55-22-21-48-38(57)18-9-19-39(58)51-34(23-28-11-3-1-4-12-28)41(60)53-35(24-29-13-5-2-6-14-29)42(61)52-33(17-10-20-49-44(46)47)40(59)54-36(43(55)62)25-30-26-50-32-16-8-7-15-31(30)32/h1-8,11-16,26,33-36,50H,9-10,17-25,27H2,(H2,45,56)(H,48,57)(H,51,58)(H,52,61)(H,53,60)(H,54,59)(H4,46,47,49)/t33-,34-,35+,36-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC1R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50374671

(CHEMBL272660)Show SMILES NC(=O)CN1CCCNC(=O)CCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:27.28,wD:16.16,49.51,38.40,(28.48,-20.35,;27.16,-19.57,;27.17,-18.03,;25.81,-20.32,;25.8,-21.86,;23.64,-21.92,;23.15,-20.32,;24.48,-19.55,;24.48,-18.01,;23.15,-17.24,;23.15,-15.7,;21.81,-18.01,;21.81,-19.55,;20.48,-20.32,;19.15,-19.55,;20.48,-21.86,;19.15,-22.63,;17.81,-21.86,;16.48,-22.63,;15.15,-21.85,;13.82,-22.62,;13.81,-24.16,;15.16,-24.93,;16.48,-24.16,;19.15,-24.17,;17.81,-24.94,;20.48,-24.94,;20.48,-26.48,;19.15,-27.25,;19.15,-28.79,;17.81,-29.56,;17.8,-31.09,;19.14,-31.87,;20.48,-31.09,;20.47,-29.55,;21.81,-27.25,;21.81,-28.79,;23.15,-26.48,;24.48,-27.25,;24.48,-28.79,;25.81,-29.56,;25.81,-31.1,;27.15,-31.87,;27.15,-33.41,;28.48,-34.18,;25.81,-34.18,;25.81,-26.48,;27.15,-27.25,;25.81,-24.94,;27.15,-24.17,;28.48,-24.94,;29.82,-24.17,;29.98,-22.63,;31.49,-22.31,;32.24,-23.66,;33.75,-23.99,;34.21,-25.46,;33.18,-26.59,;31.67,-26.26,;31.21,-24.8,;27.15,-22.63,;28.48,-21.86,)| Show InChI InChI=1S/C44H55N11O7/c45-37(56)27-55-22-10-21-48-38(57)18-19-39(58)51-34(23-28-11-3-1-4-12-28)41(60)53-35(24-29-13-5-2-6-14-29)42(61)52-33(17-9-20-49-44(46)47)40(59)54-36(43(55)62)25-30-26-50-32-16-8-7-15-31(30)32/h1-8,11-16,26,33-36,50H,9-10,17-25,27H2,(H2,45,56)(H,48,57)(H,51,58)(H,52,61)(H,53,60)(H,54,59)(H4,46,47,49)/t33-,34-,35+,36-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC5R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50374670

(CHEMBL270923)Show SMILES NC(=O)CN1CCNC(=O)c2ccccc2C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:30.32,wD:52.55,19.20,41.44,(6.67,-17.07,;5.13,-17.07,;4.36,-15.74,;4.37,-18.41,;2.83,-18.41,;2.05,-17.08,;.51,-17.09,;-.25,-18.42,;-1.79,-18.43,;-2.56,-19.76,;-2.57,-17.1,;-1.81,-15.76,;-2.59,-14.43,;-4.14,-14.45,;-4.89,-15.78,;-4.11,-17.1,;-4.87,-18.43,;-6.41,-18.44,;-4.1,-19.76,;-5.43,-20.54,;-6.76,-19.76,;-8.09,-20.54,;-9.42,-19.75,;-10.76,-20.53,;-10.76,-22.07,;-9.41,-22.83,;-8.09,-22.06,;-5.43,-22.08,;-6.76,-22.84,;-4.1,-22.84,;-4.1,-24.38,;-5.43,-25.16,;-5.43,-26.7,;-6.77,-27.46,;-6.77,-28.99,;-5.43,-29.77,;-4.1,-28.99,;-4.1,-27.45,;-2.76,-25.16,;-2.76,-26.7,;-1.42,-24.38,;-.09,-25.16,;-.09,-26.7,;1.24,-27.46,;1.24,-29,;2.58,-29.78,;2.58,-31.32,;3.91,-32.08,;1.24,-32.08,;1.24,-24.38,;2.58,-25.16,;1.24,-22.84,;2.58,-22.08,;3.91,-22.84,;5.24,-22.08,;5.41,-20.53,;6.91,-20.22,;7.67,-21.56,;9.18,-21.89,;9.64,-23.36,;8.6,-24.49,;7.1,-24.16,;6.63,-22.7,;2.06,-19.75,;.52,-19.75,)| Show InChI InChI=1S/C47H53N11O7/c48-40(59)28-58-23-22-51-41(60)33-17-7-8-18-34(33)42(61)55-37(24-29-12-3-1-4-13-29)45(64)56-38(25-30-14-5-2-6-15-30)44(63)54-36(20-11-21-52-47(49)50)43(62)57-39(46(58)65)26-31-27-53-35-19-10-9-16-32(31)35/h1-10,12-19,27,36-39,53H,11,20-26,28H2,(H2,48,59)(H,51,60)(H,54,63)(H,55,61)(H,56,64)(H,57,62)(H4,49,50,52)/t36-,37-,38+,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC4R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50121900
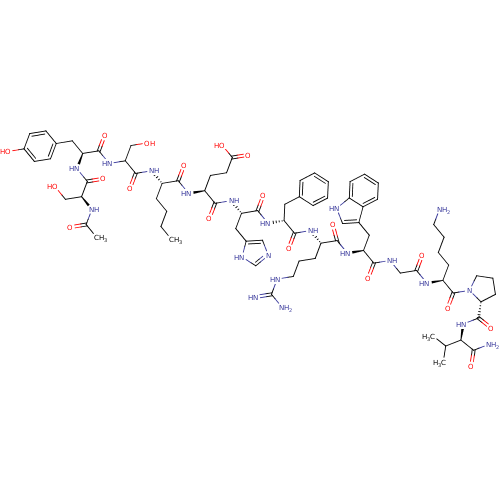
(Ac-Ser-Try-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pr...)Show SMILES CCCC[C@H](NC(=O)C(CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@@H]1C(=O)N[C@H](C(C)C)C(N)=O Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61?,62+,65+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC4R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50374669

(CHEMBL406985)Show SMILES NC(=O)CN1CCNC(=O)CCCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:27.28,wD:16.16,49.51,38.40,(13.37,-36.69,;12.04,-35.93,;12.04,-34.39,;10.72,-36.69,;10.72,-38.22,;8.06,-38.19,;8.05,-36.66,;9.38,-35.89,;9.37,-34.36,;10.7,-33.58,;8.04,-33.6,;6.71,-34.37,;6.71,-35.91,;5.38,-36.68,;4.04,-35.91,;5.38,-38.22,;4.04,-38.99,;2.71,-38.22,;1.37,-38.99,;.04,-38.21,;-1.29,-38.98,;-1.29,-40.52,;.05,-41.29,;1.38,-40.52,;4.04,-40.53,;2.71,-41.3,;5.38,-41.3,;5.38,-42.84,;4.04,-43.61,;4.04,-45.15,;2.7,-45.91,;2.7,-47.45,;4.04,-48.22,;5.38,-47.44,;5.37,-45.91,;6.71,-43.61,;6.71,-45.15,;8.04,-42.84,;9.38,-43.61,;9.38,-45.15,;10.71,-45.92,;10.71,-47.46,;12.05,-48.23,;12.05,-49.77,;13.38,-50.54,;10.71,-50.54,;10.71,-42.84,;12.05,-43.61,;10.71,-41.3,;12.05,-40.53,;13.38,-41.3,;14.72,-40.53,;14.88,-38.98,;16.39,-38.67,;17.14,-40.02,;18.64,-40.35,;19.12,-41.81,;18.08,-42.95,;16.57,-42.62,;16.11,-41.15,;12.05,-38.99,;13.38,-38.22,)| Show InChI InChI=1S/C44H55N11O7/c45-37(56)27-55-22-21-48-38(57)18-9-19-39(58)51-34(23-28-11-3-1-4-12-28)41(60)53-35(24-29-13-5-2-6-14-29)42(61)52-33(17-10-20-49-44(46)47)40(59)54-36(43(55)62)25-30-26-50-32-16-8-7-15-31(30)32/h1-8,11-16,26,33-36,50H,9-10,17-25,27H2,(H2,45,56)(H,48,57)(H,51,58)(H,52,61)(H,53,60)(H,54,59)(H4,46,47,49)/t33-,34-,35+,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC3R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50374673

(CHEMBL408398)Show SMILES NC(=O)CN1CCNC(=O)CCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:26.27,wD:15.15,48.50,37.39,(3.62,2.67,;2.29,1.89,;.96,2.65,;2.3,.35,;.97,-.43,;.99,1.1,;-.35,1.87,;-.35,3.41,;-1.68,4.18,;-1.68,5.72,;-3.01,3.41,;-3.01,1.87,;-4.35,1.1,;-5.68,1.87,;-4.35,-.44,;-5.68,-1.21,;-7.01,-.44,;-8.35,-1.21,;-9.68,-.43,;-11.01,-1.19,;-11.01,-2.74,;-9.67,-3.51,;-8.34,-2.73,;-5.68,-2.75,;-7.01,-3.52,;-4.35,-3.52,;-4.35,-5.06,;-5.68,-5.83,;-5.68,-7.37,;-7.02,-8.13,;-7.02,-9.67,;-5.69,-10.44,;-4.35,-9.66,;-4.36,-8.12,;-3.01,-5.83,;-3.01,-7.37,;-1.68,-5.06,;-.35,-5.83,;-.35,-7.37,;.99,-8.14,;.99,-9.68,;2.32,-10.45,;2.32,-11.99,;3.65,-12.76,;.99,-12.76,;.99,-5.06,;2.32,-5.83,;.99,-3.52,;2.32,-2.75,;3.65,-3.52,;4.99,-2.75,;5.16,-1.2,;6.66,-.89,;7.42,-2.23,;8.92,-2.56,;9.39,-4.03,;8.35,-5.17,;6.85,-4.84,;6.38,-3.37,;2.32,-1.21,;3.65,-.44,)| Show InChI InChI=1S/C43H53N11O7/c44-36(55)26-54-21-20-47-37(56)17-18-38(57)50-33(22-27-10-3-1-4-11-27)40(59)52-34(23-28-12-5-2-6-13-28)41(60)51-32(16-9-19-48-43(45)46)39(58)53-35(42(54)61)24-29-25-49-31-15-8-7-14-30(29)31/h1-8,10-15,25,32-35,49H,9,16-24,26H2,(H2,44,55)(H,47,56)(H,50,57)(H,51,60)(H,52,59)(H,53,58)(H4,45,46,48)/t32-,33-,34+,35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC4R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50374673

(CHEMBL408398)Show SMILES NC(=O)CN1CCNC(=O)CCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:26.27,wD:15.15,48.50,37.39,(3.62,2.67,;2.29,1.89,;.96,2.65,;2.3,.35,;.97,-.43,;.99,1.1,;-.35,1.87,;-.35,3.41,;-1.68,4.18,;-1.68,5.72,;-3.01,3.41,;-3.01,1.87,;-4.35,1.1,;-5.68,1.87,;-4.35,-.44,;-5.68,-1.21,;-7.01,-.44,;-8.35,-1.21,;-9.68,-.43,;-11.01,-1.19,;-11.01,-2.74,;-9.67,-3.51,;-8.34,-2.73,;-5.68,-2.75,;-7.01,-3.52,;-4.35,-3.52,;-4.35,-5.06,;-5.68,-5.83,;-5.68,-7.37,;-7.02,-8.13,;-7.02,-9.67,;-5.69,-10.44,;-4.35,-9.66,;-4.36,-8.12,;-3.01,-5.83,;-3.01,-7.37,;-1.68,-5.06,;-.35,-5.83,;-.35,-7.37,;.99,-8.14,;.99,-9.68,;2.32,-10.45,;2.32,-11.99,;3.65,-12.76,;.99,-12.76,;.99,-5.06,;2.32,-5.83,;.99,-3.52,;2.32,-2.75,;3.65,-3.52,;4.99,-2.75,;5.16,-1.2,;6.66,-.89,;7.42,-2.23,;8.92,-2.56,;9.39,-4.03,;8.35,-5.17,;6.85,-4.84,;6.38,-3.37,;2.32,-1.21,;3.65,-.44,)| Show InChI InChI=1S/C43H53N11O7/c44-36(55)26-54-21-20-47-37(56)17-18-38(57)50-33(22-27-10-3-1-4-11-27)40(59)52-34(23-28-12-5-2-6-13-28)41(60)51-32(16-9-19-48-43(45)46)39(58)53-35(42(54)61)24-29-25-49-31-15-8-7-14-30(29)31/h1-8,10-15,25,32-35,49H,9,16-24,26H2,(H2,44,55)(H,47,56)(H,50,57)(H,51,60)(H,52,59)(H,53,58)(H4,45,46,48)/t32-,33-,34+,35-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC5R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50121900

(Ac-Ser-Try-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pr...)Show SMILES CCCC[C@H](NC(=O)C(CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@@H]1C(=O)N[C@H](C(C)C)C(N)=O Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61?,62+,65+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC3R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50374671

(CHEMBL272660)Show SMILES NC(=O)CN1CCCNC(=O)CCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:27.28,wD:16.16,49.51,38.40,(28.48,-20.35,;27.16,-19.57,;27.17,-18.03,;25.81,-20.32,;25.8,-21.86,;23.64,-21.92,;23.15,-20.32,;24.48,-19.55,;24.48,-18.01,;23.15,-17.24,;23.15,-15.7,;21.81,-18.01,;21.81,-19.55,;20.48,-20.32,;19.15,-19.55,;20.48,-21.86,;19.15,-22.63,;17.81,-21.86,;16.48,-22.63,;15.15,-21.85,;13.82,-22.62,;13.81,-24.16,;15.16,-24.93,;16.48,-24.16,;19.15,-24.17,;17.81,-24.94,;20.48,-24.94,;20.48,-26.48,;19.15,-27.25,;19.15,-28.79,;17.81,-29.56,;17.8,-31.09,;19.14,-31.87,;20.48,-31.09,;20.47,-29.55,;21.81,-27.25,;21.81,-28.79,;23.15,-26.48,;24.48,-27.25,;24.48,-28.79,;25.81,-29.56,;25.81,-31.1,;27.15,-31.87,;27.15,-33.41,;28.48,-34.18,;25.81,-34.18,;25.81,-26.48,;27.15,-27.25,;25.81,-24.94,;27.15,-24.17,;28.48,-24.94,;29.82,-24.17,;29.98,-22.63,;31.49,-22.31,;32.24,-23.66,;33.75,-23.99,;34.21,-25.46,;33.18,-26.59,;31.67,-26.26,;31.21,-24.8,;27.15,-22.63,;28.48,-21.86,)| Show InChI InChI=1S/C44H55N11O7/c45-37(56)27-55-22-10-21-48-38(57)18-19-39(58)51-34(23-28-11-3-1-4-12-28)41(60)53-35(24-29-13-5-2-6-14-29)42(61)52-33(17-9-20-49-44(46)47)40(59)54-36(43(55)62)25-30-26-50-32-16-8-7-15-31(30)32/h1-8,11-16,26,33-36,50H,9-10,17-25,27H2,(H2,45,56)(H,48,57)(H,51,58)(H,52,61)(H,53,60)(H,54,59)(H4,46,47,49)/t33-,34-,35+,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC3R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50374670

(CHEMBL270923)Show SMILES NC(=O)CN1CCNC(=O)c2ccccc2C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:30.32,wD:52.55,19.20,41.44,(6.67,-17.07,;5.13,-17.07,;4.36,-15.74,;4.37,-18.41,;2.83,-18.41,;2.05,-17.08,;.51,-17.09,;-.25,-18.42,;-1.79,-18.43,;-2.56,-19.76,;-2.57,-17.1,;-1.81,-15.76,;-2.59,-14.43,;-4.14,-14.45,;-4.89,-15.78,;-4.11,-17.1,;-4.87,-18.43,;-6.41,-18.44,;-4.1,-19.76,;-5.43,-20.54,;-6.76,-19.76,;-8.09,-20.54,;-9.42,-19.75,;-10.76,-20.53,;-10.76,-22.07,;-9.41,-22.83,;-8.09,-22.06,;-5.43,-22.08,;-6.76,-22.84,;-4.1,-22.84,;-4.1,-24.38,;-5.43,-25.16,;-5.43,-26.7,;-6.77,-27.46,;-6.77,-28.99,;-5.43,-29.77,;-4.1,-28.99,;-4.1,-27.45,;-2.76,-25.16,;-2.76,-26.7,;-1.42,-24.38,;-.09,-25.16,;-.09,-26.7,;1.24,-27.46,;1.24,-29,;2.58,-29.78,;2.58,-31.32,;3.91,-32.08,;1.24,-32.08,;1.24,-24.38,;2.58,-25.16,;1.24,-22.84,;2.58,-22.08,;3.91,-22.84,;5.24,-22.08,;5.41,-20.53,;6.91,-20.22,;7.67,-21.56,;9.18,-21.89,;9.64,-23.36,;8.6,-24.49,;7.1,-24.16,;6.63,-22.7,;2.06,-19.75,;.52,-19.75,)| Show InChI InChI=1S/C47H53N11O7/c48-40(59)28-58-23-22-51-41(60)33-17-7-8-18-34(33)42(61)55-37(24-29-12-3-1-4-13-29)45(64)56-38(25-30-14-5-2-6-15-30)44(63)54-36(20-11-21-52-47(49)50)43(62)57-39(46(58)65)26-31-27-53-35-19-10-9-16-32(31)35/h1-10,12-19,27,36-39,53H,11,20-26,28H2,(H2,48,59)(H,51,60)(H,54,63)(H,55,61)(H,56,64)(H,57,62)(H4,49,50,52)/t36-,37-,38+,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC3R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50374672

(CHEMBL271586)Show SMILES NC(=O)CN1CCCNC(=O)CCCCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:29.30,wD:51.53,18.18,40.42,(30.64,-.68,;29.3,.09,;29.31,1.63,;27.97,-.68,;26.64,.1,;26.64,1.64,;27.98,2.4,;27.98,3.94,;25.3,3.95,;23.96,4.72,;23.96,6.26,;22.63,3.95,;21.3,4.72,;19.96,3.95,;19.96,2.41,;18.63,1.64,;17.29,2.41,;18.63,.1,;17.29,-.67,;15.96,.1,;14.63,-.67,;13.3,.11,;11.96,-.66,;11.96,-2.2,;13.31,-2.97,;14.63,-2.2,;17.29,-2.21,;15.96,-2.98,;18.63,-2.98,;18.63,-4.52,;17.29,-5.29,;17.29,-6.83,;15.95,-7.59,;15.95,-9.13,;17.29,-9.9,;18.63,-9.12,;18.62,-7.59,;19.96,-5.29,;19.96,-6.83,;21.3,-4.52,;22.63,-5.29,;22.63,-6.83,;23.96,-7.6,;23.96,-9.14,;25.3,-9.91,;25.3,-11.45,;26.63,-12.22,;23.96,-12.22,;23.96,-4.52,;25.3,-5.29,;23.96,-2.98,;25.3,-2.21,;26.63,-2.98,;28.17,-2.98,;29.08,-1.72,;30.54,-2.19,;30.53,-3.74,;31.67,-4.77,;31.35,-6.28,;29.88,-6.75,;28.74,-5.71,;29.07,-4.21,;25.3,-.67,;23.97,.1,)| Show InChI InChI=1S/C46H59N11O7/c47-39(58)29-57-24-12-23-50-40(59)20-9-10-21-41(60)53-36(25-30-13-3-1-4-14-30)43(62)55-37(26-31-15-5-2-6-16-31)44(63)54-35(19-11-22-51-46(48)49)42(61)56-38(45(57)64)27-32-28-52-34-18-8-7-17-33(32)34/h1-8,13-18,28,35-38,52H,9-12,19-27,29H2,(H2,47,58)(H,50,59)(H,53,60)(H,54,63)(H,55,62)(H,56,61)(H4,48,49,51)/t35-,36-,37+,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.75E+3 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC3R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































