 Found 908 hits with Last Name = 'wittmann' and Initial = 'hj'
Found 908 hits with Last Name = 'wittmann' and Initial = 'hj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50013847
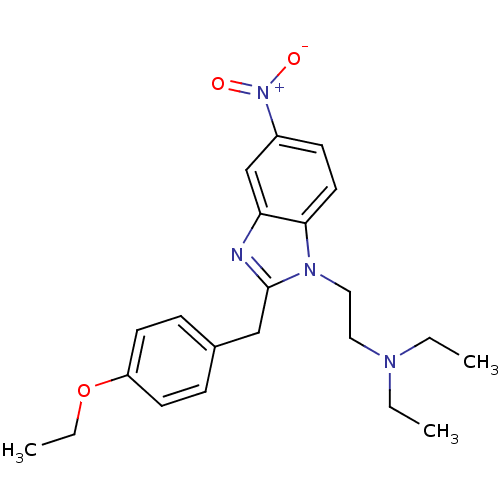
(CHEMBL312040 | Etonitazene | {2-[2-(4-Ethoxy-benzy...)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)[N+]([O-])=O)cc1 Show InChI InChI=1S/C22H28N4O3/c1-4-24(5-2)13-14-25-21-12-9-18(26(27)28)16-20(21)23-22(25)15-17-7-10-19(11-8-17)29-6-3/h7-12,16H,4-6,13-15H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Diprenorphine from human mu opioid receptor expressed in HEK293-TSA cell membranes after 4 hrs by scintillation counting method |
J Med Chem 61: 1646-1663 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01760
BindingDB Entry DOI: 10.7270/Q2RJ4MWK |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50116766

((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...)Show InChI InChI=1S/C10H17N3S/c1-2-5-12-7-3-4-8-9(6-7)14-10(11)13-8/h7,12H,2-6H2,1H3,(H2,11,13)/t7-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50601572

(CHEMBL5208722)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.C[C@@H](NC(=O)\N=C(/N)NCCCc1c[nH]cn1)c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM24226

(1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...)Show SMILES COc1ccc(CCN2CCC(CC2)Nc2nc3ccccc3n2Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C28H31FN4O/c1-34-25-12-8-21(9-13-25)14-17-32-18-15-24(16-19-32)30-28-31-26-4-2-3-5-27(26)33(28)20-22-6-10-23(29)11-7-22/h2-13,24H,14-20H2,1H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]mepyramine from human H1R expressed in Sf9 cells co-expressing RGS4 after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 6274-80 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.001
BindingDB Entry DOI: 10.7270/Q28K7BB7 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50601551
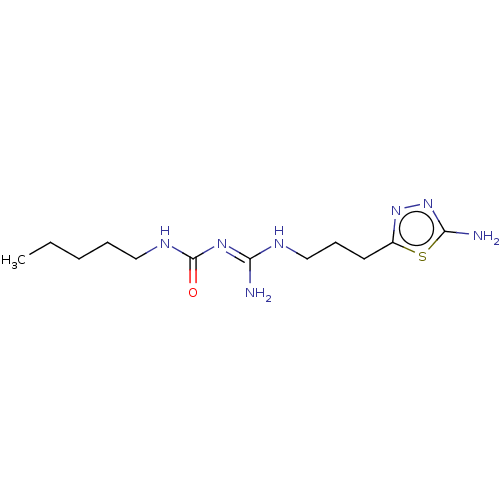
(CHEMBL5207281)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CCCCCNC(=O)\N=C(/N)NCCCc1nnc(N)s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50170164
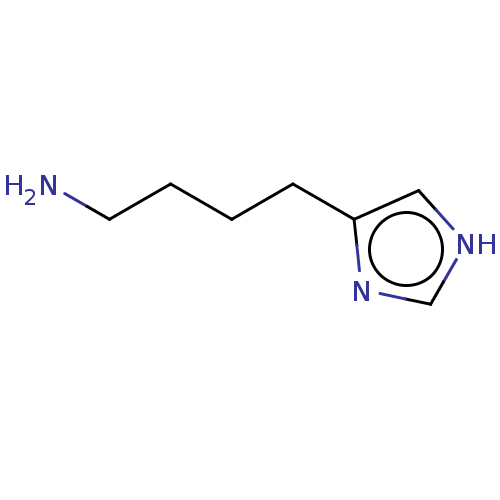
(Imbutamine)Show InChI InChI=1S/C7H13N3/c8-4-2-1-3-7-5-9-6-10-7/h5-6H,1-4,8H2,(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells incubated for 40 mins |
J Med Chem 59: 3452-70 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00120
BindingDB Entry DOI: 10.7270/Q23T9K42 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22567

(3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...)Show InChI InChI=1S/C17H23N3O/c1-19(2)12-13-20(17-6-4-5-11-18-17)14-15-7-9-16(21-3)10-8-15/h4-11H,12-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 4.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]mepyramine from human H1R expressed in Sf9 cells co-expressing RGS4 after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 6274-80 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.001
BindingDB Entry DOI: 10.7270/Q28K7BB7 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50601590
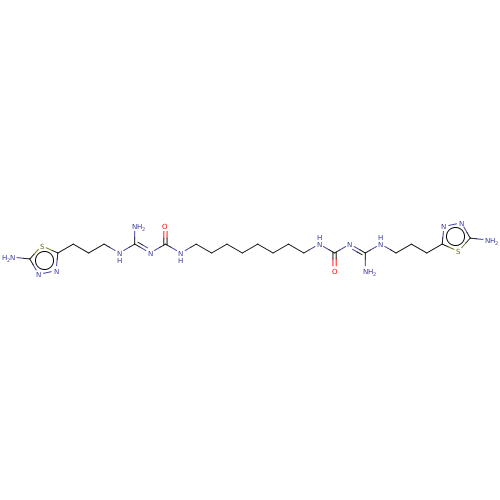
(CHEMBL5173079)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N\C(NCCCc1nnc(N)s1)=N/C(=O)NCCCCCCCCNC(=O)\N=C(/N)NCCCc1nnc(N)s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine 4 receptor expressed in HEK293T cell membranes incubated for 1 hr by radioligand binding assay |
Bioorg Med Chem Lett 26: 292-300 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.035
BindingDB Entry DOI: 10.7270/Q2Z03B0K |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50601567
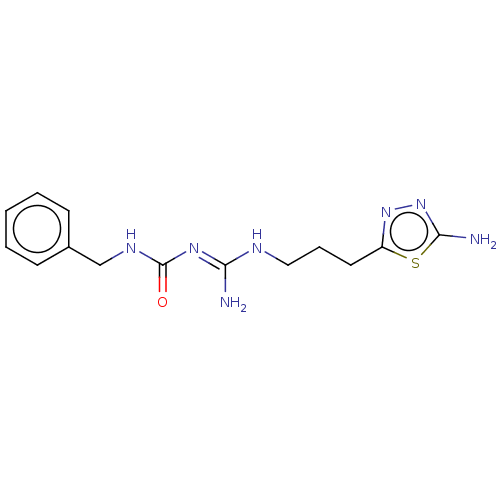
(CHEMBL5206565)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N\C(NCCCc1nnc(N)s1)=N/C(=O)NCc1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50601556

(CHEMBL5200771)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CCCCCCNC(=O)\N=C(/N)NCCCc1nnc(N)s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50601589

(CHEMBL5176229)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N\C(NCCCc1nnc(N)s1)=N/C(=O)NCCCCCCNC(=O)\N=C(/N)NCCCc1nnc(N)s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50419322

(CHEMBL1910386)Show SMILES Fc1ccc(Cn2c(NC3CCN(CCN4CCN(CC4)C(=O)c4cc5ccccc5[nH]4)CC3)nc3ccccc23)cc1 Show InChI InChI=1S/C34H38FN7O/c35-27-11-9-25(10-12-27)24-42-32-8-4-3-7-30(32)38-34(42)36-28-13-15-39(16-14-28)17-18-40-19-21-41(22-20-40)33(43)31-23-26-5-1-2-6-29(26)37-31/h1-12,23,28,37H,13-22,24H2,(H,36,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]mepyramine from human H1R expressed in Sf9 cells co-expressing RGS4 after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 6274-80 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.001
BindingDB Entry DOI: 10.7270/Q28K7BB7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50200170

(5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)N[C@H]2[C@@]3(C)CC[C@H](C3)C2(C)C)cc1 |r| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22-,27-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 90 mins |
J Med Chem 61: 1646-1663 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01760
BindingDB Entry DOI: 10.7270/Q2RJ4MWK |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50601572

(CHEMBL5208722)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.C[C@@H](NC(=O)\N=C(/N)NCCCc1c[nH]cn1)c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50601577
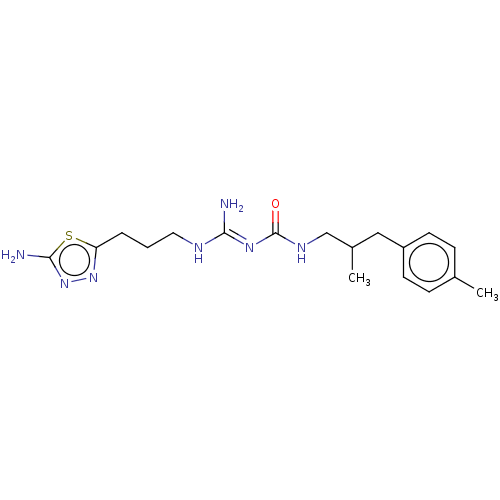
(CHEMBL5180504)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC(CNC(=O)\N=C(/N)NCCCc1nnc(N)s1)Cc1ccc(C)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50419326
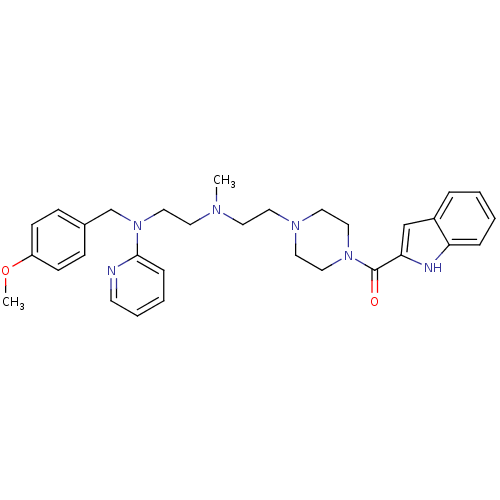
(CHEMBL1910381)Show SMILES COc1ccc(CN(CCN(C)CCN2CCN(CC2)C(=O)c2cc3ccccc3[nH]2)c2ccccn2)cc1 Show InChI InChI=1S/C31H38N6O2/c1-34(16-20-37(30-9-5-6-14-32-30)24-25-10-12-27(39-2)13-11-25)15-17-35-18-21-36(22-19-35)31(38)29-23-26-7-3-4-8-28(26)33-29/h3-14,23,33H,15-22,24H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]mepyramine from human H1R expressed in Sf9 cells co-expressing RGS4 after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 6274-80 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.001
BindingDB Entry DOI: 10.7270/Q28K7BB7 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50601576

(CHEMBL5184911)Show SMILES Cl.Cl.CC(NC(=O)\N=C(/N)NCCCc1nnc(N)s1)c1cccc(F)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50191289
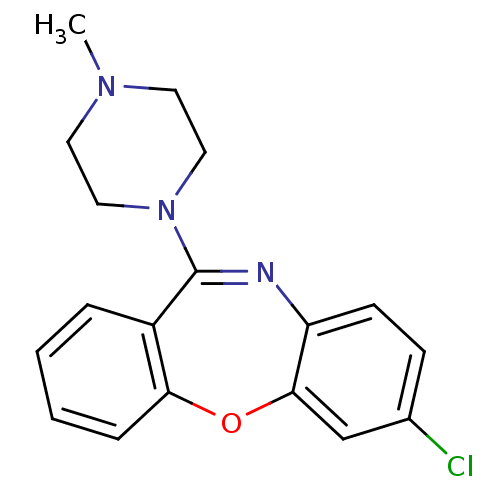
(7-chloro-11-(4-methyl-piperazin-1-yl)-dibenzo[b,f]...)Show SMILES CN1CCN(CC1)C1=Nc2ccc(Cl)cc2Oc2ccccc12 |t:8| Show InChI InChI=1S/C18H18ClN3O/c1-21-8-10-22(11-9-21)18-14-4-2-3-5-16(14)23-17-12-13(19)6-7-15(17)20-18/h2-7,12H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Displacement of [3H]mepyramine from human histamine 1 receptor expressed in Sf9 cell membranes by liquid scintillation counting |
Bioorg Med Chem Lett 26: 292-300 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.035
BindingDB Entry DOI: 10.7270/Q2Z03B0K |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50601572

(CHEMBL5208722)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.C[C@@H](NC(=O)\N=C(/N)NCCCc1c[nH]cn1)c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in human SK-N-MC cell membrane incubated for 60 mins |
J Med Chem 59: 3452-70 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00120
BindingDB Entry DOI: 10.7270/Q23T9K42 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50601573
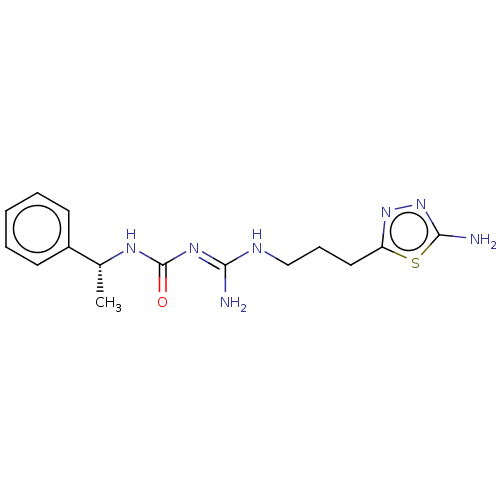
(CHEMBL5208845)Show SMILES Cl.Cl.C[C@@H](NC(=O)\N=C(/N)NCCCc1nnc(N)s1)c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50170164

(Imbutamine)Show InChI InChI=1S/C7H13N3/c8-4-2-1-3-7-5-9-6-10-7/h5-6H,1-4,8H2,(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in human SK-N-MC cell membrane incubated for 60 mins |
J Med Chem 59: 3452-70 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00120
BindingDB Entry DOI: 10.7270/Q23T9K42 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50017674

((2-Benzhydryloxy-ethyl)-dimethyl-amine | 2-(benzhy...)Show InChI InChI=1S/C17H21NO/c1-18(2)13-14-19-17(15-9-5-3-6-10-15)16-11-7-4-8-12-16/h3-12,17H,13-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 14.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]mepyramine from human H1R expressed in Sf9 cells co-expressing RGS4 after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 6274-80 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.001
BindingDB Entry DOI: 10.7270/Q28K7BB7 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50601583
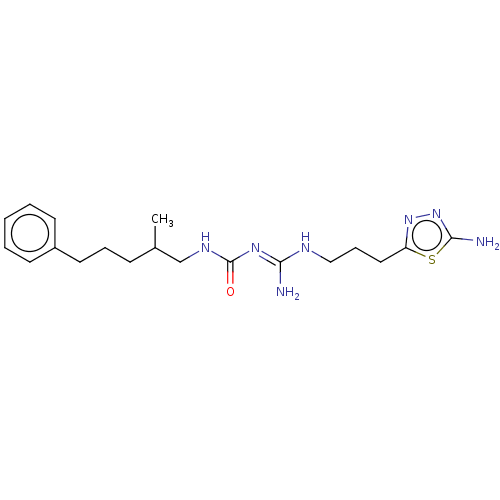
(CHEMBL5198795)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC(CCCc1ccccc1)CNC(=O)\N=C(/N)NCCCc1nnc(N)s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50170165
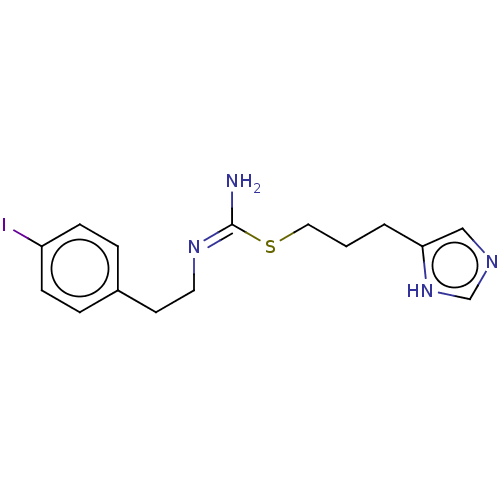
(Iodophenpropit)Show InChI InChI=1S/C15H19IN4S/c16-13-5-3-12(4-6-13)7-8-19-15(17)21-9-1-2-14-10-18-11-20-14/h3-6,10-11H,1-2,7-9H2,(H2,17,19)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in human SK-N-MC cell membrane incubated for 60 mins |
J Med Chem 59: 3452-70 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00120
BindingDB Entry DOI: 10.7270/Q23T9K42 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H4R expressed in Sf9 cells co-expressing RGS19, Galphai2 and Gbeta1gamma2 after 60 mins by liquid scintillat... |
Bioorg Med Chem Lett 21: 6274-80 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.001
BindingDB Entry DOI: 10.7270/Q28K7BB7 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50601563

(CHEMBL5204599)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC(CNC(=O)\N=C(/N)NCCCc1nnc(N)s1)C1CCCCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50200170

(5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)N[C@H]2[C@@]3(C)CC[C@H](C3)C2(C)C)cc1 |r| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22-,27-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method |
J Med Chem 61: 1646-1663 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01760
BindingDB Entry DOI: 10.7270/Q2RJ4MWK |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50601552

(CHEMBL5183205)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CCCCCNC(=O)\N=C(/N)NCCCc1cnc(N)s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50121205

(CHEBI:18295 | Histamine)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50116766

((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...)Show InChI InChI=1S/C10H17N3S/c1-2-5-12-7-3-4-8-9(6-7)14-10(11)13-8/h7,12H,2-6H2,1H3,(H2,11,13)/t7-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50121205

(CHEBI:18295 | Histamine)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50601568
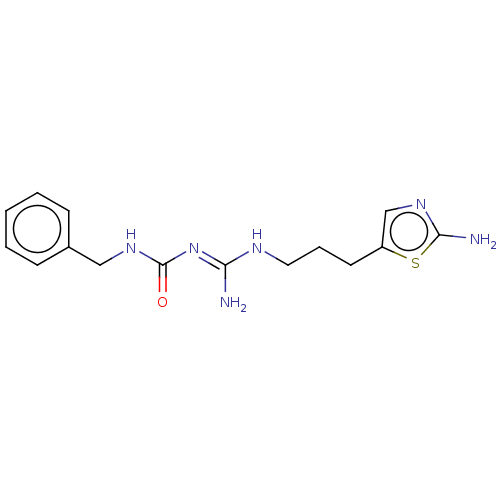
(CHEMBL5178472)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N\C(NCCCc1cnc(N)s1)=N/C(=O)NCc1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50601552

(CHEMBL5183205)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CCCCCNC(=O)\N=C(/N)NCCCc1cnc(N)s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50601559
(CHEMBL5176780)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.NCCCCCCCCNC(=O)\N=C(/N)NCCCc1nnc(N)s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Binding affinity to histamine 4 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 292-300 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.035
BindingDB Entry DOI: 10.7270/Q2Z03B0K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50244276
(2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benz...)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCC(C)C)C(=O)N(CC)CC)cc1 Show InChI InChI=1S/C26H35N3O2/c1-6-28(7-2)26(30)21-11-14-24-23(18-21)27-25(29(24)16-15-19(4)5)17-20-9-12-22(13-10-20)31-8-3/h9-14,18-19H,6-8,15-17H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method |
J Med Chem 61: 1646-1663 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01760
BindingDB Entry DOI: 10.7270/Q2RJ4MWK |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50412494

(CHEMBL452847 | VUF-10497)Show InChI InChI=1S/C18H20ClN5S/c1-23-6-8-24(9-7-23)18-21-16-5-4-13(19)11-15(16)17(22-18)20-12-14-3-2-10-25-14/h2-5,10-11H,6-9,12H2,1H3,(H,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine 4 receptor expressed in Sf9 cell membranes by liquid scintillation counting |
Bioorg Med Chem Lett 26: 292-300 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.035
BindingDB Entry DOI: 10.7270/Q2Z03B0K |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50601574
(CHEMBL5201074)Show SMILES Cl.Cl.C[C@@H](NC(=O)\N=C(/N)NCCCc1ncn[nH]1)c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50601589

(CHEMBL5176229)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N\C(NCCCc1nnc(N)s1)=N/C(=O)NCCCCCCNC(=O)\N=C(/N)NCCCc1nnc(N)s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50601579

(CHEMBL5202592)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC(CNC(=O)\N=C(/N)NCCCc1cnc(N)s1)Cc1ccc(C)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in sf9 cells co-expressing mammalian Galphai2 and Gbeta1gamma2 |
J Med Chem 59: 3452-70 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00120
BindingDB Entry DOI: 10.7270/Q23T9K42 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50601568

(CHEMBL5178472)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N\C(NCCCc1cnc(N)s1)=N/C(=O)NCc1ccccc1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50170126

(CHEMBL3805361)Show SMILES N#C\N=C(\NCCSc1ccccc1)NC[C@H]1CC[C@@H](C1)c1c[nH]cn1 |r| Show InChI InChI=1S/C19H24N6S/c20-13-24-19(22-8-9-26-17-4-2-1-3-5-17)23-11-15-6-7-16(10-15)18-12-21-14-25-18/h1-5,12,14-16H,6-11H2,(H,21,25)(H2,22,23,24)/t15-,16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-PI294 from human histamine H4 receptor expressed in sf9 cell membrane co-expressing mammalian Galphai2 and Gbeta1gamma2 incuba... |
J Med Chem 59: 3452-70 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00120
BindingDB Entry DOI: 10.7270/Q23T9K42 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50170126

(CHEMBL3805361)Show SMILES N#C\N=C(\NCCSc1ccccc1)NC[C@H]1CC[C@@H](C1)c1c[nH]cn1 |r| Show InChI InChI=1S/C19H24N6S/c20-13-24-19(22-8-9-26-17-4-2-1-3-5-17)23-11-15-6-7-16(10-15)18-12-21-14-25-18/h1-5,12,14-16H,6-11H2,(H,21,25)(H2,22,23,24)/t15-,16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-PI294 from human histamine H4 receptor expressed in sf9 cell membrane co-expressing mammalian Galphai2 and Gbeta1gamma2 incuba... |
J Med Chem 59: 3452-70 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00120
BindingDB Entry DOI: 10.7270/Q23T9K42 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50419325
(CHEMBL1910382)Show SMILES COc1ccc(CN(CCN(C)CCN2CCN(CC2)C(=O)c2cc3cc(Cl)ccc3[nH]2)c2ccccn2)cc1 Show InChI InChI=1S/C31H37ClN6O2/c1-35(14-18-38(30-5-3-4-12-33-30)23-24-6-9-27(40-2)10-7-24)13-15-36-16-19-37(20-17-36)31(39)29-22-25-21-26(32)8-11-28(25)34-29/h3-12,21-22,34H,13-20,23H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]mepyramine from human H1R expressed in Sf9 cells co-expressing RGS4 after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 6274-80 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.001
BindingDB Entry DOI: 10.7270/Q28K7BB7 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50191289

(7-chloro-11-(4-methyl-piperazin-1-yl)-dibenzo[b,f]...)Show SMILES CN1CCN(CC1)C1=Nc2ccc(Cl)cc2Oc2ccccc12 |t:8| Show InChI InChI=1S/C18H18ClN3O/c1-21-8-10-22(11-9-21)18-14-4-2-3-5-16(14)23-17-12-13(19)6-7-15(17)20-18/h2-7,12H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine 4 receptor expressed in Sf9 cell membranes by liquid scintillation counting |
Bioorg Med Chem Lett 26: 292-300 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.035
BindingDB Entry DOI: 10.7270/Q2Z03B0K |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50601582
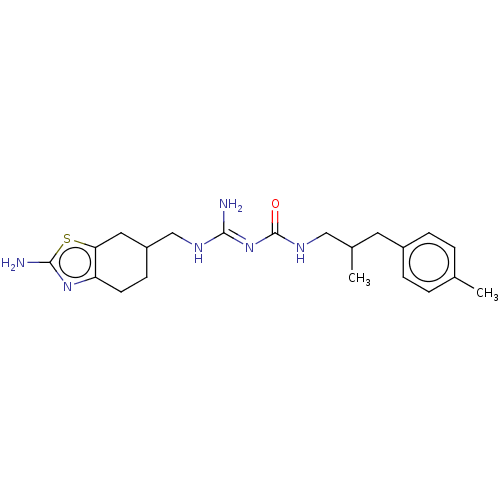
(CHEMBL5208113)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC(CNC(=O)\N=C(/N)NCC1CCc2nc(N)sc2C1)Cc1ccc(C)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50601586

(CHEMBL5194860)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC(CCCc1ccccc1)CNC(=O)\N=C(/N)NCC1CCc2nc(N)sc2C1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data