 Found 2231 hits with Last Name = 'wityak' and Initial = 'j'
Found 2231 hits with Last Name = 'wityak' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50288406
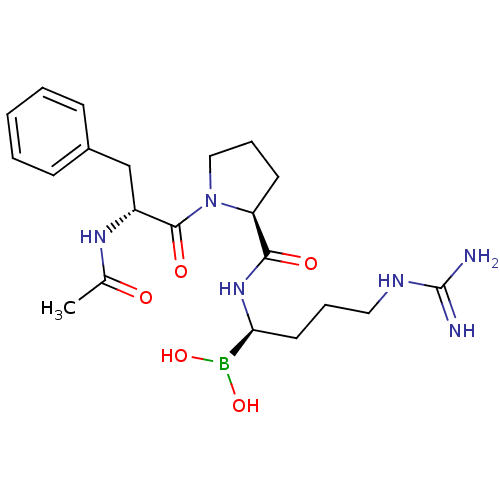
(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)B(O)O Show InChI InChI=1S/C21H33BN6O5/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25)/t16-,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine protease 1
(Homo sapiens (Human)) | BDBM50288406

(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)B(O)O Show InChI InChI=1S/C21H33BN6O5/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25)/t16-,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16318
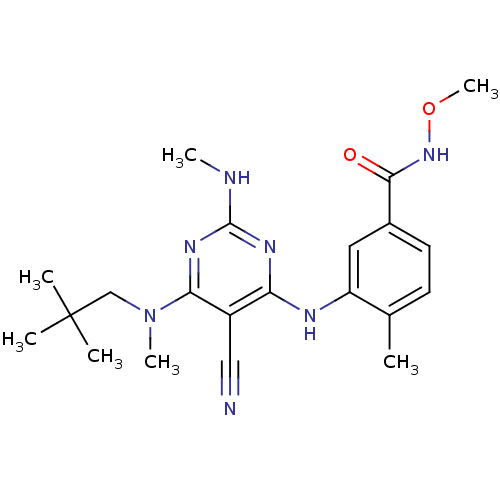
(3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]-...)Show SMILES CNc1nc(Nc2cc(ccc2C)C(=O)NOC)c(C#N)c(n1)N(C)CC(C)(C)C Show InChI InChI=1S/C21H29N7O2/c1-13-8-9-14(19(29)27-30-7)10-16(13)24-17-15(11-22)18(26-20(23-5)25-17)28(6)12-21(2,3)4/h8-10H,12H2,1-7H3,(H,27,29)(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0470 | -58.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16319
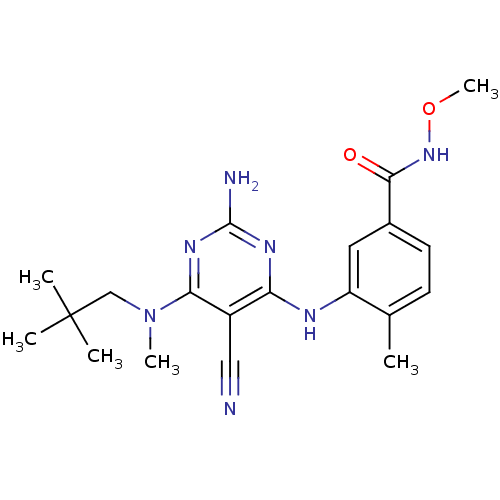
(3-({2-amino-5-cyano-6-[(2,2-dimethylpropyl)(methyl...)Show SMILES CONC(=O)c1ccc(C)c(Nc2nc(N)nc(N(C)CC(C)(C)C)c2C#N)c1 Show InChI InChI=1S/C20H27N7O2/c1-12-7-8-13(18(28)26-29-6)9-15(12)23-16-14(10-21)17(25-19(22)24-16)27(5)11-20(2,3)4/h7-9H,11H2,1-6H3,(H,26,28)(H3,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | -58.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16329
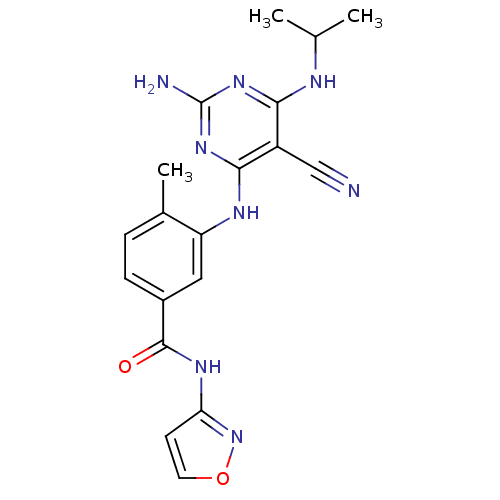
(3-({2-amino-5-cyano-6-[(1-methylethyl)amino]pyrimi...)Show SMILES CC(C)Nc1nc(N)nc(Nc2cc(ccc2C)C(=O)Nc2ccon2)c1C#N Show InChI InChI=1S/C19H20N8O2/c1-10(2)22-16-13(9-20)17(26-19(21)25-16)23-14-8-12(5-4-11(14)3)18(28)24-15-6-7-29-27-15/h4-8,10H,1-3H3,(H,24,27,28)(H4,21,22,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0570 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16317
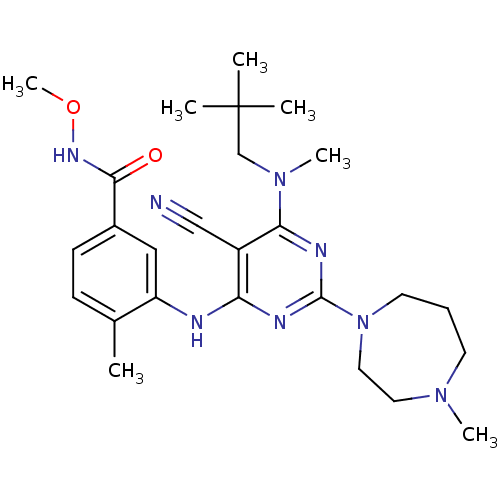
(3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]-...)Show SMILES CONC(=O)c1ccc(C)c(Nc2nc(nc(N(C)CC(C)(C)C)c2C#N)N2CCCN(C)CC2)c1 Show InChI InChI=1S/C26H38N8O2/c1-18-9-10-19(24(35)31-36-7)15-21(18)28-22-20(16-27)23(33(6)17-26(2,3)4)30-25(29-22)34-12-8-11-32(5)13-14-34/h9-10,15H,8,11-14,17H2,1-7H3,(H,31,35)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0570 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50289586

(3-Methyl-2'-sulfamoyl-biphenyl-4-carboxylic acid [...)Show SMILES Cc1cc(ccc1C(=O)N[C@@H](CCCNC(N)=N)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1)-c1ccccc1S(N)(=O)=O |TLB:30:28:25:23| Show InChI InChI=1S/C29H40BN5O5S/c1-17-14-18(21-8-5-6-9-22(21)41(33,37)38)11-12-20(17)26(36)35-25(10-7-13-34-27(31)32)30-39-24-16-19-15-23(28(19,2)3)29(24,4)40-30/h5-6,8-9,11-12,14,19,23-25H,7,10,13,15-16H2,1-4H3,(H,35,36)(H4,31,32,34)(H2,33,37,38)/t19?,23?,24-,25+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16320
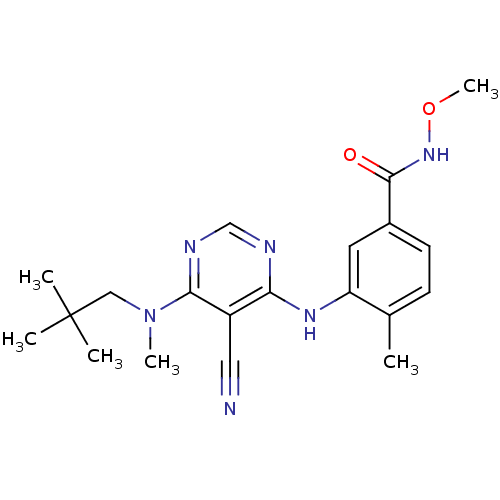
(3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]p...)Show SMILES CONC(=O)c1ccc(C)c(Nc2ncnc(N(C)CC(C)(C)C)c2C#N)c1 Show InChI InChI=1S/C20H26N6O2/c1-13-7-8-14(19(27)25-28-6)9-16(13)24-17-15(10-21)18(23-12-22-17)26(5)11-20(2,3)4/h7-9,12H,11H2,1-6H3,(H,25,27)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16330
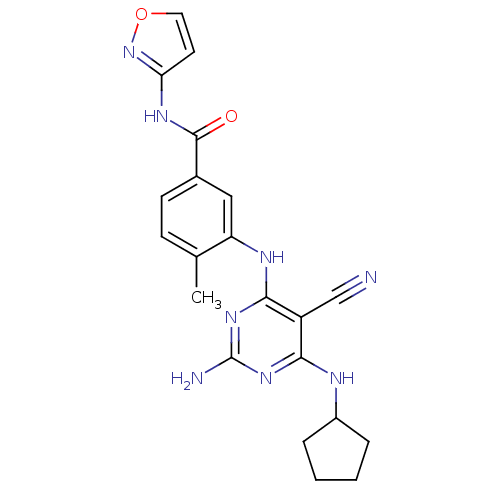
(3-{[2-amino-5-cyano-6-(cyclopentylamino)pyrimidin-...)Show SMILES Cc1ccc(cc1Nc1nc(N)nc(NC2CCCC2)c1C#N)C(=O)Nc1ccon1 Show InChI InChI=1S/C21H22N8O2/c1-12-6-7-13(20(30)26-17-8-9-31-29-17)10-16(12)25-19-15(11-22)18(27-21(23)28-19)24-14-4-2-3-5-14/h6-10,14H,2-5H2,1H3,(H,26,29,30)(H4,23,24,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50084682

(1-[3-(4-Carbamimidoyl-phenyl)-2-(2-methyl-1,2,3,4-...)Show SMILES Cc1cc(ccc1C(=O)N[C@@H](CCCNC(N)=N)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1)-c1ccccc1S(=O)(=O)NC(C)(C)C |TLB:30:28:25:23| Show InChI InChI=1S/C33H48BN5O5S/c1-20-17-21(24-11-8-9-12-25(24)45(41,42)39-31(2,3)4)14-15-23(20)29(40)38-28(13-10-16-37-30(35)36)34-43-27-19-22-18-26(32(22,5)6)33(27,7)44-34/h8-9,11-12,14-15,17,22,26-28,39H,10,13,16,18-19H2,1-7H3,(H,38,40)(H4,35,36,37)/t22?,26?,27-,28+,33+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289591
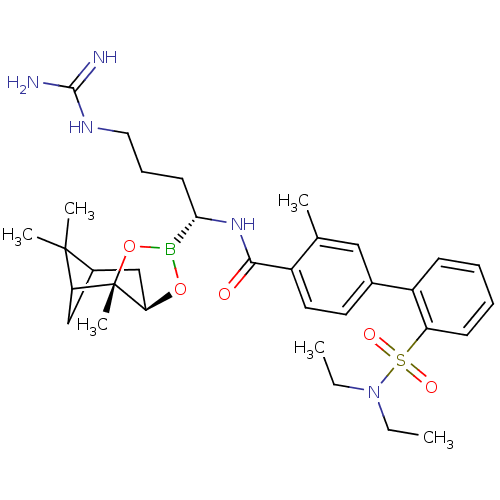
(2'-Diethylsulfamoyl-3-methyl-biphenyl-4-carboxylic...)Show SMILES CCN(CC)S(=O)(=O)c1ccccc1-c1ccc(C(=O)N[C@@H](CCCNC(N)=N)B2O[C@@H]3CC4CC(C4(C)C)[C@]3(C)O2)c(C)c1 |TLB:41:39:36:34| Show InChI InChI=1S/C33H48BN5O5S/c1-7-39(8-2)45(41,42)26-13-10-9-12-25(26)22-15-16-24(21(3)18-22)30(40)38-29(14-11-17-37-31(35)36)34-43-28-20-23-19-27(32(23,4)5)33(28,6)44-34/h9-10,12-13,15-16,18,23,27-29H,7-8,11,14,17,19-20H2,1-6H3,(H,38,40)(H4,35,36,37)/t23?,27?,28-,29+,33+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16325
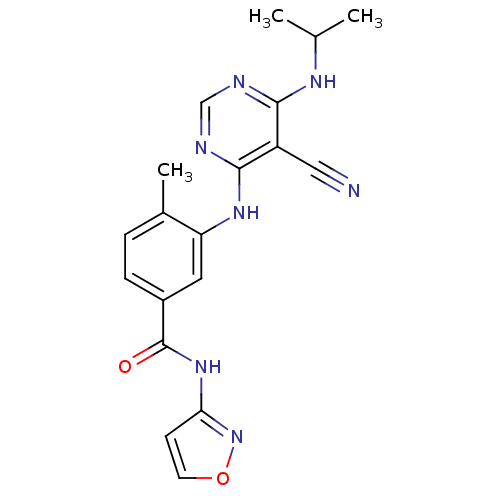
(3-({5-cyano-6-[(1-methylethyl)amino]pyrimidin-4-yl...)Show SMILES CC(C)Nc1ncnc(Nc2cc(ccc2C)C(=O)Nc2ccon2)c1C#N Show InChI InChI=1S/C19H19N7O2/c1-11(2)23-17-14(9-20)18(22-10-21-17)24-15-8-13(5-4-12(15)3)19(27)25-16-6-7-28-26-16/h4-8,10-11H,1-3H3,(H,25,26,27)(H2,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289587
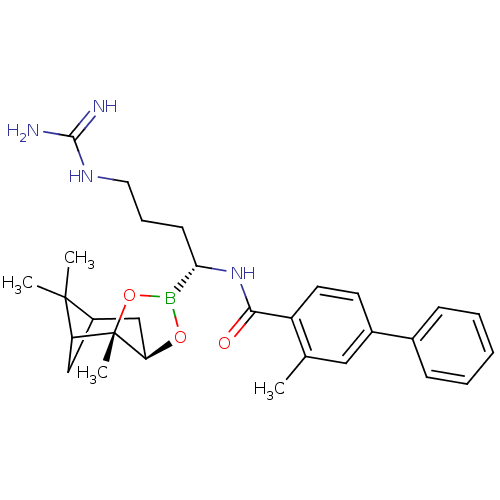
(3-Methyl-biphenyl-4-carboxylic acid [(R)-4-guanidi...)Show SMILES Cc1cc(ccc1C(=O)N[C@@H](CCCNC(N)=N)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1)-c1ccccc1 |TLB:30:28:25:23| Show InChI InChI=1S/C29H39BN4O3/c1-18-15-20(19-9-6-5-7-10-19)12-13-22(18)26(35)34-25(11-8-14-33-27(31)32)30-36-24-17-21-16-23(28(21,2)3)29(24,4)37-30/h5-7,9-10,12-13,15,21,23-25H,8,11,14,16-17H2,1-4H3,(H,34,35)(H4,31,32,33)/t21?,23?,24-,25+,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16324
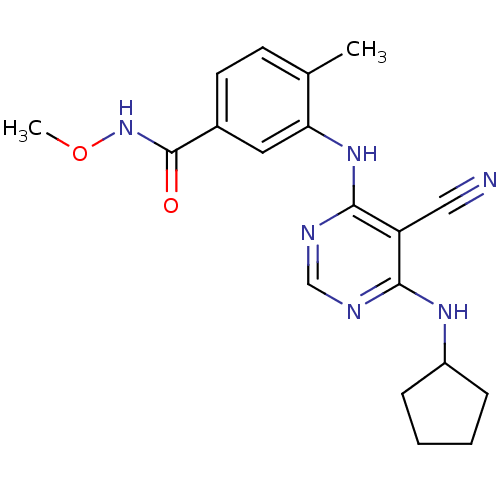
(3-{[5-cyano-6-(cyclopentylamino)pyrimidin-4-yl]ami...)Show SMILES CONC(=O)c1ccc(C)c(Nc2ncnc(NC3CCCC3)c2C#N)c1 Show InChI InChI=1S/C19H22N6O2/c1-12-7-8-13(19(26)25-27-2)9-16(12)24-18-15(10-20)17(21-11-22-18)23-14-5-3-4-6-14/h7-9,11,14H,3-6H2,1-2H3,(H,25,26)(H2,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289575
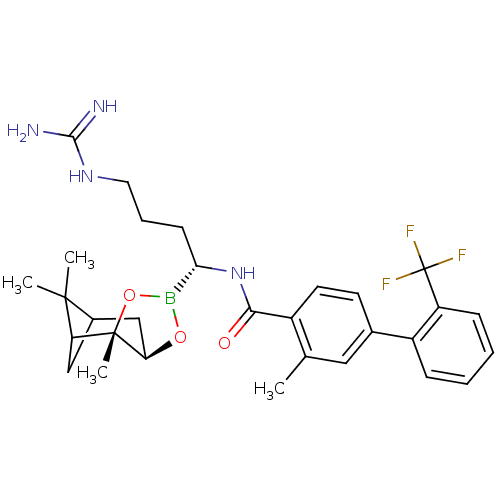
(3-Methyl-2'-trifluoromethyl-biphenyl-4-carboxylic ...)Show SMILES Cc1cc(ccc1C(=O)N[C@@H](CCCNC(N)=N)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1)-c1ccccc1C(F)(F)F |TLB:30:28:25:23| Show InChI InChI=1S/C30H38BF3N4O3/c1-17-14-18(21-8-5-6-9-22(21)30(32,33)34)11-12-20(17)26(39)38-25(10-7-13-37-27(35)36)31-40-24-16-19-15-23(28(19,2)3)29(24,4)41-31/h5-6,8-9,11-12,14,19,23-25H,7,10,13,15-16H2,1-4H3,(H,38,39)(H4,35,36,37)/t19?,23?,24-,25+,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50131114
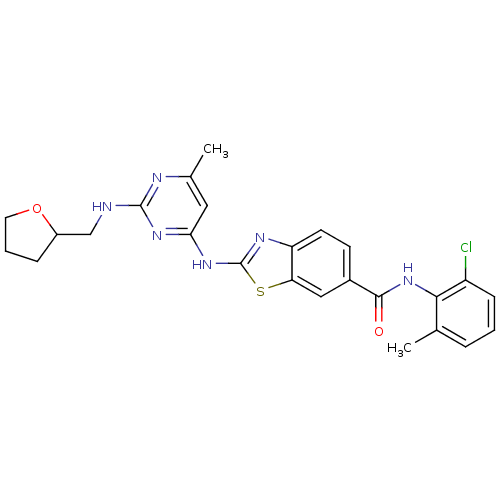
(2-{6-Methyl-2-[(tetrahydro-furan-2-ylmethyl)-amino...)Show SMILES Cc1cc(Nc2nc3ccc(cc3s2)C(=O)Nc2c(C)cccc2Cl)nc(NCC2CCCO2)n1 Show InChI InChI=1S/C25H25ClN6O2S/c1-14-5-3-7-18(26)22(14)32-23(33)16-8-9-19-20(12-16)35-25(29-19)31-21-11-15(2)28-24(30-21)27-13-17-6-4-10-34-17/h3,5,7-9,11-12,17H,4,6,10,13H2,1-2H3,(H,32,33)(H2,27,28,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
Bioorg Med Chem Lett 13: 2587-90 (2003)
BindingDB Entry DOI: 10.7270/Q2FX78V4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16323
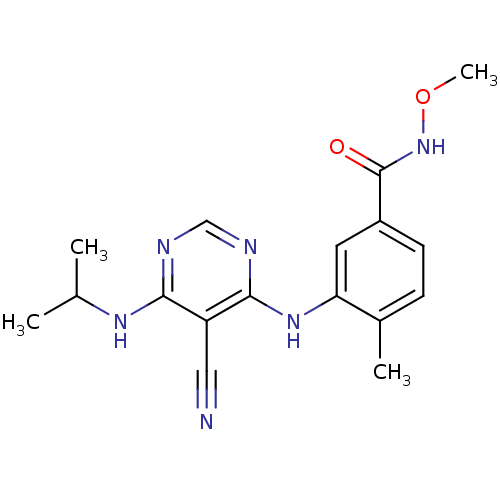
(3-({5-cyano-6-[(1-methylethyl)amino]pyrimidin-4-yl...)Show InChI InChI=1S/C17H20N6O2/c1-10(2)21-15-13(8-18)16(20-9-19-15)22-14-7-12(6-5-11(14)3)17(24)23-25-4/h5-7,9-10H,1-4H3,(H,23,24)(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | -52.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50289591

(2'-Diethylsulfamoyl-3-methyl-biphenyl-4-carboxylic...)Show SMILES CCN(CC)S(=O)(=O)c1ccccc1-c1ccc(C(=O)N[C@@H](CCCNC(N)=N)B2O[C@@H]3CC4CC(C4(C)C)[C@]3(C)O2)c(C)c1 |TLB:41:39:36:34| Show InChI InChI=1S/C33H48BN5O5S/c1-7-39(8-2)45(41,42)26-13-10-9-12-25(26)22-15-16-24(21(3)18-22)30(40)38-29(14-11-17-37-31(35)36)34-43-28-20-23-19-27(32(23,4)5)33(28,6)44-34/h9-10,12-13,15-16,18,23,27-29H,7-8,11,14,17,19-20H2,1-6H3,(H,38,40)(H4,35,36,37)/t23?,27?,28-,29+,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50289582

(3-Methyl-2'-methylsulfonylcarbamate-biphenyl-4-car...)Show SMILES COC(=O)NS(=O)(=O)c1ccccc1-c1ccc(C(=O)N[C@@H](CCCNC(N)=N)B2O[C@@H]3CC4CC(C4(C)C)[C@]3(C)O2)c(C)c1 |TLB:41:39:36:34| Show InChI InChI=1S/C31H42BN5O7S/c1-18-15-19(22-9-6-7-10-23(22)45(40,41)37-29(39)42-5)12-13-21(18)27(38)36-26(11-8-14-35-28(33)34)32-43-25-17-20-16-24(30(20,2)3)31(25,4)44-32/h6-7,9-10,12-13,15,20,24-26H,8,11,14,16-17H2,1-5H3,(H,36,38)(H,37,39)(H4,33,34,35)/t20?,24?,25-,26+,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50084682

(1-[3-(4-Carbamimidoyl-phenyl)-2-(2-methyl-1,2,3,4-...)Show SMILES Cc1cc(ccc1C(=O)N[C@@H](CCCNC(N)=N)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1)-c1ccccc1S(=O)(=O)NC(C)(C)C |TLB:30:28:25:23| Show InChI InChI=1S/C33H48BN5O5S/c1-20-17-21(24-11-8-9-12-25(24)45(41,42)39-31(2,3)4)14-15-23(20)29(40)38-28(13-10-16-37-30(35)36)34-43-27-19-22-18-26(32(22,5)6)33(27,7)44-34/h8-9,11-12,14-15,17,22,26-28,39H,10,13,16,18-19H2,1-7H3,(H,38,40)(H4,35,36,37)/t22?,26?,27-,28+,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM13357
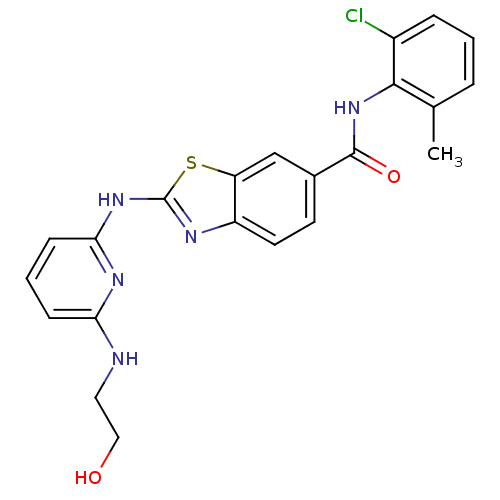
(CHEMBL312933 | N-(2-chloro-6-methylphenyl)-2-({6-[...)Show SMILES Cc1cccc(Cl)c1NC(=O)c1ccc2nc(Nc3cccc(NCCO)n3)sc2c1 Show InChI InChI=1S/C22H20ClN5O2S/c1-13-4-2-5-15(23)20(13)28-21(30)14-8-9-16-17(12-14)31-22(25-16)27-19-7-3-6-18(26-19)24-10-11-29/h2-9,12,29H,10-11H2,1H3,(H,28,30)(H2,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
Bioorg Med Chem Lett 13: 2587-90 (2003)
BindingDB Entry DOI: 10.7270/Q2FX78V4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289586

(3-Methyl-2'-sulfamoyl-biphenyl-4-carboxylic acid [...)Show SMILES Cc1cc(ccc1C(=O)N[C@@H](CCCNC(N)=N)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1)-c1ccccc1S(N)(=O)=O |TLB:30:28:25:23| Show InChI InChI=1S/C29H40BN5O5S/c1-17-14-18(21-8-5-6-9-22(21)41(33,37)38)11-12-20(17)26(36)35-25(10-7-13-34-27(31)32)30-39-24-16-19-15-23(28(19,2)3)29(24,4)40-30/h5-6,8-9,11-12,14,19,23-25H,7,10,13,15-16H2,1-4H3,(H,35,36)(H4,31,32,34)(H2,33,37,38)/t19?,23?,24-,25+,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289576
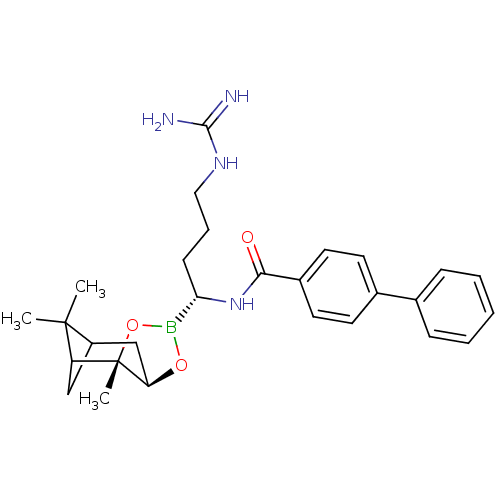
(Biphenyl-4-carboxylic acid [(R)-4-guanidino-1-((2S...)Show SMILES CC1(C)C2CC1[C@]1(C)OB(O[C@@H]1C2)[C@H](CCCNC(N)=N)NC(=O)c1ccc(cc1)-c1ccccc1 |THB:8:6:1:4| Show InChI InChI=1S/C28H37BN4O3/c1-27(2)21-16-22(27)28(3)23(17-21)35-29(36-28)24(10-7-15-32-26(30)31)33-25(34)20-13-11-19(12-14-20)18-8-5-4-6-9-18/h4-6,8-9,11-14,21-24H,7,10,15-17H2,1-3H3,(H,33,34)(H4,30,31,32)/t21?,22?,23-,24+,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16322
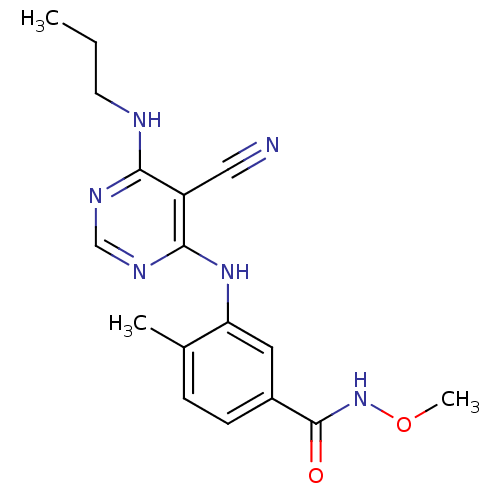
(3-{[5-cyano-6-(propylamino)pyrimidin-4-yl]amino}-N...)Show InChI InChI=1S/C17H20N6O2/c1-4-7-19-15-13(9-18)16(21-10-20-15)22-14-8-12(6-5-11(14)2)17(24)23-25-3/h5-6,8,10H,4,7H2,1-3H3,(H,23,24)(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.970 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50131114

(2-{6-Methyl-2-[(tetrahydro-furan-2-ylmethyl)-amino...)Show SMILES Cc1cc(Nc2nc3ccc(cc3s2)C(=O)Nc2c(C)cccc2Cl)nc(NCC2CCCO2)n1 Show InChI InChI=1S/C25H25ClN6O2S/c1-14-5-3-7-18(26)22(14)32-23(33)16-8-9-19-20(12-16)35-25(29-19)31-21-11-15(2)28-24(30-21)27-13-17-6-4-10-34-17/h3,5,7-9,11-12,17H,4,6,10,13H2,1-2H3,(H,32,33)(H2,27,28,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Fyn protein kinase |
Bioorg Med Chem Lett 13: 2587-90 (2003)
BindingDB Entry DOI: 10.7270/Q2FX78V4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289582

(3-Methyl-2'-methylsulfonylcarbamate-biphenyl-4-car...)Show SMILES COC(=O)NS(=O)(=O)c1ccccc1-c1ccc(C(=O)N[C@@H](CCCNC(N)=N)B2O[C@@H]3CC4CC(C4(C)C)[C@]3(C)O2)c(C)c1 |TLB:41:39:36:34| Show InChI InChI=1S/C31H42BN5O7S/c1-18-15-19(22-9-6-7-10-23(22)45(40,41)37-29(39)42-5)12-13-21(18)27(38)36-26(11-8-14-35-28(33)34)32-43-25-17-20-16-24(30(20,2)3)31(25,4)44-32/h6-7,9-10,12-13,15,20,24-26H,8,11,14,16-17H2,1-5H3,(H,36,38)(H,37,39)(H4,33,34,35)/t20?,24?,25-,26+,31+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289598

(3-Fluoro-biphenyl-4-carboxylic acid [(R)-4-guanidi...)Show SMILES CC1(C)C2CC1[C@]1(C)OB(O[C@@H]1C2)[C@H](CCCNC(N)=N)NC(=O)c1ccc(cc1F)-c1ccccc1 |THB:8:6:1:4| Show InChI InChI=1S/C28H36BFN4O3/c1-27(2)19-15-22(27)28(3)23(16-19)36-29(37-28)24(10-7-13-33-26(31)32)34-25(35)20-12-11-18(14-21(20)30)17-8-5-4-6-9-17/h4-6,8-9,11-12,14,19,22-24H,7,10,13,15-16H2,1-3H3,(H,34,35)(H4,31,32,33)/t19?,22?,23-,24+,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289588
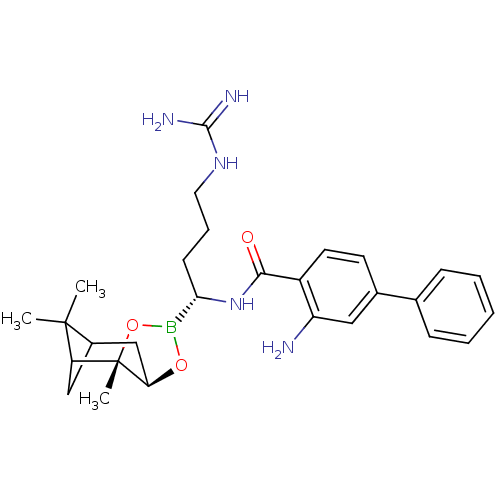
(3-Amino-biphenyl-4-carboxylic acid [(R)-4-guanidin...)Show SMILES CC1(C)C2CC1[C@]1(C)OB(O[C@@H]1C2)[C@H](CCCNC(N)=N)NC(=O)c1ccc(cc1N)-c1ccccc1 |THB:8:6:1:4| Show InChI InChI=1S/C28H38BN5O3/c1-27(2)19-15-22(27)28(3)23(16-19)36-29(37-28)24(10-7-13-33-26(31)32)34-25(35)20-12-11-18(14-21(20)30)17-8-5-4-6-9-17/h4-6,8-9,11-12,14,19,22-24H,7,10,13,15-16,30H2,1-3H3,(H,34,35)(H4,31,32,33)/t19?,22?,23-,24+,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16331
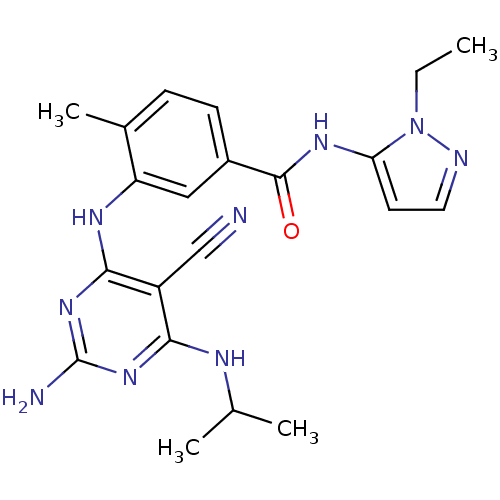
(3-({2-amino-5-cyano-6-[(1-methylethyl)amino]pyrimi...)Show SMILES CCn1nccc1NC(=O)c1ccc(C)c(Nc2nc(N)nc(NC(C)C)c2C#N)c1 Show InChI InChI=1S/C21H25N9O/c1-5-30-17(8-9-24-30)27-20(31)14-7-6-13(4)16(10-14)26-19-15(11-22)18(25-12(2)3)28-21(23)29-19/h6-10,12H,5H2,1-4H3,(H,27,31)(H4,23,25,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | -49.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16326
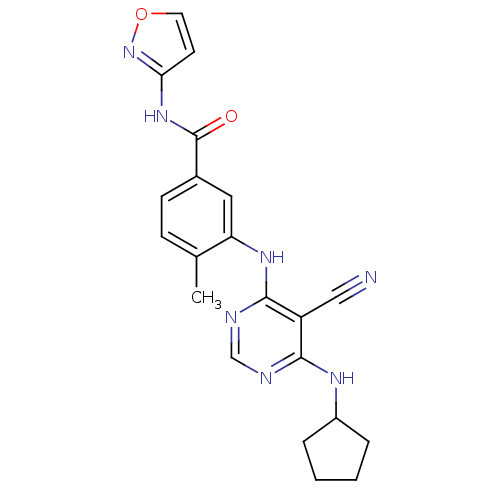
(3-{[5-cyano-6-(cyclopentylamino)pyrimidin-4-yl]ami...)Show SMILES Cc1ccc(cc1Nc1ncnc(NC2CCCC2)c1C#N)C(=O)Nc1ccon1 Show InChI InChI=1S/C21H21N7O2/c1-13-6-7-14(21(29)27-18-8-9-30-28-18)10-17(13)26-20-16(11-22)19(23-12-24-20)25-15-4-2-3-5-15/h6-10,12,15H,2-5H2,1H3,(H,27,28,29)(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289596

(CHEMBL264370 | N-[(1S)-4-{[(Z)-amino(imino)methyl]...)Show SMILES NC(=N)NCCC[C@H](NC(=O)c1ccc(cc1)-c1ccccc1)B(O)O Show InChI InChI=1S/C18H23BN4O3/c20-18(21)22-12-4-7-16(19(25)26)23-17(24)15-10-8-14(9-11-15)13-5-2-1-3-6-13/h1-3,5-6,8-11,16,25-26H,4,7,12H2,(H,23,24)(H4,20,21,22)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16332
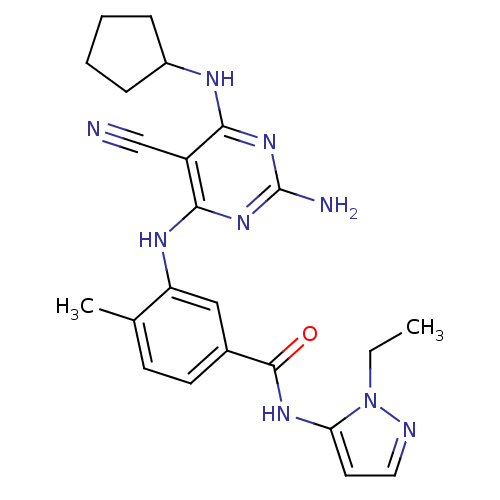
(3-{[2-amino-5-cyano-6-(cyclopentylamino)pyrimidin-...)Show SMILES CCn1nccc1NC(=O)c1ccc(C)c(Nc2nc(N)nc(NC3CCCC3)c2C#N)c1 Show InChI InChI=1S/C23H27N9O/c1-3-32-19(10-11-26-32)29-22(33)15-9-8-14(2)18(12-15)28-21-17(13-24)20(30-23(25)31-21)27-16-6-4-5-7-16/h8-12,16H,3-7H2,1-2H3,(H,29,33)(H4,25,27,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | -49.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM13357

(CHEMBL312933 | N-(2-chloro-6-methylphenyl)-2-({6-[...)Show SMILES Cc1cccc(Cl)c1NC(=O)c1ccc2nc(Nc3cccc(NCCO)n3)sc2c1 Show InChI InChI=1S/C22H20ClN5O2S/c1-13-4-2-5-15(23)20(13)28-21(30)14-8-9-16-17(12-14)31-22(25-16)27-19-7-3-6-18(26-19)24-10-11-29/h2-9,12,29H,10-11H2,1H3,(H,28,30)(H2,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Fyn protein kinase |
Bioorg Med Chem Lett 13: 2587-90 (2003)
BindingDB Entry DOI: 10.7270/Q2FX78V4 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50289575

(3-Methyl-2'-trifluoromethyl-biphenyl-4-carboxylic ...)Show SMILES Cc1cc(ccc1C(=O)N[C@@H](CCCNC(N)=N)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1)-c1ccccc1C(F)(F)F |TLB:30:28:25:23| Show InChI InChI=1S/C30H38BF3N4O3/c1-17-14-18(21-8-5-6-9-22(21)30(32,33)34)11-12-20(17)26(39)38-25(10-7-13-37-27(35)36)31-40-24-16-19-15-23(28(19,2)3)29(24,4)41-31/h5-6,8-9,11-12,14,19,23-25H,7,10,13,15-16H2,1-4H3,(H,38,39)(H4,35,36,37)/t19?,23?,24-,25+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50131114

(2-{6-Methyl-2-[(tetrahydro-furan-2-ylmethyl)-amino...)Show SMILES Cc1cc(Nc2nc3ccc(cc3s2)C(=O)Nc2c(C)cccc2Cl)nc(NCC2CCCO2)n1 Show InChI InChI=1S/C25H25ClN6O2S/c1-14-5-3-7-18(26)22(14)32-23(33)16-8-9-19-20(12-16)35-25(29-19)31-21-11-15(2)28-24(30-21)27-13-17-6-4-10-34-17/h3,5,7-9,11-12,17H,4,6,10,13H2,1-2H3,(H,32,33)(H2,27,28,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 13: 2587-90 (2003)
BindingDB Entry DOI: 10.7270/Q2FX78V4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289579

(3'-Methanesulfonylamino-biphenyl-3-carboxylic acid...)Show SMILES CC1(C)C2CC1[C@]1(C)OB(O[C@@H]1C2)[C@H](CCCNC(N)=N)NC(=O)c1cccc(c1)-c1cccc(NS(C)(=O)=O)c1 |THB:8:6:1:4| Show InChI InChI=1S/C29H40BN5O5S/c1-28(2)21-16-23(28)29(3)24(17-21)39-30(40-29)25(12-7-13-33-27(31)32)34-26(36)20-10-5-8-18(14-20)19-9-6-11-22(15-19)35-41(4,37)38/h5-6,8-11,14-15,21,23-25,35H,7,12-13,16-17H2,1-4H3,(H,34,36)(H4,31,32,33)/t21?,23?,24-,25+,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16316
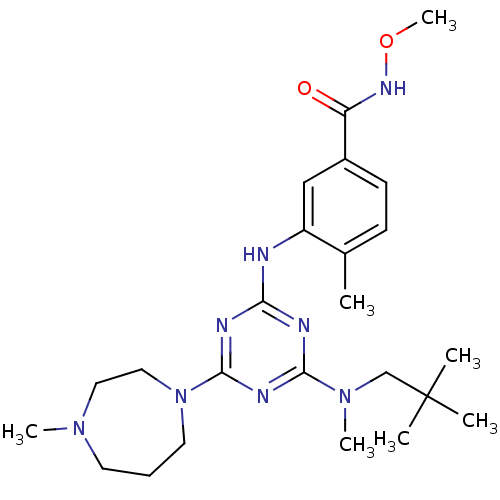
(3-({4-[(2,2-dimethylpropyl)(methyl)amino]-6-(4-met...)Show SMILES CONC(=O)c1ccc(C)c(Nc2nc(nc(n2)N2CCCN(C)CC2)N(C)CC(C)(C)C)c1 Show InChI InChI=1S/C24H38N8O2/c1-17-9-10-18(20(33)29-34-7)15-19(17)25-21-26-22(31(6)16-24(2,3)4)28-23(27-21)32-12-8-11-30(5)13-14-32/h9-10,15H,8,11-14,16H2,1-7H3,(H,29,33)(H,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | -47.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50289579

(3'-Methanesulfonylamino-biphenyl-3-carboxylic acid...)Show SMILES CC1(C)C2CC1[C@]1(C)OB(O[C@@H]1C2)[C@H](CCCNC(N)=N)NC(=O)c1cccc(c1)-c1cccc(NS(C)(=O)=O)c1 |THB:8:6:1:4| Show InChI InChI=1S/C29H40BN5O5S/c1-28(2)21-16-23(28)29(3)24(17-21)39-30(40-29)25(12-7-13-33-27(31)32)34-26(36)20-10-5-8-18(14-20)19-9-6-11-22(15-19)35-41(4,37)38/h5-6,8-11,14-15,21,23-25,35H,7,12-13,16-17H2,1-4H3,(H,34,36)(H4,31,32,33)/t21?,23?,24-,25+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50289587

(3-Methyl-biphenyl-4-carboxylic acid [(R)-4-guanidi...)Show SMILES Cc1cc(ccc1C(=O)N[C@@H](CCCNC(N)=N)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1)-c1ccccc1 |TLB:30:28:25:23| Show InChI InChI=1S/C29H39BN4O3/c1-18-15-20(19-9-6-5-7-10-19)12-13-22(18)26(35)34-25(11-8-14-33-27(31)32)30-36-24-17-21-16-23(28(21,2)3)29(24,4)37-30/h5-7,9-10,12-13,15,21,23-25H,8,11,14,16-17H2,1-4H3,(H,34,35)(H4,31,32,33)/t21?,23?,24-,25+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289584
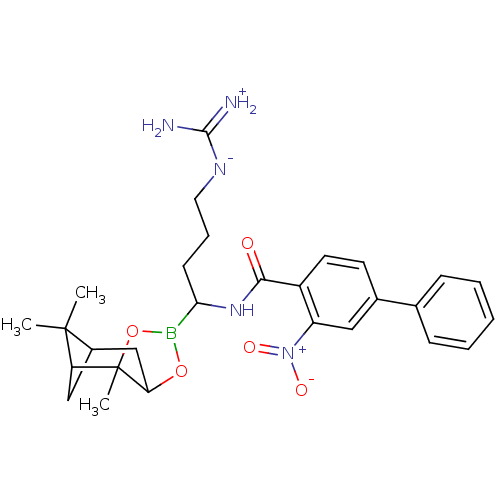
(3-Nitro-biphenyl-4-carboxylic acid [(R)-4-guanidin...)Show SMILES CC1(C)C2CC1C1(C)OB(OC1C2)C(CCC[N-]C(N)=[NH2+])NC(=O)c1ccc(cc1[N+]([O-])=O)-c1ccccc1 |THB:8:6:1:4| Show InChI InChI=1S/C28H36BN5O5/c1-27(2)19-15-22(27)28(3)23(16-19)38-29(39-28)24(10-7-13-32-26(30)31)33-25(35)20-12-11-18(14-21(20)34(36)37)17-8-5-4-6-9-17/h4-6,8-9,11-12,14,19,22-24H,7,10,13,15-16H2,1-3H3,(H5,30,31,32,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50289595
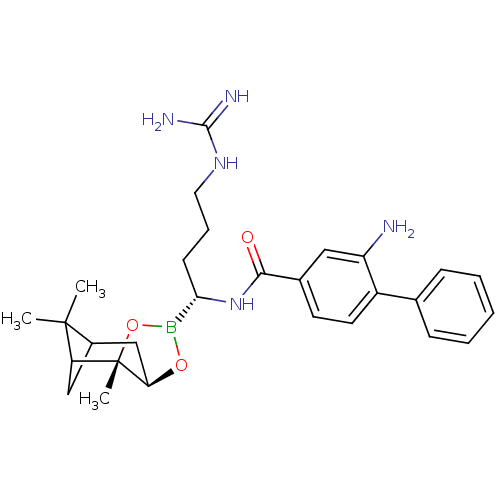
(2-Amino-biphenyl-4-carboxylic acid [(R)-4-guanidin...)Show SMILES CC1(C)C2CC1[C@]1(C)OB(O[C@@H]1C2)[C@H](CCCNC(N)=N)NC(=O)c1ccc(c(N)c1)-c1ccccc1 |THB:8:6:1:4| Show InChI InChI=1S/C28H38BN5O3/c1-27(2)19-15-22(27)28(3)23(16-19)36-29(37-28)24(10-7-13-33-26(31)32)34-25(35)18-11-12-20(21(30)14-18)17-8-5-4-6-9-17/h4-6,8-9,11-12,14,19,22-24H,7,10,13,15-16,30H2,1-3H3,(H,34,35)(H4,31,32,33)/t19?,22?,23-,24+,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50289581

(2-Nitro-biphenyl-4-carboxylic acid [(R)-4-guanidin...)Show SMILES CC1(C)C2CC1C1(C)OB(OC1C2)C(CCC[N-]C(N)=[NH2+])NC(=O)c1ccc(-c2ccccc2)c(c1)[N+]([O-])=O |THB:8:6:1:4| Show InChI InChI=1S/C28H36BN5O5/c1-27(2)19-15-22(27)28(3)23(16-19)38-29(39-28)24(10-7-13-32-26(30)31)33-25(35)18-11-12-20(21(14-18)34(36)37)17-8-5-4-6-9-17/h4-6,8-9,11-12,14,19,22-24H,7,10,13,15-16H2,1-3H3,(H5,30,31,32,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289581

(2-Nitro-biphenyl-4-carboxylic acid [(R)-4-guanidin...)Show SMILES CC1(C)C2CC1C1(C)OB(OC1C2)C(CCC[N-]C(N)=[NH2+])NC(=O)c1ccc(-c2ccccc2)c(c1)[N+]([O-])=O |THB:8:6:1:4| Show InChI InChI=1S/C28H36BN5O5/c1-27(2)19-15-22(27)28(3)23(16-19)38-29(39-28)24(10-7-13-32-26(30)31)33-25(35)18-11-12-20(21(14-18)34(36)37)17-8-5-4-6-9-17/h4-6,8-9,11-12,14,19,22-24H,7,10,13,15-16H2,1-3H3,(H5,30,31,32,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50289588

(3-Amino-biphenyl-4-carboxylic acid [(R)-4-guanidin...)Show SMILES CC1(C)C2CC1[C@]1(C)OB(O[C@@H]1C2)[C@H](CCCNC(N)=N)NC(=O)c1ccc(cc1N)-c1ccccc1 |THB:8:6:1:4| Show InChI InChI=1S/C28H38BN5O3/c1-27(2)19-15-22(27)28(3)23(16-19)36-29(37-28)24(10-7-13-33-26(31)32)34-25(35)20-12-11-18(14-21(20)30)17-8-5-4-6-9-17/h4-6,8-9,11-12,14,19,22-24H,7,10,13,15-16,30H2,1-3H3,(H,34,35)(H4,31,32,33)/t19?,22?,23-,24+,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289595

(2-Amino-biphenyl-4-carboxylic acid [(R)-4-guanidin...)Show SMILES CC1(C)C2CC1[C@]1(C)OB(O[C@@H]1C2)[C@H](CCCNC(N)=N)NC(=O)c1ccc(c(N)c1)-c1ccccc1 |THB:8:6:1:4| Show InChI InChI=1S/C28H38BN5O3/c1-27(2)19-15-22(27)28(3)23(16-19)36-29(37-28)24(10-7-13-33-26(31)32)34-25(35)18-11-12-20(21(30)14-18)17-8-5-4-6-9-17/h4-6,8-9,11-12,14,19,22-24H,7,10,13,15-16,30H2,1-3H3,(H,34,35)(H4,31,32,33)/t19?,22?,23-,24+,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50129307
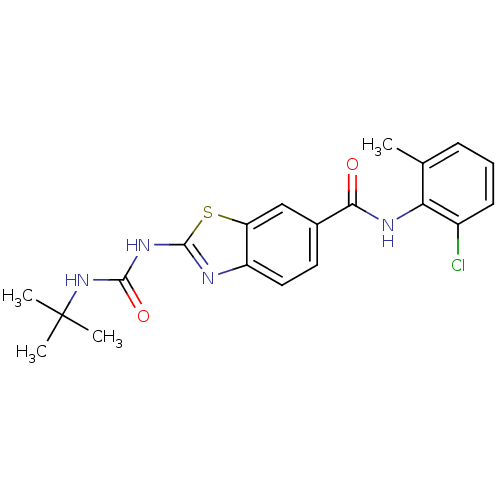
(2-(3-tert-Butyl-ureido)-benzothiazole-6-carboxylic...)Show SMILES Cc1cccc(Cl)c1NC(=O)c1ccc2nc(NC(=O)NC(C)(C)C)sc2c1 Show InChI InChI=1S/C20H21ClN4O2S/c1-11-6-5-7-13(21)16(11)23-17(26)12-8-9-14-15(10-12)28-19(22-14)24-18(27)25-20(2,3)4/h5-10H,1-4H3,(H,23,26)(H2,22,24,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
Bioorg Med Chem Lett 13: 2587-90 (2003)
BindingDB Entry DOI: 10.7270/Q2FX78V4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50131131

(2-(6-Methylamino-pyrimidin-4-ylamino)-benzothiazol...)Show SMILES CNc1cc(Nc2nc3ccc(cc3s2)C(=O)Nc2c(C)cccc2Cl)ncn1 Show InChI InChI=1S/C20H17ClN6OS/c1-11-4-3-5-13(21)18(11)27-19(28)12-6-7-14-15(8-12)29-20(25-14)26-17-9-16(22-2)23-10-24-17/h3-10H,1-2H3,(H,27,28)(H2,22,23,24,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
Bioorg Med Chem Lett 13: 2587-90 (2003)
BindingDB Entry DOI: 10.7270/Q2FX78V4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289594

(2-Biphenyl-4-yl-N-[(R)-4-guanidino-1-((2S,6R)-2,9,...)Show SMILES CC1(C)C2CC1[C@]1(C)OB(O[C@@H]1C2)[C@H](CCCNC(N)=N)NC(=O)Cc1ccc(cc1)-c1ccccc1 |THB:8:6:1:4| Show InChI InChI=1S/C29H39BN4O3/c1-28(2)22-17-23(28)29(3)24(18-22)36-30(37-29)25(10-7-15-33-27(31)32)34-26(35)16-19-11-13-21(14-12-19)20-8-5-4-6-9-20/h4-6,8-9,11-14,22-25H,7,10,15-18H2,1-3H3,(H,34,35)(H4,31,32,33)/t22?,23?,24-,25+,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50289589
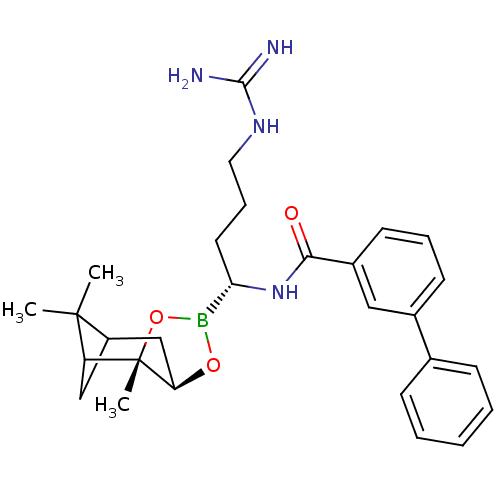
(Biphenyl-3-carboxylic acid [(R)-4-guanidino-1-((2S...)Show SMILES CC1(C)C2CC1[C@]1(C)OB(O[C@@H]1C2)[C@H](CCCNC(N)=N)NC(=O)c1cccc(c1)-c1ccccc1 |THB:8:6:1:4| Show InChI InChI=1S/C28H37BN4O3/c1-27(2)21-16-22(27)28(3)23(17-21)35-29(36-28)24(13-8-14-32-26(30)31)33-25(34)20-12-7-11-19(15-20)18-9-5-4-6-10-18/h4-7,9-12,15,21-24H,8,13-14,16-17H2,1-3H3,(H,33,34)(H4,30,31,32)/t21?,22?,23-,24+,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289580

(CHEMBL290720 | {3'-[(R)-4-Guanidino-1-((2S,6R)-2,9...)Show SMILES CC(C)(C)OC(=O)Nc1cccc(c1)-c1cccc(c1)C(=O)N[C@@H](CCCNC(N)=N)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1 |TLB:43:41:38:36| Show InChI InChI=1S/C33H46BN5O5/c1-31(2,3)42-30(41)38-24-13-8-11-21(17-24)20-10-7-12-22(16-20)28(40)39-27(14-9-15-37-29(35)36)34-43-26-19-23-18-25(32(23,4)5)33(26,6)44-34/h7-8,10-13,16-17,23,25-27H,9,14-15,18-19H2,1-6H3,(H,38,41)(H,39,40)(H4,35,36,37)/t23?,25?,26-,27+,33+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data