 Found 2469 hits with Last Name = 'wolf' and Initial = 'm'
Found 2469 hits with Last Name = 'wolf' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50306528
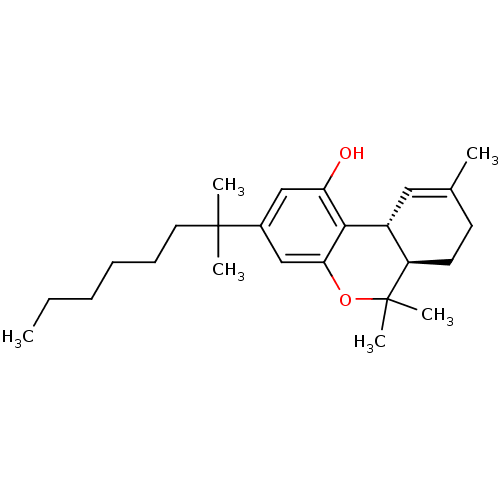
((6aR,10aR)-6,6,9-trimethyl-3-(2-methyloctan-2-yl)-...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:15| Show InChI InChI=1S/C25H38O2/c1-7-8-9-10-13-24(3,4)18-15-21(26)23-19-14-17(2)11-12-20(19)25(5,6)27-22(23)16-18/h14-16,19-20,26H,7-13H2,1-6H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from CB2 receptor |
Bioorg Med Chem Lett 20: 1424-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.092
BindingDB Entry DOI: 10.7270/Q2PC32GV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50306528

((6aR,10aR)-6,6,9-trimethyl-3-(2-methyloctan-2-yl)-...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:15| Show InChI InChI=1S/C25H38O2/c1-7-8-9-10-13-24(3,4)18-15-21(26)23-19-14-17(2)11-12-20(19)25(5,6)27-22(23)16-18/h14-16,19-20,26H,7-13H2,1-6H3/t19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from CB1 receptor |
Bioorg Med Chem Lett 20: 1424-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.092
BindingDB Entry DOI: 10.7270/Q2PC32GV |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50575178
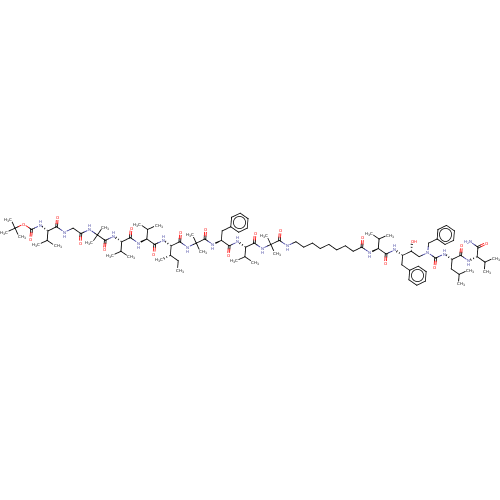
(CHEMBL4853450)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)C(C)(C)NC(=O)CNC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)C)C(C)C)C(C)C)C(=O)NC(C)(C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)NC(C)(C)C(=O)NCCCCCCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(N)=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Noncompetitive inhibition of gamma secretase (unknown origin) expressed in HEK293 cells assessed as reduction of A-beta40 levels using varying level ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01395
BindingDB Entry DOI: 10.7270/Q2HX1HGF |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50575197
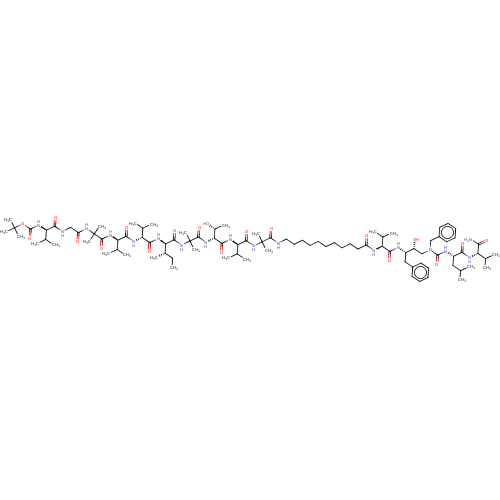
(CHEMBL4868549)Show SMILES CC[C@H](C)[C@@H](NC(=O)[C@H](NC(=O)[C@H](NC(=O)C(C)(C)NC(=O)CNC(=O)[C@H](NC(=O)OC(C)(C)C)C(C)C)C(C)C)C(C)C)C(=O)NC(C)(C)C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H](C(C)C)C(=O)NC(C)(C)C(=O)NCCCCCCCCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(N)=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Noncompetitive inhibition of gamma secretase (unknown origin) expressed in HEK293 cells assessed as reduction of A-beta40 levels using varying level ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01395
BindingDB Entry DOI: 10.7270/Q2HX1HGF |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50476859
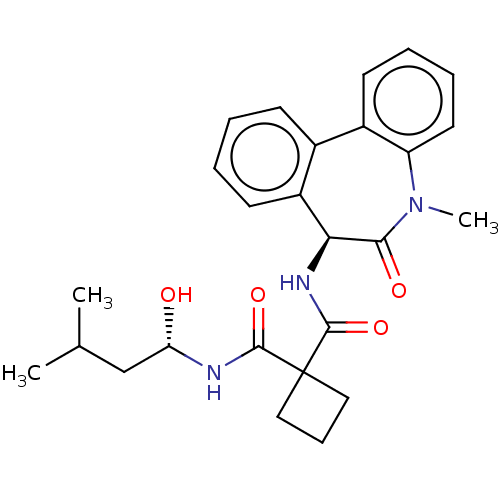
(CHEMBL232938)Show SMILES CC(C)C[C@H](O)NC(=O)C1(CCC1)C(=O)N[C@H]1c2ccccc2-c2ccccc2N(C)C1=O Show InChI InChI=1S/C26H31N3O4/c1-16(2)15-21(30)27-24(32)26(13-8-14-26)25(33)28-22-19-11-5-4-9-17(19)18-10-6-7-12-20(18)29(3)23(22)31/h4-7,9-12,16,21-22,30H,8,13-15H2,1-3H3,(H,27,32)(H,28,33)/t21-,22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes |
Bioorg Med Chem Lett 17: 3910-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.102
BindingDB Entry DOI: 10.7270/Q2M90CFP |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50476878

(CHEMBL399204)Show SMILES CC(C)[C@H](O)NC(=O)C1(CCC1)C(=O)N[C@H]1N=C(c2ccc(cc2)C(F)(F)F)c2ccccc2N(C)C1=O |t:17| Show InChI InChI=1S/C27H29F3N4O4/c1-15(2)22(35)33-25(38)26(13-6-14-26)24(37)32-21-23(36)34(3)19-8-5-4-7-18(19)20(31-21)16-9-11-17(12-10-16)27(28,29)30/h4-5,7-12,15,21-22,35H,6,13-14H2,1-3H3,(H,32,37)(H,33,38)/t21-,22+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes |
Bioorg Med Chem Lett 17: 3910-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.102
BindingDB Entry DOI: 10.7270/Q2M90CFP |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50575187

(CHEMBL4848116)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)C(C)(C)NC(=O)CNC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)C)C(C)C)C(C)C)C(=O)NC(C)(C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)NC(C)(C)C(=O)NCC(=O)NCC(=O)NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(N)=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Noncompetitive inhibition of gamma secretase (unknown origin) expressed in HEK293 cells assessed as reduction of A-beta40 levels using varying level ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01395
BindingDB Entry DOI: 10.7270/Q2HX1HGF |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50476861
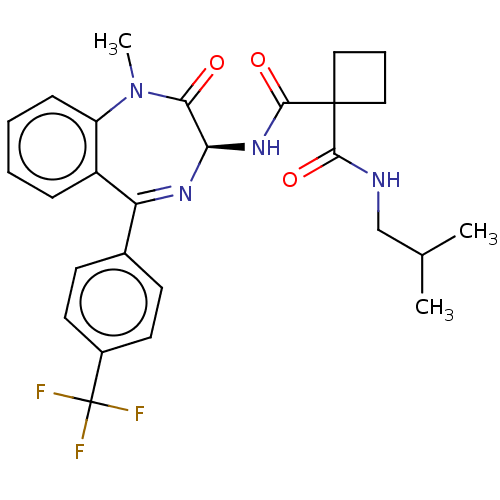
(CHEMBL401044)Show SMILES CC(C)CNC(=O)C1(CCC1)C(=O)N[C@H]1N=C(c2ccc(cc2)C(F)(F)F)c2ccccc2N(C)C1=O |t:16| Show InChI InChI=1S/C27H29F3N4O3/c1-16(2)15-31-24(36)26(13-6-14-26)25(37)33-22-23(35)34(3)20-8-5-4-7-19(20)21(32-22)17-9-11-18(12-10-17)27(28,29)30/h4-5,7-12,16,22H,6,13-15H2,1-3H3,(H,31,36)(H,33,37)/t22-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes |
Bioorg Med Chem Lett 17: 3910-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.102
BindingDB Entry DOI: 10.7270/Q2M90CFP |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50476880

(CHEMBL450279)Show SMILES CN1c2ccccc2-c2ccccc2[C@H](NC(=O)C2(CCC2)C(=O)NCCC2CCCC2)C1=O Show InChI InChI=1S/C28H33N3O3/c1-31-23-14-7-6-12-21(23)20-11-4-5-13-22(20)24(25(31)32)30-27(34)28(16-8-17-28)26(33)29-18-15-19-9-2-3-10-19/h4-7,11-14,19,24H,2-3,8-10,15-18H2,1H3,(H,29,33)(H,30,34)/t24-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes |
Bioorg Med Chem Lett 17: 3910-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.102
BindingDB Entry DOI: 10.7270/Q2M90CFP |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313984
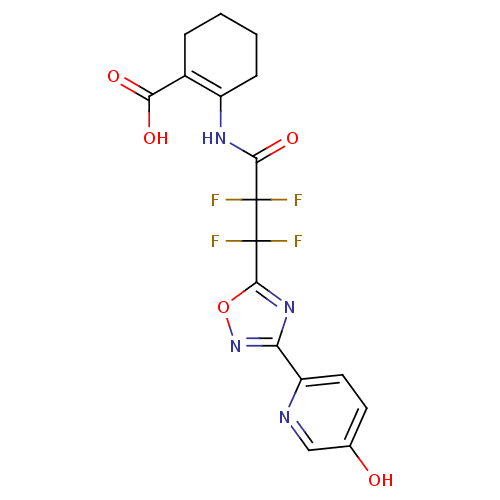
(2-(2,2,3,3-tetrafluoro-3-(3-(5-hydroxypyridin-2-yl...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)C(F)(F)C(F)(F)c1nc(no1)-c1ccc(O)cn1 |t:3| Show InChI InChI=1S/C17H14F4N4O5/c18-16(19,14(29)23-10-4-2-1-3-9(10)13(27)28)17(20,21)15-24-12(25-30-15)11-6-5-8(26)7-22-11/h5-7,26H,1-4H2,(H,23,29)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50476871
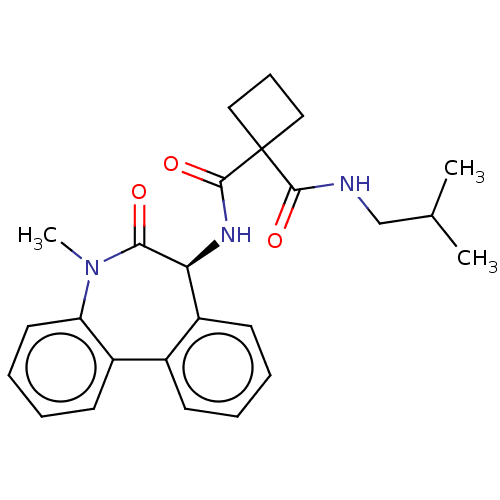
(CHEMBL232182)Show SMILES CC(C)CNC(=O)C1(CCC1)C(=O)N[C@H]1c2ccccc2-c2ccccc2N(C)C1=O Show InChI InChI=1S/C25H29N3O3/c1-16(2)15-26-23(30)25(13-8-14-25)24(31)27-21-19-11-5-4-9-17(19)18-10-6-7-12-20(18)28(3)22(21)29/h4-7,9-12,16,21H,8,13-15H2,1-3H3,(H,26,30)(H,27,31)/t21-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes |
Bioorg Med Chem Lett 17: 3910-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.102
BindingDB Entry DOI: 10.7270/Q2M90CFP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190788
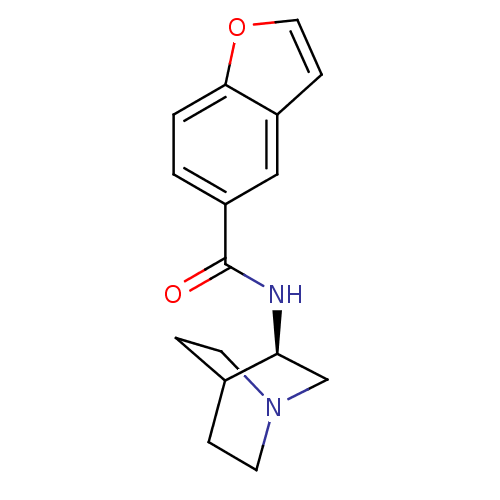
(CHEMBL378471 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2occc2c1 |wU:3.2,(-3.91,-15.15,;-3.91,-16.69,;-2.57,-17.45,;-1.24,-16.67,;-1.25,-15.14,;.09,-14.37,;1.43,-15.13,;1.43,-16.67,;.1,-17.45,;-.78,-16.3,;.04,-15.5,;-5.24,-17.46,;-5.23,-19.02,;-6.57,-19.79,;-7.91,-19.02,;-9.37,-19.49,;-10.28,-18.24,;-9.37,-17,;-7.9,-17.48,;-6.58,-16.7,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50476864

(CHEMBL429438)Show SMILES CC(C)CNC(=O)C1(CCCC1)C(=O)N[C@H]1N=C(c2ccc(cc2)C(F)(F)F)c2ccccc2N(C)C1=O |t:17| Show InChI InChI=1S/C28H31F3N4O3/c1-17(2)16-32-25(37)27(14-6-7-15-27)26(38)34-23-24(36)35(3)21-9-5-4-8-20(21)22(33-23)18-10-12-19(13-11-18)28(29,30)31/h4-5,8-13,17,23H,6-7,14-16H2,1-3H3,(H,32,37)(H,34,38)/t23-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes |
Bioorg Med Chem Lett 17: 3910-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.102
BindingDB Entry DOI: 10.7270/Q2M90CFP |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50476860
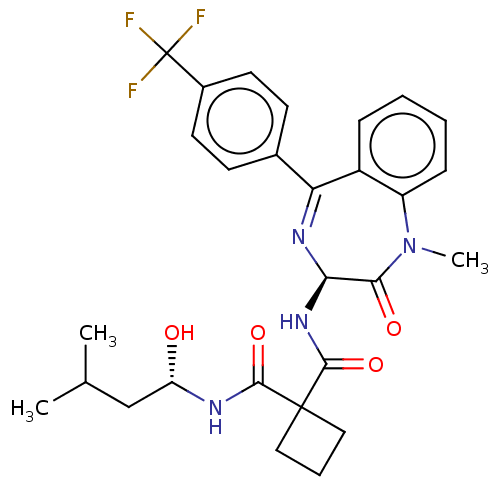
(CHEMBL232936)Show SMILES CC(C)C[C@H](O)NC(=O)C1(CCC1)C(=O)N[C@H]1N=C(c2ccc(cc2)C(F)(F)F)c2ccccc2N(C)C1=O |t:18| Show InChI InChI=1S/C28H31F3N4O4/c1-16(2)15-21(36)32-25(38)27(13-6-14-27)26(39)34-23-24(37)35(3)20-8-5-4-7-19(20)22(33-23)17-9-11-18(12-10-17)28(29,30)31/h4-5,7-12,16,21,23,36H,6,13-15H2,1-3H3,(H,32,38)(H,34,39)/t21-,23+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes |
Bioorg Med Chem Lett 17: 3910-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.102
BindingDB Entry DOI: 10.7270/Q2M90CFP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190785
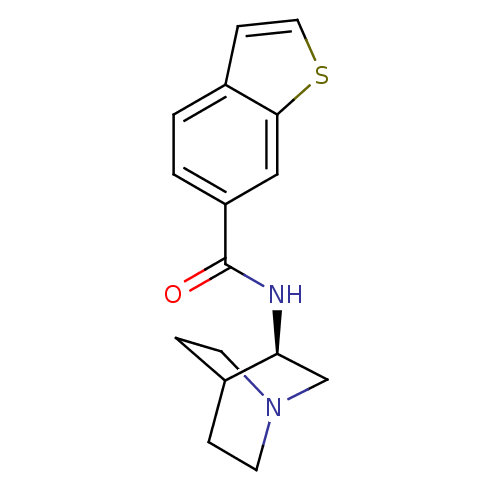
(CHEMBL378349 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccsc2c1 |wU:3.2,(28.02,-3.67,;28.02,-5.21,;29.36,-5.97,;30.69,-5.19,;30.68,-3.66,;32.02,-2.89,;33.36,-3.65,;33.36,-5.2,;32.03,-5.97,;31.15,-4.82,;31.97,-4.02,;26.69,-5.98,;26.7,-7.54,;25.35,-8.31,;24.02,-7.54,;22.56,-8.01,;21.65,-6.76,;22.56,-5.52,;24.03,-6,;25.35,-5.22,)| Show InChI InChI=1S/C16H18N2OS/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50476870

(CHEMBL232346)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)C2(CCC2)C(=O)NCCC2CCCC2)C1=O)c1ccc(cc1)C(F)(F)F |c:9| Show InChI InChI=1S/C30H33F3N4O3/c1-37-23-10-5-4-9-22(23)24(20-11-13-21(14-12-20)30(31,32)33)35-25(26(37)38)36-28(40)29(16-6-17-29)27(39)34-18-15-19-7-2-3-8-19/h4-5,9-14,19,25H,2-3,6-8,15-18H2,1H3,(H,34,39)(H,36,40)/t25-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes |
Bioorg Med Chem Lett 17: 3910-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.102
BindingDB Entry DOI: 10.7270/Q2M90CFP |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM209909
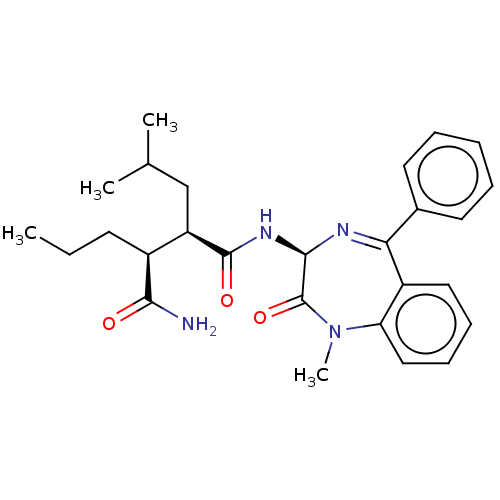
(US9273014, Comparative Compound 45 | US9427442, Co...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1N=C(c2ccccc2)c2ccccc2N(C)C1=O)C(N)=O |r,t:13| Show InChI InChI=1S/C27H34N4O3/c1-5-11-19(24(28)32)21(16-17(2)3)26(33)30-25-27(34)31(4)22-15-10-9-14-20(22)23(29-25)18-12-7-6-8-13-18/h6-10,12-15,17,19,21,25H,5,11,16H2,1-4H3,(H2,28,32)(H,30,33)/t19-,21+,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes |
Bioorg Med Chem Lett 17: 3910-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.102
BindingDB Entry DOI: 10.7270/Q2M90CFP |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50575179
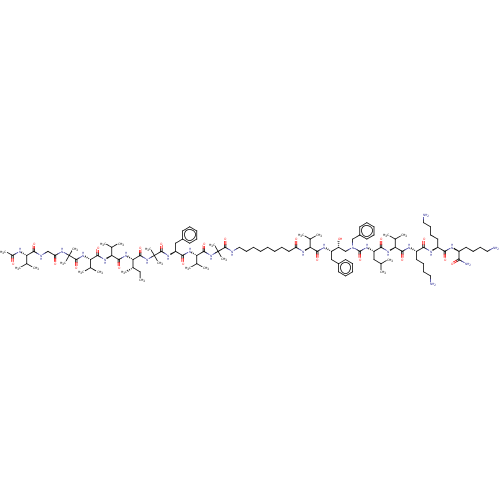
(CHEMBL4851959)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)C(C)(C)NC(=O)CNC(=O)[C@@H](NC(C)=O)C(C)C)C(C)C)C(C)C)C(=O)NC(C)(C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)NC(C)(C)C(=O)NCCCCCCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(N)=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Noncompetitive inhibition of gamma secretase (unknown origin) expressed in HEK293 cells assessed as reduction of A-beta40 levels using varying level ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01395
BindingDB Entry DOI: 10.7270/Q2HX1HGF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50306530
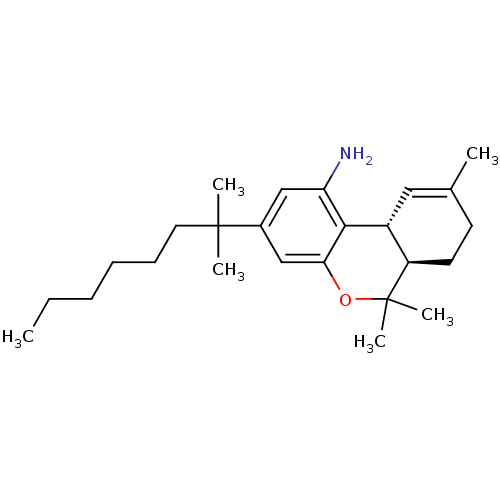
((6aR,10aR)-6,6,9-trimethyl-3-(2-methyloctan-2-yl)-...)Show SMILES CCCCCCC(C)(C)c1cc(N)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:15| Show InChI InChI=1S/C25H39NO/c1-7-8-9-10-13-24(3,4)18-15-21(26)23-19-14-17(2)11-12-20(19)25(5,6)27-22(23)16-18/h14-16,19-20H,7-13,26H2,1-6H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from CB2 receptor |
Bioorg Med Chem Lett 20: 1424-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.092
BindingDB Entry DOI: 10.7270/Q2PC32GV |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50377050

(CHEMBL403858 | PH-709829)Show SMILES O=C(N[C@@H]1C[C@H]2CCN(C2)C1)c1cc2ccoc2cn1 |TLB:2:3:9:7.6| Show InChI InChI=1S/C15H17N3O2/c19-15(13-6-11-2-4-20-14(11)7-16-13)17-12-5-10-1-3-18(8-10)9-12/h2,4,6-7,10,12H,1,3,5,8-9H2,(H,17,19)/t10-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha-7 nACh receptor in rat brain |
Bioorg Med Chem Lett 18: 3611-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.070
BindingDB Entry DOI: 10.7270/Q2KD1ZS9 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313977
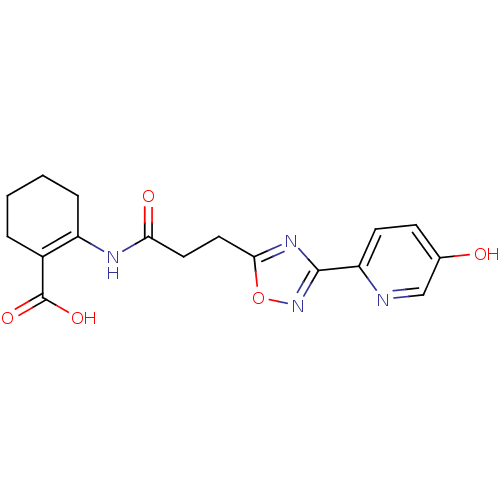
(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1nc(no1)-c1ccc(O)cn1 |t:3| Show InChI InChI=1S/C17H18N4O5/c22-10-5-6-13(18-9-10)16-20-15(26-21-16)8-7-14(23)19-12-4-2-1-3-11(12)17(24)25/h5-6,9,22H,1-4,7-8H2,(H,19,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50476872

(CHEMBL401045)Show SMILES CC(C)CNC(=O)C1(CCCC1)C(=O)N[C@H]1c2ccccc2-c2ccccc2N(C)C1=O Show InChI InChI=1S/C26H31N3O3/c1-17(2)16-27-24(31)26(14-8-9-15-26)25(32)28-22-20-12-5-4-10-18(20)19-11-6-7-13-21(19)29(3)23(22)30/h4-7,10-13,17,22H,8-9,14-16H2,1-3H3,(H,27,31)(H,28,32)/t22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes |
Bioorg Med Chem Lett 17: 3910-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.102
BindingDB Entry DOI: 10.7270/Q2M90CFP |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50476865

(CHEMBL392265)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)C2(CCC2)C(=O)N[C@@H](O)C2CCCCC2)C1=O)c1ccc(cc1)C(F)(F)F |c:9| Show InChI InChI=1S/C30H33F3N4O4/c1-37-22-11-6-5-10-21(22)23(18-12-14-20(15-13-18)30(31,32)33)34-24(26(37)39)35-27(40)29(16-7-17-29)28(41)36-25(38)19-8-3-2-4-9-19/h5-6,10-15,19,24-25,38H,2-4,7-9,16-17H2,1H3,(H,35,40)(H,36,41)/t24-,25+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes |
Bioorg Med Chem Lett 17: 3910-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.102
BindingDB Entry DOI: 10.7270/Q2M90CFP |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50476857
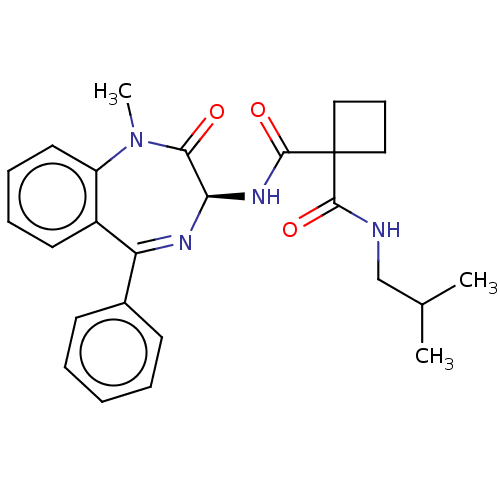
(CHEMBL449960)Show SMILES CC(C)CNC(=O)C1(CCC1)C(=O)N[C@H]1N=C(c2ccccc2)c2ccccc2N(C)C1=O |t:16| Show InChI InChI=1S/C26H30N4O3/c1-17(2)16-27-24(32)26(14-9-15-26)25(33)29-22-23(31)30(3)20-13-8-7-12-19(20)21(28-22)18-10-5-4-6-11-18/h4-8,10-13,17,22H,9,14-16H2,1-3H3,(H,27,32)(H,29,33)/t22-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes |
Bioorg Med Chem Lett 17: 3910-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.102
BindingDB Entry DOI: 10.7270/Q2M90CFP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006222

((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |r,t:13| Show InChI InChI=1S/C11H13N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h1,3-4,6,8-9,17-19H,2H2,(H2,12,13,14)/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy) rate of inactivation by NpcA |
J Med Chem 35: 1782-91 (1992)
BindingDB Entry DOI: 10.7270/Q23J3BW0 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50476862

(CHEMBL232739)Show SMILES CC(C)[C@H](O)NC(=O)C1(CCC1)C(=O)N[C@H]1c2ccccc2-c2ccccc2N(C)C1=O Show InChI InChI=1S/C25H29N3O4/c1-15(2)21(29)27-24(32)25(13-8-14-25)23(31)26-20-18-11-5-4-9-16(18)17-10-6-7-12-19(17)28(3)22(20)30/h4-7,9-12,15,20-21,29H,8,13-14H2,1-3H3,(H,26,31)(H,27,32)/t20-,21-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes |
Bioorg Med Chem Lett 17: 3910-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.102
BindingDB Entry DOI: 10.7270/Q2M90CFP |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus (rat)) | BDBM84745

(CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...)Show InChI InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3/t17-/m0/s1 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of rat NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313976
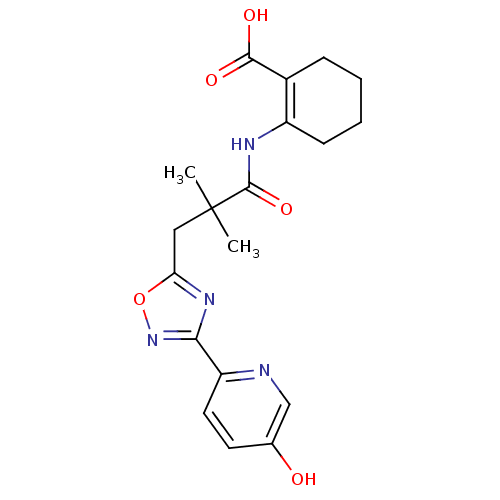
(2-({3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...)Show SMILES CC(C)(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:21| Show InChI InChI=1S/C19H22N4O5/c1-19(2,18(27)21-13-6-4-3-5-12(13)17(25)26)9-15-22-16(23-28-15)14-8-7-11(24)10-20-14/h7-8,10,24H,3-6,9H2,1-2H3,(H,21,27)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23533
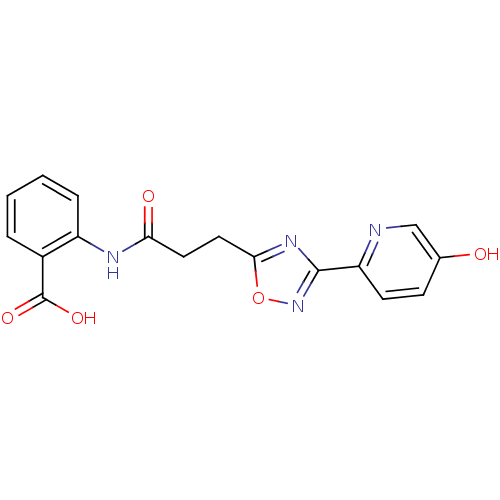
(2-{3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1nc(no1)-c1ccc(O)cn1 Show InChI InChI=1S/C17H14N4O5/c22-10-5-6-13(18-9-10)16-20-15(26-21-16)8-7-14(23)19-12-4-2-1-3-11(12)17(24)25/h1-6,9,22H,7-8H2,(H,19,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50476856
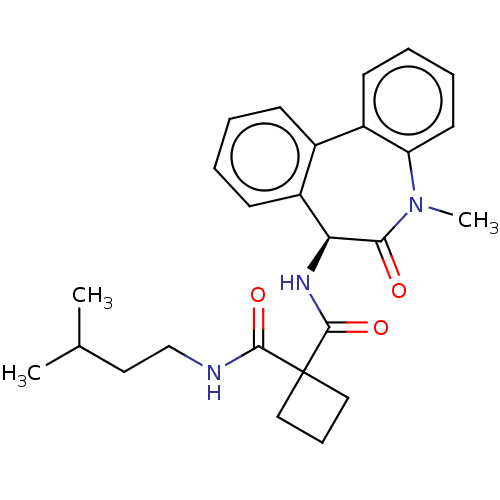
(CHEMBL232138)Show SMILES CC(C)CCNC(=O)C1(CCC1)C(=O)N[C@H]1c2ccccc2-c2ccccc2N(C)C1=O Show InChI InChI=1S/C26H31N3O3/c1-17(2)13-16-27-24(31)26(14-8-15-26)25(32)28-22-20-11-5-4-9-18(20)19-10-6-7-12-21(19)29(3)23(22)30/h4-7,9-12,17,22H,8,13-16H2,1-3H3,(H,27,31)(H,28,32)/t22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes |
Bioorg Med Chem Lett 17: 3910-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.102
BindingDB Entry DOI: 10.7270/Q2M90CFP |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401743
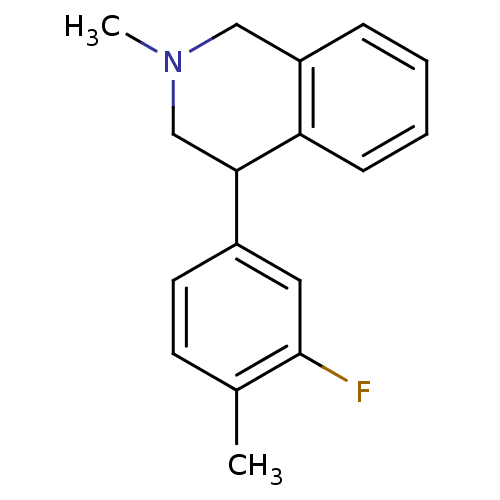
(CHEMBL2206526)Show InChI InChI=1S/C17H18FN/c1-12-7-8-13(9-17(12)18)16-11-19(2)10-14-5-3-4-6-15(14)16/h3-9,16H,10-11H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50476866
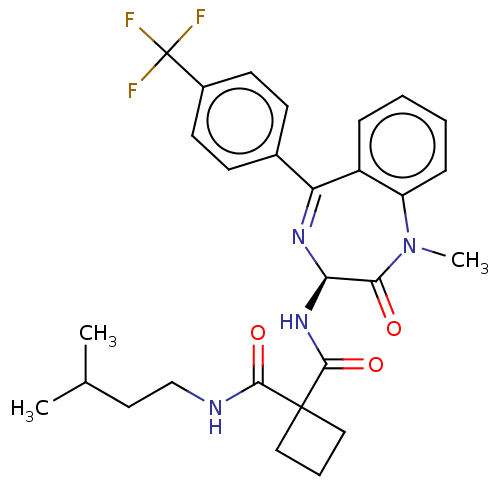
(CHEMBL231941)Show SMILES CC(C)CCNC(=O)C1(CCC1)C(=O)N[C@H]1N=C(c2ccc(cc2)C(F)(F)F)c2ccccc2N(C)C1=O |t:17| Show InChI InChI=1S/C28H31F3N4O3/c1-17(2)13-16-32-25(37)27(14-6-15-27)26(38)34-23-24(36)35(3)21-8-5-4-7-20(21)22(33-23)18-9-11-19(12-10-18)28(29,30)31/h4-5,7-12,17,23H,6,13-16H2,1-3H3,(H,32,37)(H,34,38)/t23-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes |
Bioorg Med Chem Lett 17: 3910-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.102
BindingDB Entry DOI: 10.7270/Q2M90CFP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190793
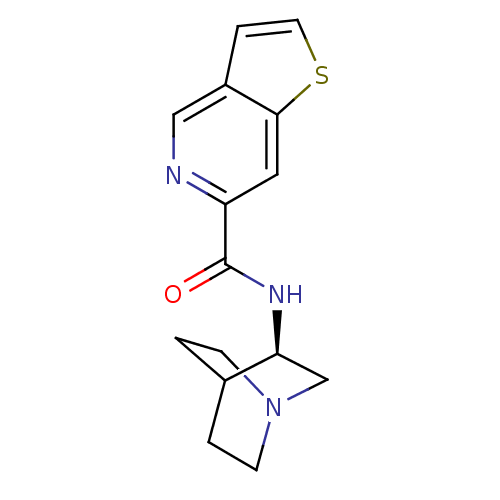
(CHEMBL268939 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2sccc2cn1 |wU:3.2,(26.77,-30.5,;26.77,-32.04,;28.11,-32.81,;29.44,-32.03,;29.43,-30.5,;30.77,-29.72,;32.11,-30.49,;32.11,-32.03,;30.78,-32.8,;29.9,-31.66,;30.72,-30.86,;25.44,-32.82,;24.1,-32.06,;22.78,-32.83,;21.31,-32.36,;20.4,-33.6,;21.31,-34.85,;22.77,-34.37,;24.1,-35.14,;25.45,-34.37,)| Show InChI InChI=1S/C15H17N3OS/c19-15(12-7-14-11(8-16-12)3-6-20-14)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401732
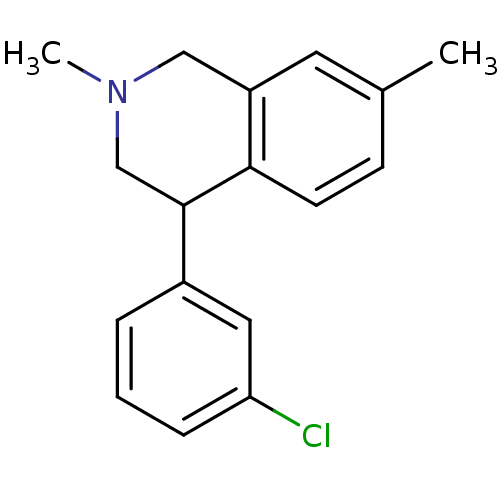
(CHEMBL2206507)Show InChI InChI=1S/C17H18ClN/c1-12-6-7-16-14(8-12)10-19(2)11-17(16)13-4-3-5-15(18)9-13/h3-9,17H,10-11H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50306529
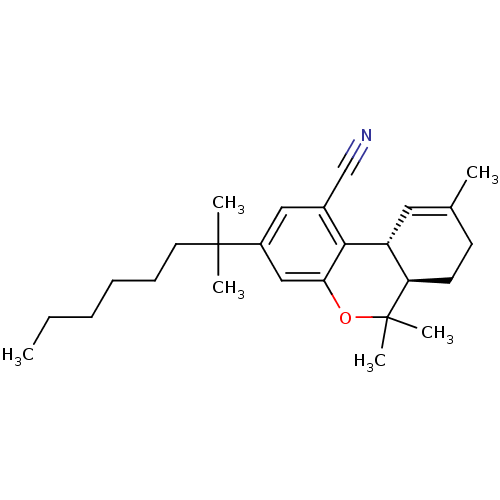
((6aR,10aR)-6,6,9-trimethyl-3-(2-methyloctan-2-yl)-...)Show SMILES CCCCCCC(C)(C)c1cc2OC(C)(C)[C@@H]3CCC(C)=C[C@H]3c2c(c1)C#N |r,c:20| Show InChI InChI=1S/C26H37NO/c1-7-8-9-10-13-25(3,4)20-15-19(17-27)24-21-14-18(2)11-12-22(21)26(5,6)28-23(24)16-20/h14-16,21-22H,7-13H2,1-6H3/t21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from CB2 receptor |
Bioorg Med Chem Lett 20: 1424-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.092
BindingDB Entry DOI: 10.7270/Q2PC32GV |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401731
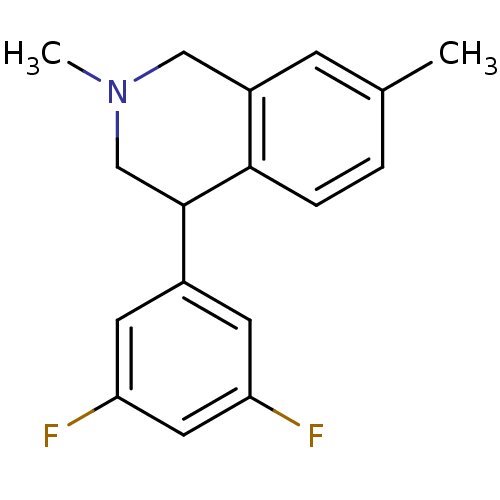
(CHEMBL2206508)Show InChI InChI=1S/C17H17F2N/c1-11-3-4-16-13(5-11)9-20(2)10-17(16)12-6-14(18)8-15(19)7-12/h3-8,17H,9-10H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM84745

(CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...)Show InChI InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3/t17-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 5.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50377049
(CHEMBL258031)Show SMILES O=C(N[C@@H]1C[C@H]2CCN(C2)C1)c1ccc2occc2c1 |TLB:2:3:9:7.6| Show InChI InChI=1S/C16H18N2O2/c19-16(13-1-2-15-12(8-13)4-6-20-15)17-14-7-11-3-5-18(9-11)10-14/h1-2,4,6,8,11,14H,3,5,7,9-10H2,(H,17,19)/t11-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha-7 nACh receptor in rat brain |
Bioorg Med Chem Lett 18: 3611-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.070
BindingDB Entry DOI: 10.7270/Q2KD1ZS9 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50476875

(CHEMBL232183)Show SMILES CC(C)CNC(=O)C1(CCCC1)C(=O)N[C@H]1N=C(c2ccccc2)c2ccccc2N(C)C1=O |t:17| Show InChI InChI=1S/C27H32N4O3/c1-18(2)17-28-25(33)27(15-9-10-16-27)26(34)30-23-24(32)31(3)21-14-8-7-13-20(21)22(29-23)19-11-5-4-6-12-19/h4-8,11-14,18,23H,9-10,15-17H2,1-3H3,(H,28,33)(H,30,34)/t23-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes |
Bioorg Med Chem Lett 17: 3910-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.102
BindingDB Entry DOI: 10.7270/Q2M90CFP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190791
(CHEMBL210865 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccsc2cn1 |wU:3.2,(11.37,-32.25,;11.38,-33.79,;12.72,-34.55,;14.05,-33.78,;14.04,-32.24,;15.38,-31.47,;16.72,-32.24,;16.71,-33.78,;15.39,-34.55,;14.51,-33.4,;15.33,-32.61,;10.05,-34.57,;8.71,-33.8,;7.38,-34.58,;5.92,-34.1,;5.01,-35.35,;5.92,-36.59,;7.38,-36.12,;8.71,-36.89,;10.06,-36.12,)| Show InChI InChI=1S/C15H17N3OS/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus (rat)) | BDBM50401730

(CHEMBL2206509)Show InChI InChI=1S/C17H18FN/c1-12-3-8-16-14(9-12)10-19(2)11-17(16)13-4-6-15(18)7-5-13/h3-9,17H,10-11H2,1-2H3/t17-/m0/s1 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of rat NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190789
(CHEMBL208565 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2sccc2c1 |wU:3.2,(11.98,-5.28,;11.99,-6.82,;13.33,-7.58,;14.66,-6.8,;14.65,-5.27,;15.99,-4.5,;17.32,-5.26,;17.32,-6.8,;16,-7.58,;15.12,-6.43,;15.94,-5.63,;10.66,-7.59,;10.66,-9.15,;9.32,-9.92,;7.99,-9.15,;6.52,-9.62,;5.62,-8.37,;6.53,-7.13,;7.99,-7.61,;9.32,-6.83,)| Show InChI InChI=1S/C16H18N2OS/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50190786
((R)-N-(quinuclidin-3-yl)furo[2,3-c]pyridine-5-carb...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccoc2cn1 |wU:3.2,(-5.16,-23.46,;-5.16,-25,;-3.82,-25.76,;-2.49,-24.99,;-2.5,-23.45,;-1.16,-22.68,;.18,-23.45,;.18,-24.99,;-1.15,-25.76,;-2.03,-24.61,;-1.21,-23.81,;-6.49,-25.78,;-7.83,-25.01,;-9.15,-25.79,;-10.62,-25.31,;-11.52,-26.55,;-10.62,-27.8,;-9.16,-27.33,;-7.82,-28.1,;-6.48,-27.33,)| Show InChI InChI=1S/C15H17N3O2/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha-7 nACh receptor in human IMR32 cells |
Bioorg Med Chem Lett 18: 3611-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.070
BindingDB Entry DOI: 10.7270/Q2KD1ZS9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50377048

(CHEMBL403857)Show SMILES O=C(N[C@@H]1C[C@H]2CCN(C2)C1)c1cc2sccc2cn1 |TLB:2:3:9:7.6| Show InChI InChI=1S/C15H17N3OS/c19-15(13-6-14-11(7-16-13)2-4-20-14)17-12-5-10-1-3-18(8-10)9-12/h2,4,6-7,10,12H,1,3,5,8-9H2,(H,17,19)/t10-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha-7 nACh receptor in rat brain |
Bioorg Med Chem Lett 18: 3611-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.070
BindingDB Entry DOI: 10.7270/Q2KD1ZS9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401730

(CHEMBL2206509)Show InChI InChI=1S/C17H18FN/c1-12-3-8-16-14(9-12)10-19(2)11-17(16)13-4-6-15(18)7-5-13/h3-9,17H,10-11H2,1-2H3/t17-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401744
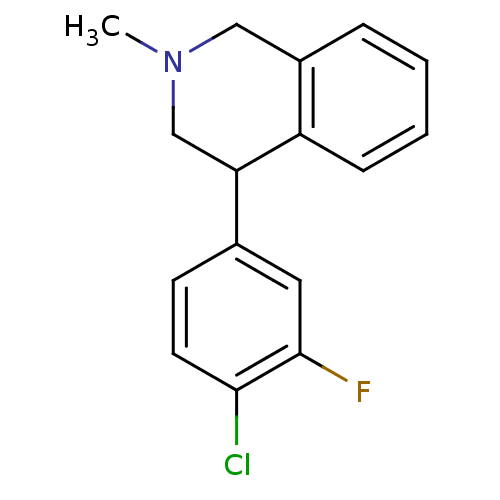
(CHEMBL2206525)Show InChI InChI=1S/C16H15ClFN/c1-19-9-12-4-2-3-5-13(12)14(10-19)11-6-7-15(17)16(18)8-11/h2-8,14H,9-10H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401737

(CHEMBL2206532)Show InChI InChI=1S/C18H21NO/c1-13-5-4-6-14(9-13)18-12-19(2)11-15-10-16(20-3)7-8-17(15)18/h4-10,18H,11-12H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313978

(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES CC(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:20| Show InChI InChI=1S/C18H20N4O5/c1-10(17(24)20-13-5-3-2-4-12(13)18(25)26)8-15-21-16(22-27-15)14-7-6-11(23)9-19-14/h6-7,9-10,23H,2-5,8H2,1H3,(H,20,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313978

(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES CC(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:20| Show InChI InChI=1S/C18H20N4O5/c1-10(17(24)20-13-5-3-2-4-12(13)18(25)26)8-15-21-16(22-27-15)14-7-6-11(23)9-19-14/h6-7,9-10,23H,2-5,8H2,1H3,(H,20,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190786

((R)-N-(quinuclidin-3-yl)furo[2,3-c]pyridine-5-carb...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccoc2cn1 |wU:3.2,(-5.16,-23.46,;-5.16,-25,;-3.82,-25.76,;-2.49,-24.99,;-2.5,-23.45,;-1.16,-22.68,;.18,-23.45,;.18,-24.99,;-1.15,-25.76,;-2.03,-24.61,;-1.21,-23.81,;-6.49,-25.78,;-7.83,-25.01,;-9.15,-25.79,;-10.62,-25.31,;-11.52,-26.55,;-10.62,-27.8,;-9.16,-27.33,;-7.82,-28.1,;-6.48,-27.33,)| Show InChI InChI=1S/C15H17N3O2/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data