

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50161121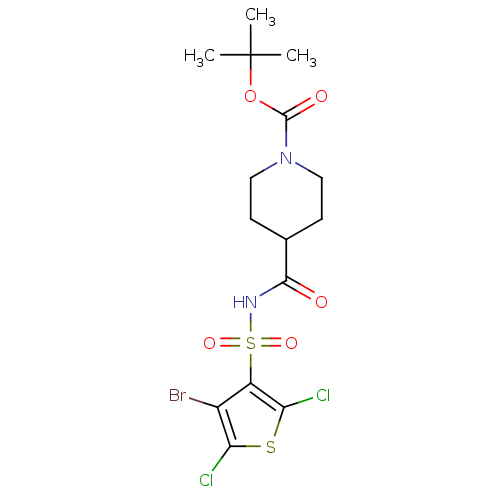 (4-(4-Bromo-2,5-dichloro-thiophene-3-sulfonylaminoc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibitory constant against human Steroid sulfatase | Bioorg Med Chem Lett 15: 1235-8 (2005) Article DOI: 10.1016/j.bmcl.2004.11.069 BindingDB Entry DOI: 10.7270/Q2RF5THG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50134333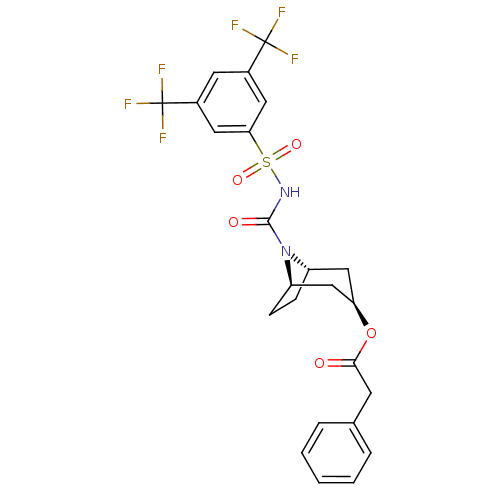 (CHEMBL331089 | Phenyl-acetic acid (1R,3R,5S)-8-(3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna Curated by ChEMBL | Assay Description Inhibitory activity against Steroid sulfatase expressed in CHO cells | Bioorg Med Chem Lett 13: 3673-7 (2003) BindingDB Entry DOI: 10.7270/Q27S7N5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50134333 (CHEMBL331089 | Phenyl-acetic acid (1R,3R,5S)-8-(3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibitory constant against human steroid sulfatase | Bioorg Med Chem Lett 15: 1235-8 (2005) Article DOI: 10.1016/j.bmcl.2004.11.069 BindingDB Entry DOI: 10.7270/Q2RF5THG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50135162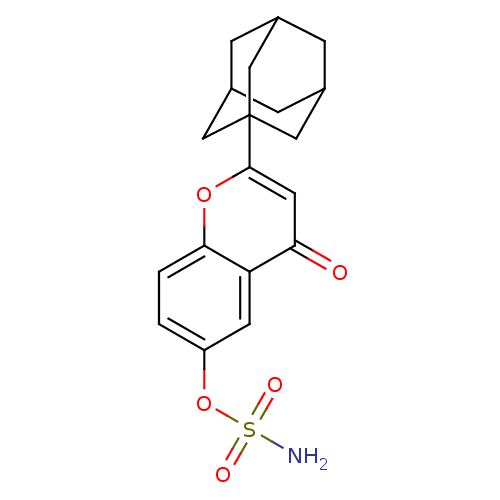 (CHEMBL147705 | Sulfamic acid 2-adamantan-1-yl-4-ox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna Curated by ChEMBL | Assay Description Inhibitory activity against human steroid sulfatase | J Med Chem 47: 4268-76 (2004) Article DOI: 10.1021/jm0407916 BindingDB Entry DOI: 10.7270/Q2XW4KK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50118560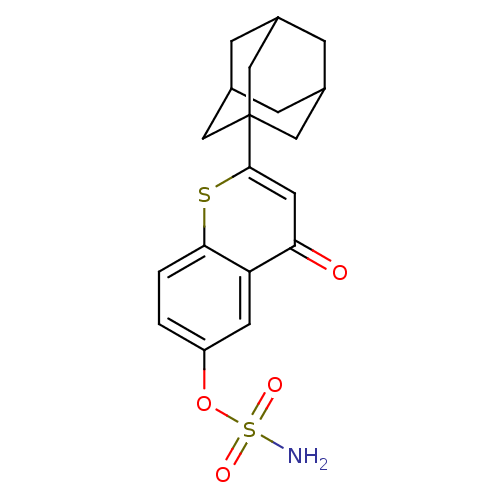 (CHEMBL262050 | Sulfamic acid 2-adamantan-1-yl-4-ox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna Curated by ChEMBL | Assay Description Inhibitory activity against human steroid sulfatase | J Med Chem 47: 4268-76 (2004) Article DOI: 10.1021/jm0407916 BindingDB Entry DOI: 10.7270/Q2XW4KK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50161124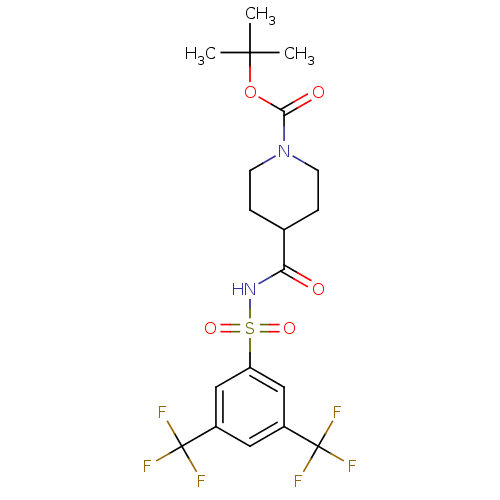 (4-(3,5-Bis-trifluoromethyl-benzenesulfonylaminocar...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibitory constant against human steroid sulfatase | Bioorg Med Chem Lett 15: 1235-8 (2005) Article DOI: 10.1016/j.bmcl.2004.11.069 BindingDB Entry DOI: 10.7270/Q2RF5THG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50134329 (CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory constant against human steroid sulfatase in CHO cells | Bioorg Med Chem Lett 13: 4313-6 (2003) BindingDB Entry DOI: 10.7270/Q28S4QGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50151142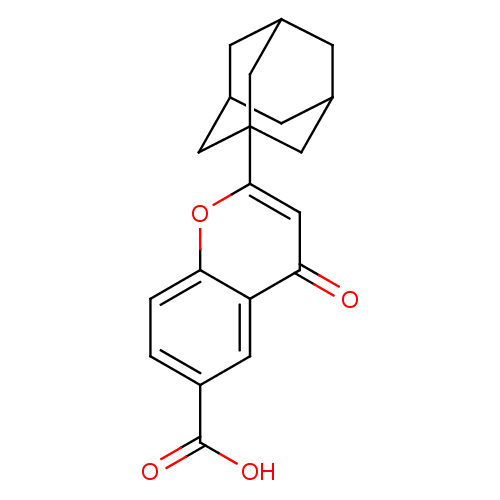 (2-Adamantan-1-yl-4-oxo-4H-chromene-6-carboxylic ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna Curated by ChEMBL | Assay Description Inhibitory activity against human steroid sulfatase | J Med Chem 47: 4268-76 (2004) Article DOI: 10.1021/jm0407916 BindingDB Entry DOI: 10.7270/Q2XW4KK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50151137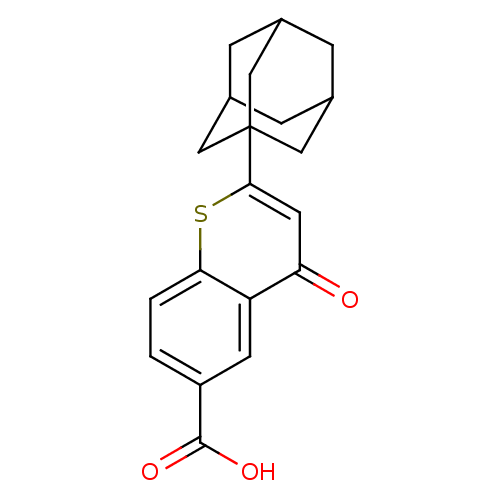 (2-Adamantan-1-yl-4-oxo-4H-thiochromene-6-carboxyli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna Curated by ChEMBL | Assay Description Inhibitory activity against human steroid sulfatase | J Med Chem 47: 4268-76 (2004) Article DOI: 10.1021/jm0407916 BindingDB Entry DOI: 10.7270/Q2XW4KK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50134332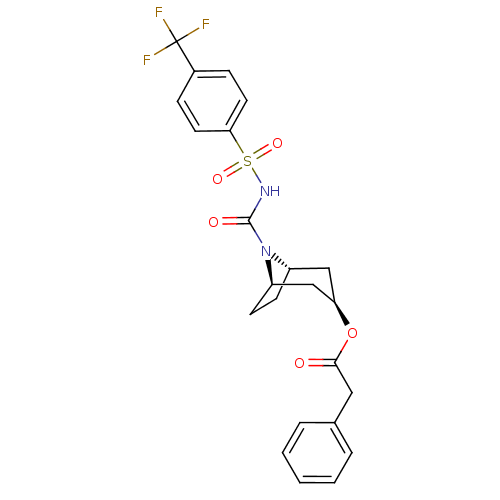 (CHEMBL332811 | Phenyl-acetic acid (1R,3R,5S)-8-(4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna Curated by ChEMBL | Assay Description Inhibitory constant against purified human Steroid sulfatase | Bioorg Med Chem Lett 13: 3673-7 (2003) BindingDB Entry DOI: 10.7270/Q27S7N5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50134329 (CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna Curated by ChEMBL | Assay Description Inhibitory activity against human steroid sulfatase | J Med Chem 47: 4268-76 (2004) Article DOI: 10.1021/jm0407916 BindingDB Entry DOI: 10.7270/Q2XW4KK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50134329 (CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibitory constant against human steroid sulfatase | Bioorg Med Chem Lett 15: 1235-8 (2005) Article DOI: 10.1016/j.bmcl.2004.11.069 BindingDB Entry DOI: 10.7270/Q2RF5THG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50134329 (CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna Curated by ChEMBL | Assay Description Inhibitory activity against purified human Steroid sulfatase | Bioorg Med Chem Lett 13: 3673-7 (2003) BindingDB Entry DOI: 10.7270/Q27S7N5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50134335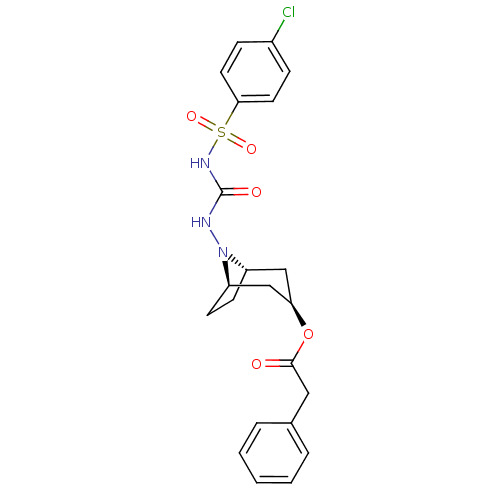 (8-[N-(4-chlorophenylsulfonamido)-carbonyl amino]-8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna Curated by ChEMBL | Assay Description Inhibitory activity against Steroid sulfatase expressed in CHO cells | Bioorg Med Chem Lett 13: 3673-7 (2003) BindingDB Entry DOI: 10.7270/Q27S7N5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50134325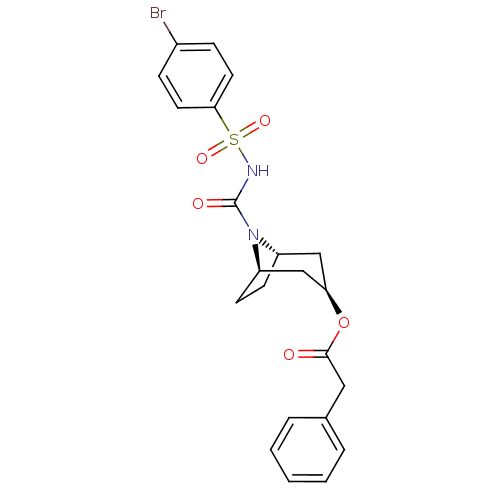 (CHEMBL440159 | Phenyl-acetic acid (1R,3R,5S)-8-(4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna Curated by ChEMBL | Assay Description Inhibitory activity against Steroid sulfatase expressed in CHO cells | Bioorg Med Chem Lett 13: 3673-7 (2003) BindingDB Entry DOI: 10.7270/Q27S7N5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50151135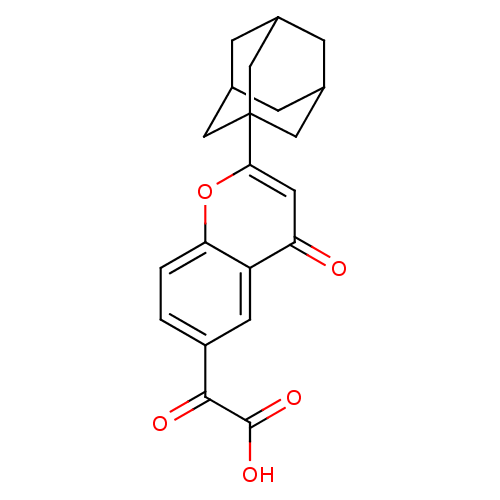 ((2-Adamantan-1-yl-4-oxo-4H-chromen-6-yl)-oxo-aceti...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna Curated by ChEMBL | Assay Description Inhibitory activity against human steroid sulfatase | J Med Chem 47: 4268-76 (2004) Article DOI: 10.1021/jm0407916 BindingDB Entry DOI: 10.7270/Q2XW4KK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50134328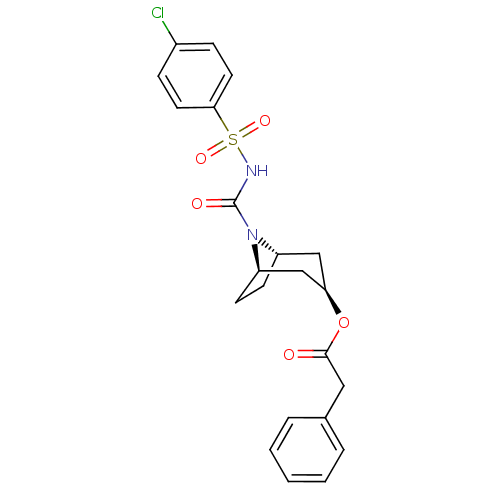 (CHEMBL124349 | Phenyl-acetic acid (1R,3R,5S)-8-(4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna Curated by ChEMBL | Assay Description Inhibitory activity against Steroid sulfatase expressed in CHO cells | Bioorg Med Chem Lett 13: 3673-7 (2003) BindingDB Entry DOI: 10.7270/Q27S7N5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50134328 (CHEMBL124349 | Phenyl-acetic acid (1R,3R,5S)-8-(4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibitory constant against human steroid sulfatase | Bioorg Med Chem Lett 15: 1235-8 (2005) Article DOI: 10.1016/j.bmcl.2004.11.069 BindingDB Entry DOI: 10.7270/Q2RF5THG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM50361254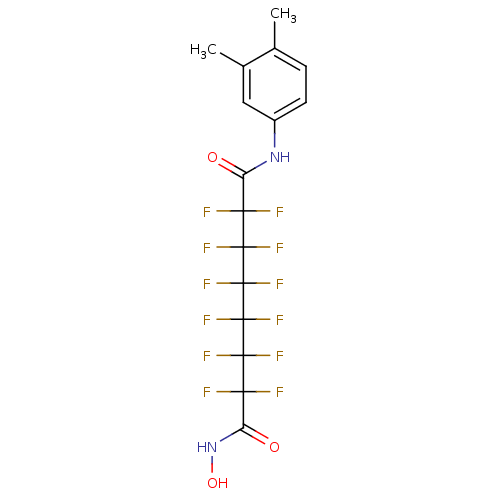 (CHEMBL1934896) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Inhibitory potency against Varicella zoster virus ribonucleotide reductase | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50151140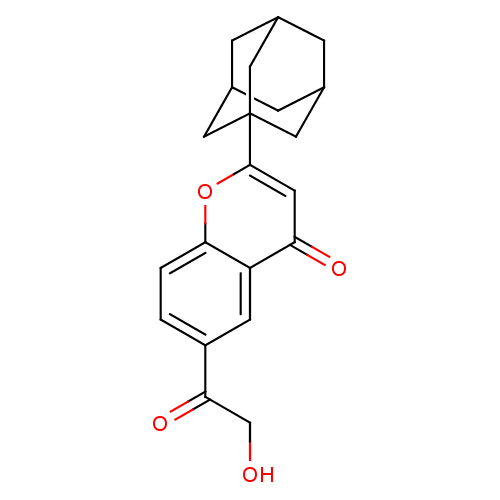 (2-Adamantan-1-yl-6-(2-hydroxy-acetyl)-chromen-4-on...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna Curated by ChEMBL | Assay Description Inhibitory activity against human steroid sulfatase | J Med Chem 47: 4268-76 (2004) Article DOI: 10.1021/jm0407916 BindingDB Entry DOI: 10.7270/Q2XW4KK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50134337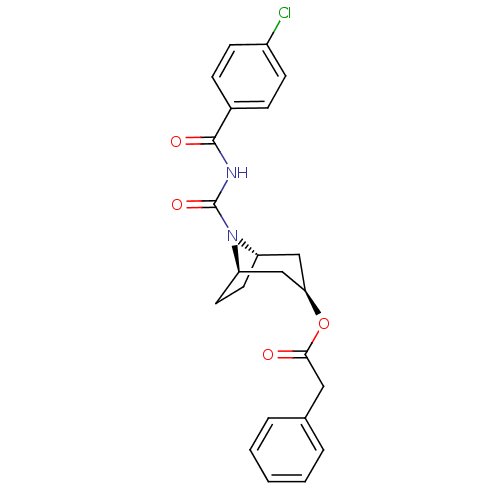 (CHEMBL121068 | Phenyl-acetic acid (1R,3R,5S)-8-(4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna Curated by ChEMBL | Assay Description Inhibitory constant against purified human Steroid sulfatase | Bioorg Med Chem Lett 13: 3673-7 (2003) BindingDB Entry DOI: 10.7270/Q27S7N5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50136297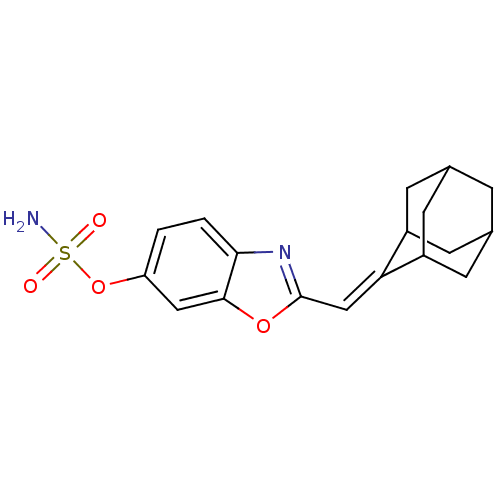 (CHEMBL136112 | Sulfamic acid 2-adamantan-2-ylidene...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory constant against human steroid sulfatase in CHO cells | Bioorg Med Chem Lett 13: 4313-6 (2003) BindingDB Entry DOI: 10.7270/Q28S4QGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361254 (CHEMBL1934896) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Inhibition of human HDAC1 using Boc-Lys(Ac)-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins by trypsi... | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50361254 (CHEMBL1934896) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 4 receptor in rat striatum by [3H]GR-113808 displacement. | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Inhibition of human HDAC5 using Boc-Lys(trifluoroacetyl)-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 m... | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM50361248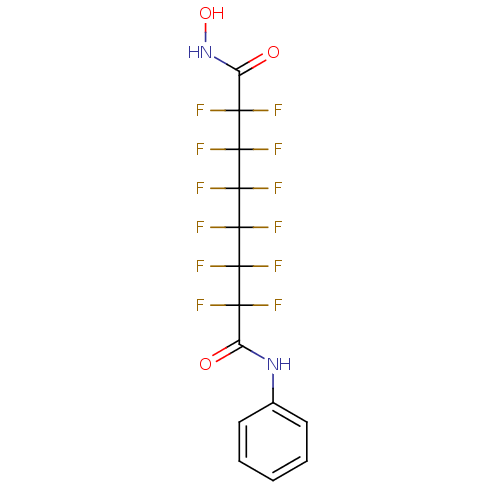 (CHEMBL1934890) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Inhibition of human HDAC5 using Boc-Lys(trifluoroacetyl)-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 m... | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Inhibition of human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 m... | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50161118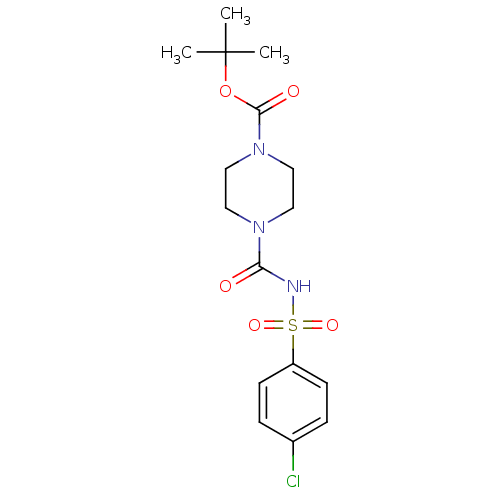 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-piperazi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibitory constant against human steroid sulfatase | Bioorg Med Chem Lett 15: 1235-8 (2005) Article DOI: 10.1016/j.bmcl.2004.11.069 BindingDB Entry DOI: 10.7270/Q2RF5THG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50161119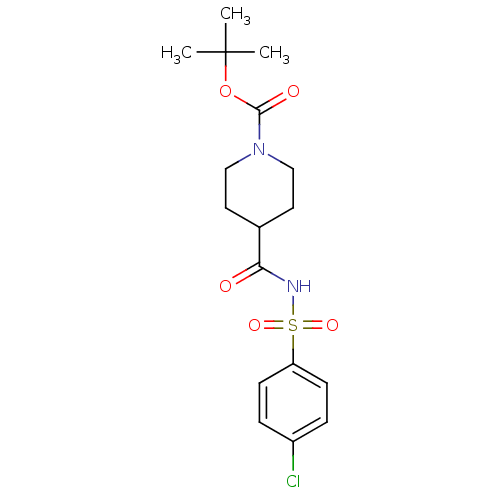 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-piperidi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibitory constant against human steroid sulfatase | Bioorg Med Chem Lett 15: 1235-8 (2005) Article DOI: 10.1016/j.bmcl.2004.11.069 BindingDB Entry DOI: 10.7270/Q2RF5THG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50361254 (CHEMBL1934896) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Inhibitory potency against Varicella zoster virus ribonucleotide reductase | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50151134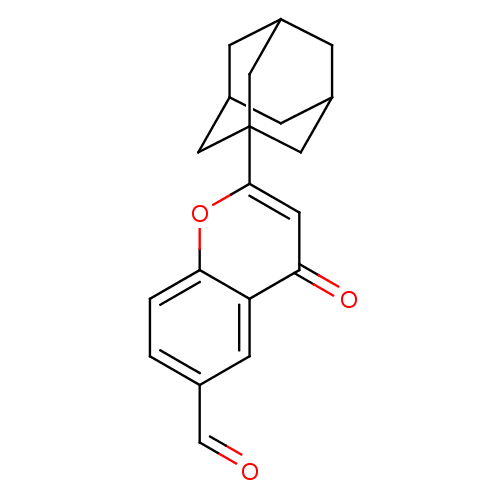 (2-Adamantan-1-yl-4-oxo-4H-chromene-6-carbaldehyde ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna Curated by ChEMBL | Assay Description Inhibitory activity against human steroid sulfatase | J Med Chem 47: 4268-76 (2004) Article DOI: 10.1021/jm0407916 BindingDB Entry DOI: 10.7270/Q2XW4KK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50151141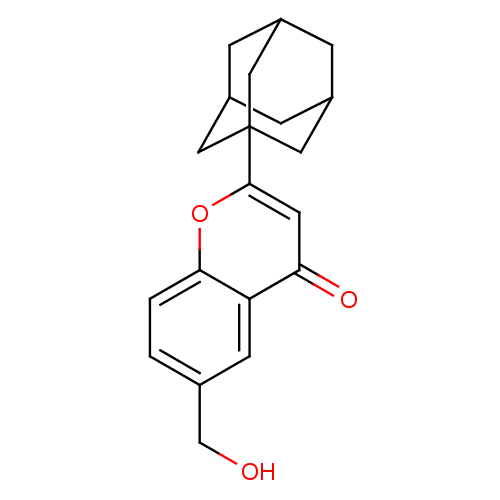 (2-Adamantan-1-yl-6-hydroxymethyl-chromen-4-one | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna Curated by ChEMBL | Assay Description Inhibitory activity against human steroid sulfatase | J Med Chem 47: 4268-76 (2004) Article DOI: 10.1021/jm0407916 BindingDB Entry DOI: 10.7270/Q2XW4KK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50361252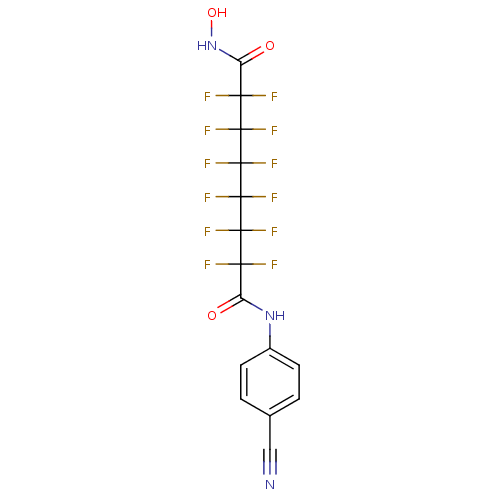 (CHEMBL1934894) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Inhibition of human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 m... | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50361253 (CHEMBL1934895) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Inhibition of human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 m... | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50235708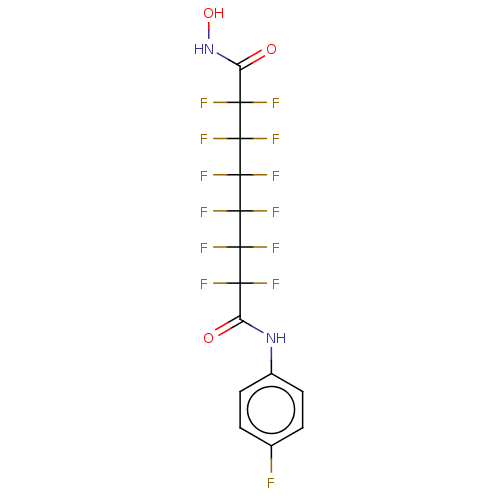 (CHEMBL4079958) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Inhibition of human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 m... | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50235705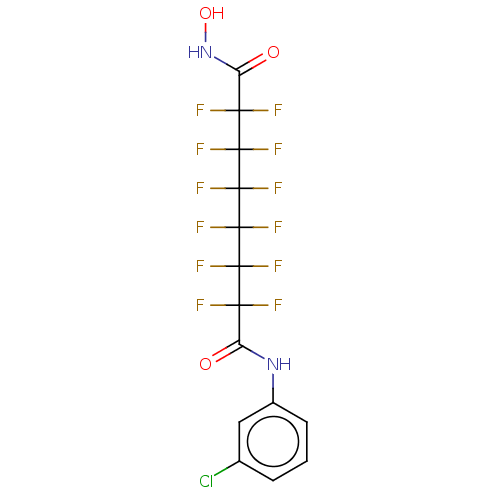 (CHEMBL4093113) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Inhibition of human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 m... | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50235708 (CHEMBL4079958) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Inhibition of human HDAC1 using Boc-Lys(Ac)-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins by trypsi... | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50235702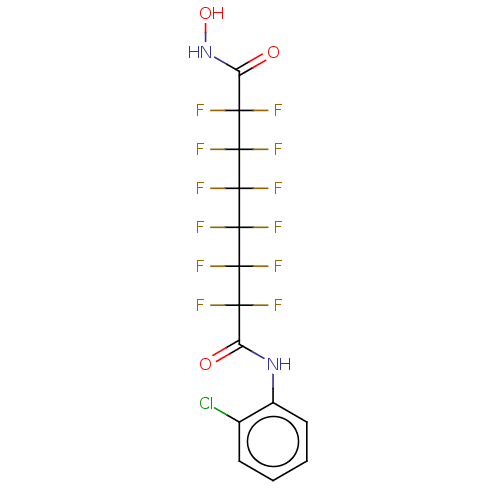 (CHEMBL4104197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Inhibition of human HDAC1 using Boc-Lys(Ac)-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins by trypsi... | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50361262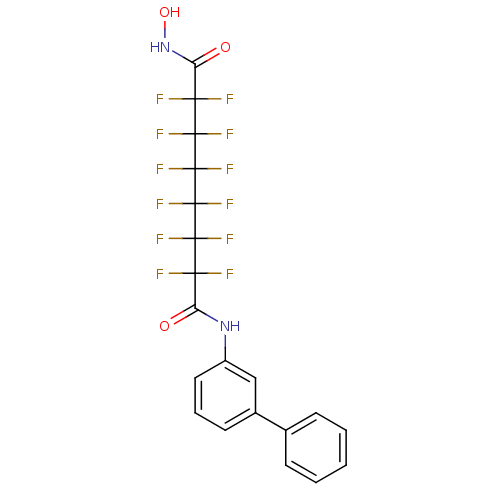 (CHEMBL1934904) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Inhibition of human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 m... | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50361255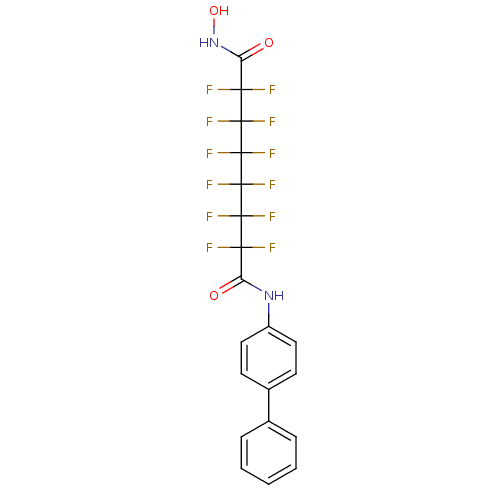 (CHEMBL1934897) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Inhibition of human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 m... | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50361263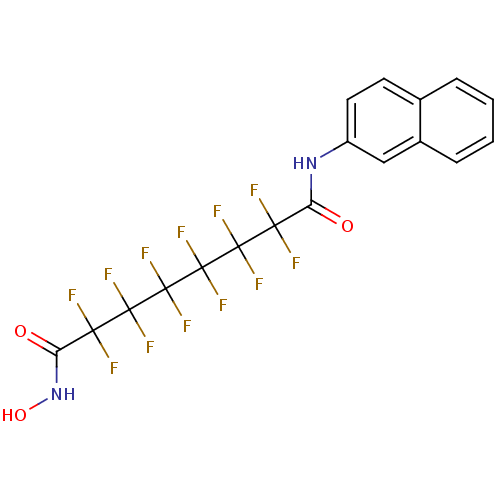 (CHEMBL1934905) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Inhibition of human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 m... | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50361261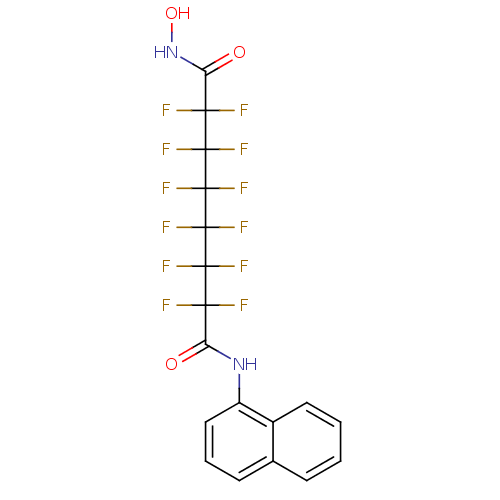 (CHEMBL1934903) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Inhibition of human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 m... | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50361256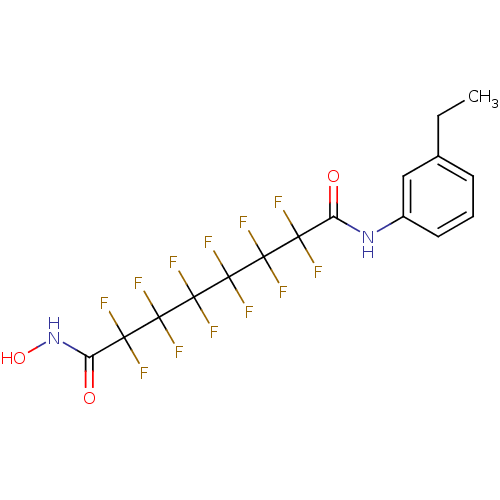 (CHEMBL1934898) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Inhibition of human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 m... | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM50361267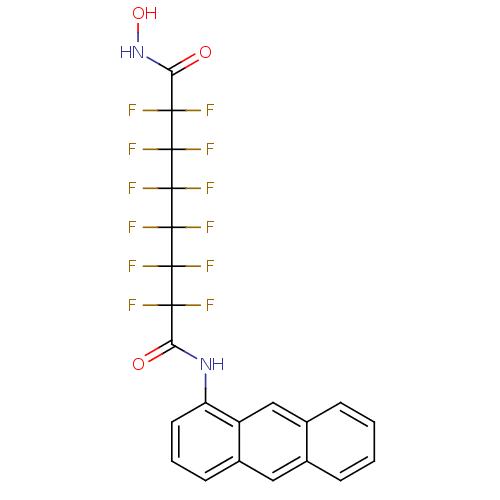 (CHEMBL1934909) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 3 receptor in rat entorhinal cortex by [3H]BRL-43694 displacement. | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50361250 (CHEMBL1934892) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description In vitro relaxation of carbachol pre-contracted rat oesophageal TMM. | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50235706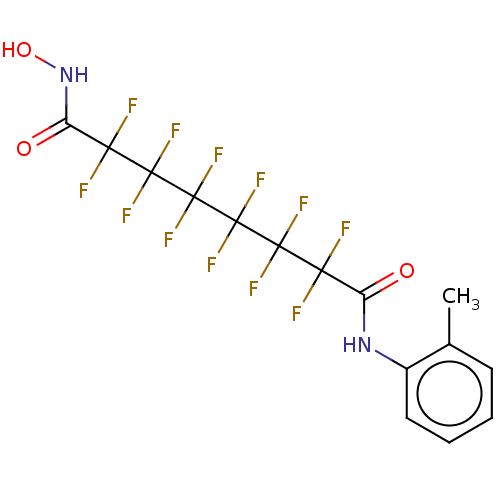 (CHEMBL4080874) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 3 receptor in rat entorhinal cortex by [3H]BRL-43694 displacement. | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50235708 (CHEMBL4079958) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 3 receptor in rat entorhinal cortex by [3H]BRL-43694 displacement. | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50235707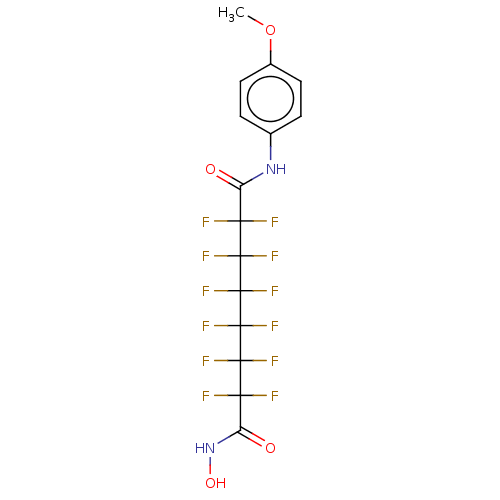 (CHEMBL4095542) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 3 receptor in rat entorhinal cortex by [3H]BRL-43694 displacement. | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50361264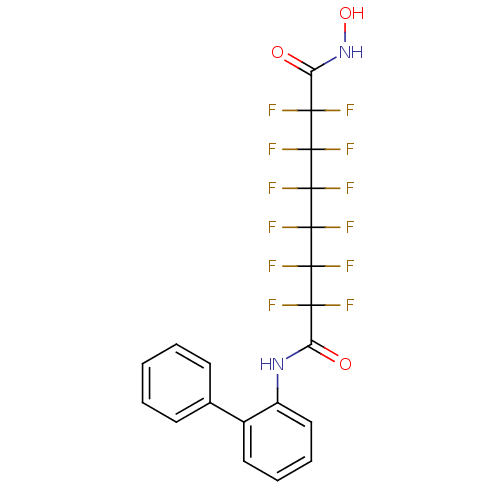 (CHEMBL1934906) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 3 receptor in rat entorhinal cortex by [3H]BRL-43694 displacement. | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM50361265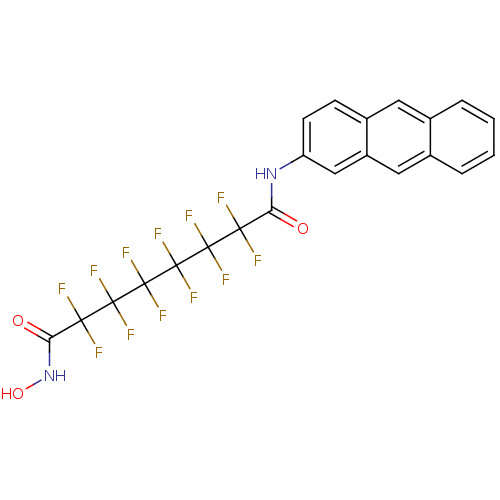 (CHEMBL1934907) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 3 receptor in rat entorhinal cortex by [3H]BRL-43694 displacement. | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 522 total ) | Next | Last >> |