

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118030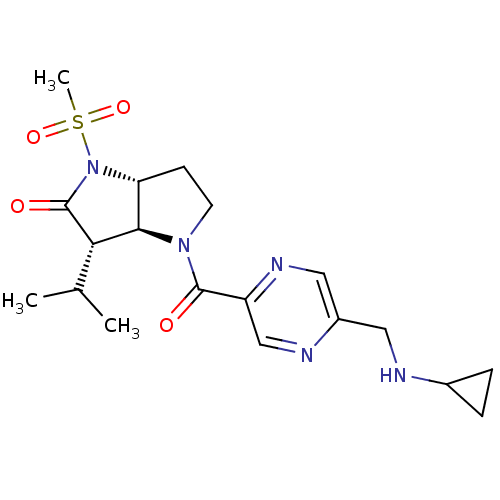 (4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118028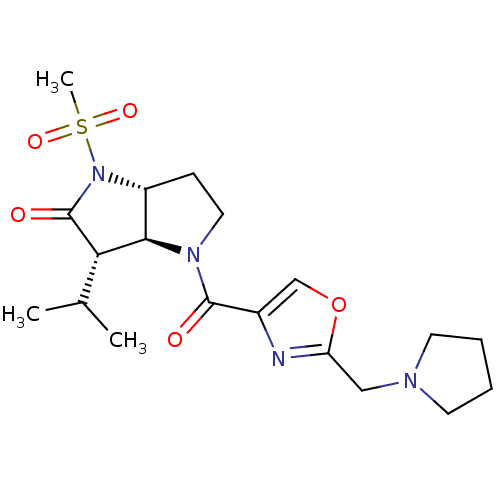 (3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118028 (3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.160 | n/a | n/a | n/a | n/a | 4.90 | 3.05E+4 | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118029 (3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | 0.00000207 | 6.63E+3 | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118029 (3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118027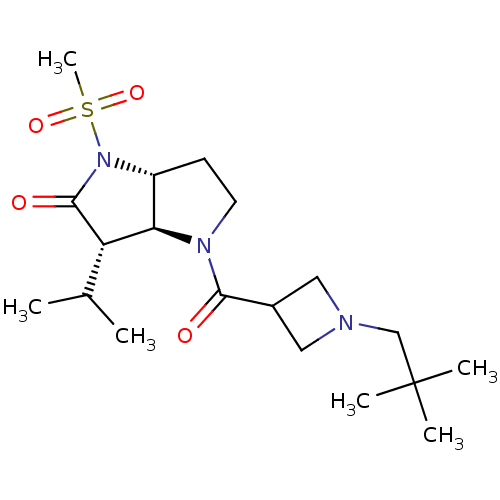 (4-[1-(2,2-Dimethyl-propyl)-azetidine-3-carbonyl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118027 (4-[1-(2,2-Dimethyl-propyl)-azetidine-3-carbonyl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.73 | n/a | n/a | n/a | n/a | 0.0000109 | 6.31E+3 | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50097655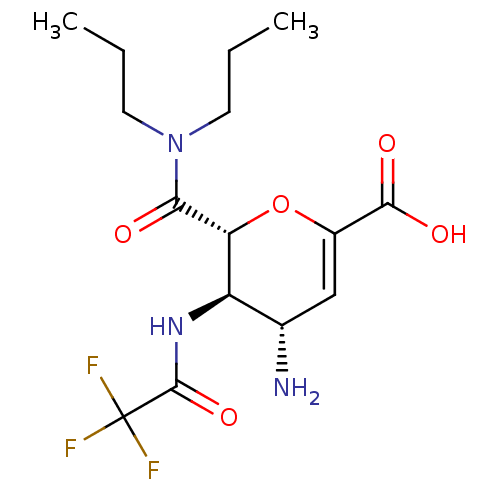 ((4S,5R,6R)-4-Amino-6-dipropylcarbamoyl-5-(2,2,2-tr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza A sialidase (Aichi) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50097648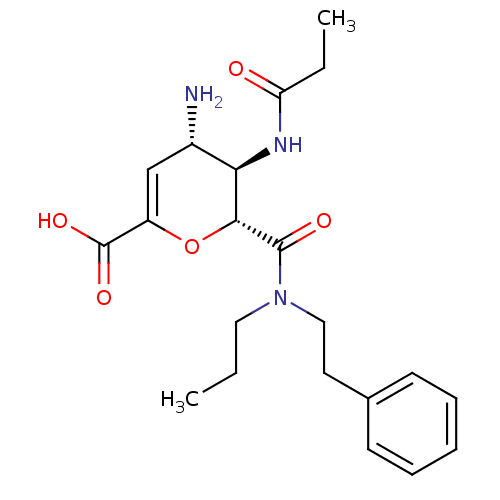 ((4S,5R,6R)-4-Amino-6-(phenethyl-propyl-carbamoyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza A sialidase (Aichi) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza A sialidase (Aichi) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4945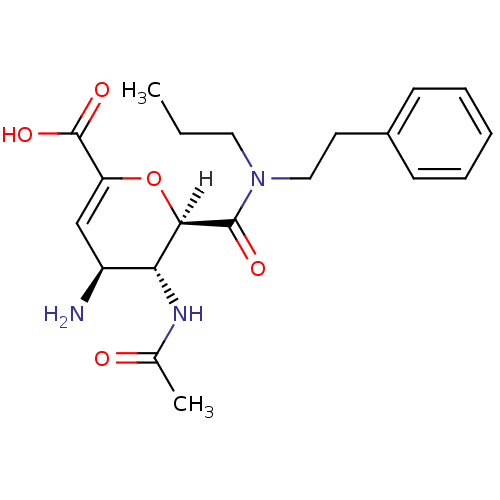 ((2R,3R,4S)-4-amino-3-acetamido-2-[(2-phenylethyl)(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited Curated by ChEMBL | Assay Description Compound was tested for inhibitory concentration against Influenza sialidase type A | J Med Chem 41: 798-807 (1998) Article DOI: 10.1021/jm9703754 BindingDB Entry DOI: 10.7270/Q2M32WFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4945 ((2R,3R,4S)-4-amino-3-acetamido-2-[(2-phenylethyl)(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza A sialidase (Aichi) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4945 ((2R,3R,4S)-4-amino-3-acetamido-2-[(2-phenylethyl)(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza A sialidase (Aichi) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50097659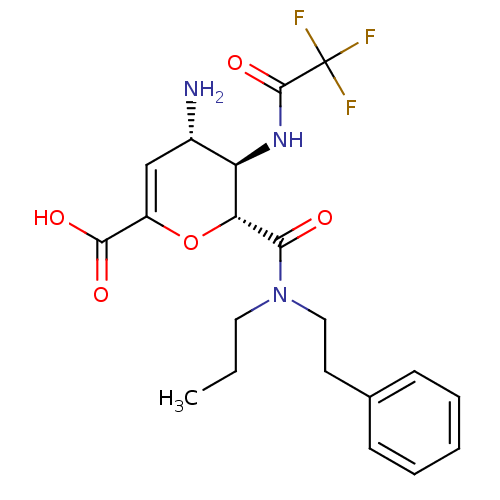 ((4S,5R,6R)-4-Amino-6-(phenethyl-propyl-carbamoyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza A sialidase (Aichi) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited Curated by ChEMBL | Assay Description Compound was tested for inhibitory concentration against Influenza sialidase type B | J Med Chem 41: 798-807 (1998) Article DOI: 10.1021/jm9703754 BindingDB Entry DOI: 10.7270/Q2M32WFK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza B sialidase (Victoria) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50097654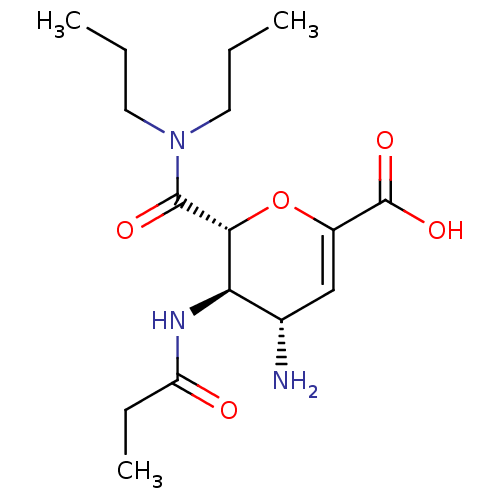 ((4S,5R,6R)-4-Amino-6-dipropylcarbamoyl-5-propionyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza A sialidase (Aichi) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited Curated by ChEMBL | Assay Description Compound was tested for inhibitory concentration against Influenza sialidase type A | J Med Chem 41: 798-807 (1998) Article DOI: 10.1021/jm9703754 BindingDB Entry DOI: 10.7270/Q2M32WFK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4952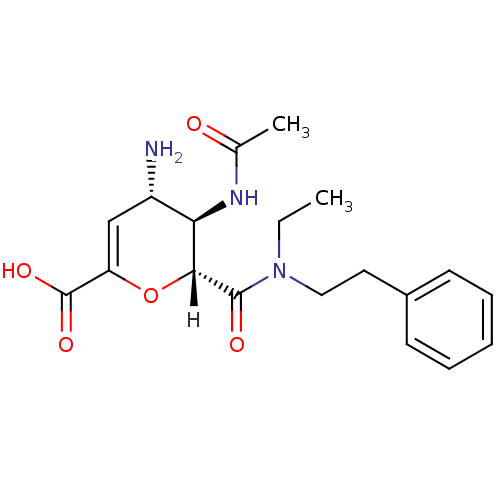 ((2R,3R,4S)-4-amino-3-acetamido-2-[ethyl(2-phenylet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited Curated by ChEMBL | Assay Description Compound was tested for inhibitory concentration against Influenza sialidase type A | J Med Chem 41: 798-807 (1998) Article DOI: 10.1021/jm9703754 BindingDB Entry DOI: 10.7270/Q2M32WFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50097650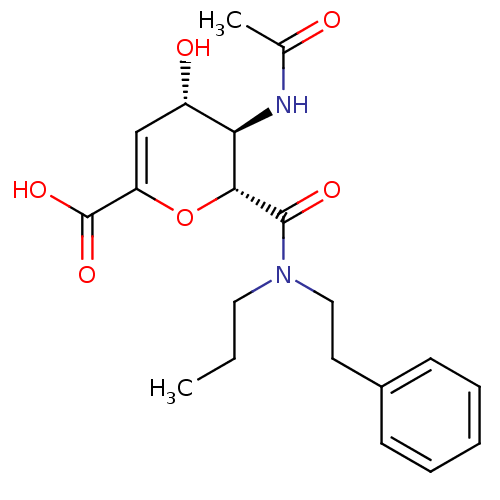 ((4S,5R,6R)-5-Acetylamino-4-hydroxy-6-(phenethyl-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza A sialidase (Aichi) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50097661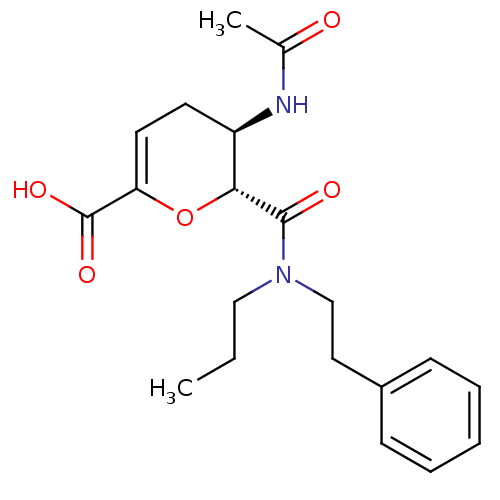 ((5R,6R)-5-Acetylamino-6-(phenethyl-propyl-carbamoy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza A sialidase (Aichi) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118028 (3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118030 (4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4929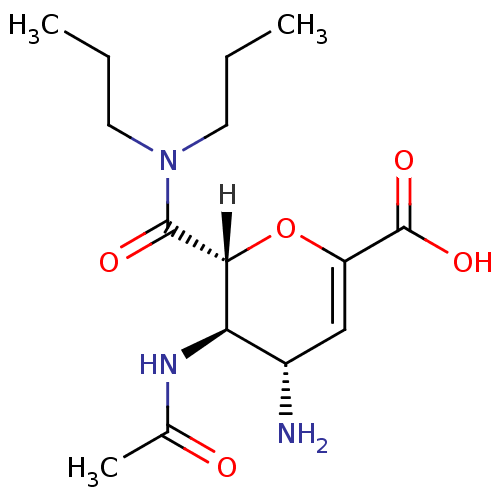 ((2R,3R,4S)-4-amino-2-(dipropylcarbamoyl)-3-acetami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza A sialidase (Aichi) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4958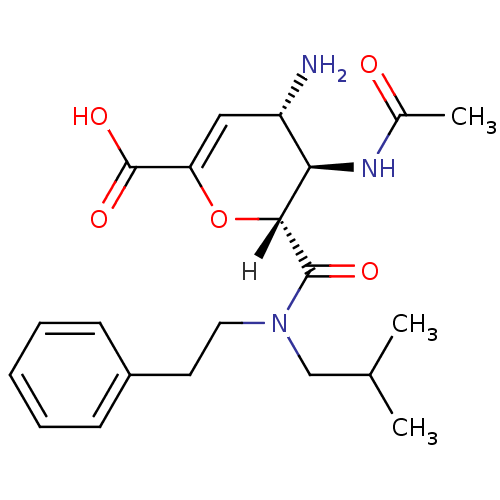 ((2R,3R,4S)-4-amino-3-acetamido-2-[(2-methylpropyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited Curated by ChEMBL | Assay Description Compound was tested for inhibitory concentration against Influenza sialidase type A | J Med Chem 41: 798-807 (1998) Article DOI: 10.1021/jm9703754 BindingDB Entry DOI: 10.7270/Q2M32WFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50097649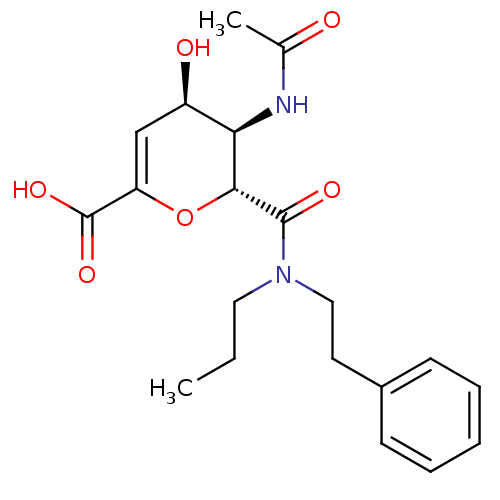 ((4R,5R,6R)-5-Acetylamino-4-hydroxy-6-(phenethyl-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza A sialidase (Aichi) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118029 (3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118029 (3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against dog neutrophil elastase using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50118029 (3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Chymotrypsinogen using selectivity assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118027 (4-[1-(2,2-Dimethyl-propyl)-azetidine-3-carbonyl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50097656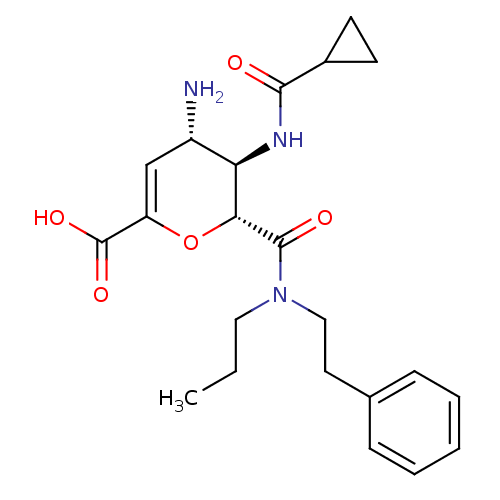 ((4S,5R,6R)-4-Amino-5-(cyclopropanecarbonyl-amino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza A sialidase (Aichi) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50097659 ((4S,5R,6R)-4-Amino-6-(phenethyl-propyl-carbamoyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza B sialidase (Victoria) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50097658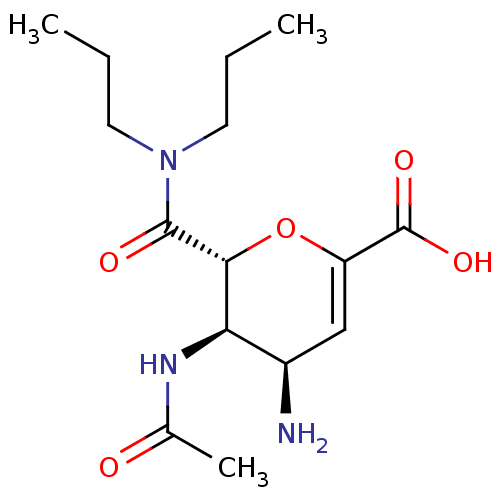 ((4R,5R,6R)-5-Acetylamino-4-amino-6-dipropylcarbamo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza A sialidase (Aichi) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118030 (4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118027 (4-[1-(2,2-Dimethyl-propyl)-azetidine-3-carbonyl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against dog neutrophil elastase using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118027 (4-[1-(2,2-Dimethyl-propyl)-azetidine-3-carbonyl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50097655 ((4S,5R,6R)-4-Amino-6-dipropylcarbamoyl-5-(2,2,2-tr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza B sialidase (Victoria) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50097660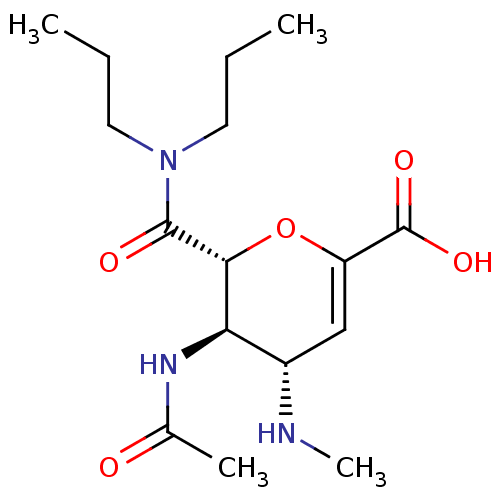 ((4S,5R,6R)-5-Acetylamino-6-dipropylcarbamoyl-4-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza A sialidase (Aichi) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118028 (3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118030 (4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against dog neutrophil elastase using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4951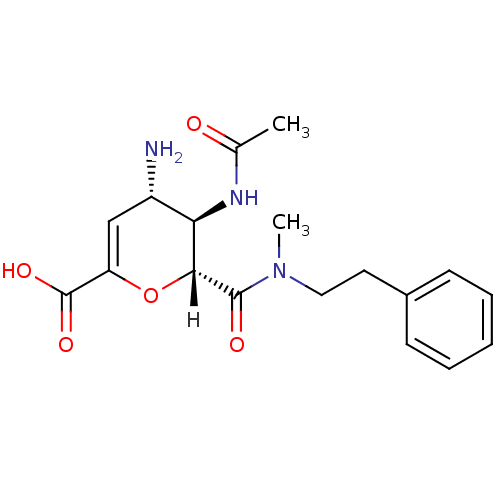 ((2R,3R,4S)-4-amino-3-acetamido-2-[methyl(2-phenyle...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited Curated by ChEMBL | Assay Description Compound was tested for inhibitory concentration against Influenza sialidase type A | J Med Chem 41: 798-807 (1998) Article DOI: 10.1021/jm9703754 BindingDB Entry DOI: 10.7270/Q2M32WFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50063303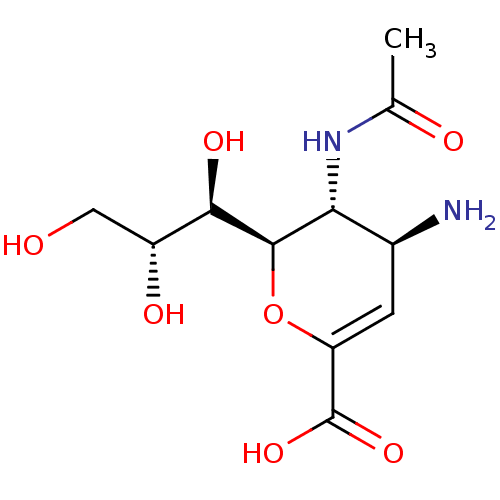 ((2R,3R,4S)-3-acetamido-4-amino-2-((1R,2R)-1,2,3-tr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited Curated by ChEMBL | Assay Description Compound was tested for inhibitory concentration against Influenza sialidase type A | J Med Chem 41: 798-807 (1998) Article DOI: 10.1021/jm9703754 BindingDB Entry DOI: 10.7270/Q2M32WFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118028 (3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against dog neutrophil elastase using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50063303 ((2R,3R,4S)-3-acetamido-4-amino-2-((1R,2R)-1,2,3-tr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited Curated by ChEMBL | Assay Description Compound was tested for inhibitory concentration against Influenza sialidase type B | J Med Chem 41: 798-807 (1998) Article DOI: 10.1021/jm9703754 BindingDB Entry DOI: 10.7270/Q2M32WFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50097651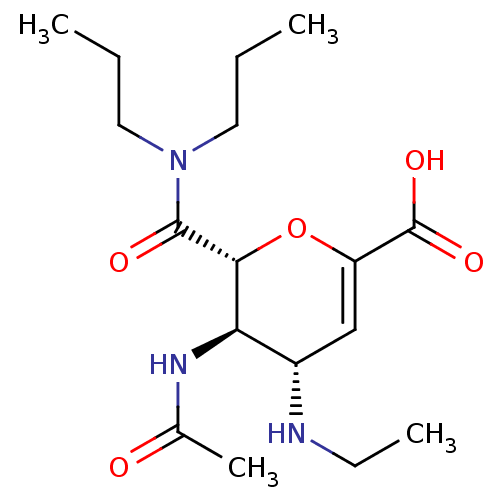 ((4S,5R,6R)-5-Acetylamino-6-dipropylcarbamoyl-4-eth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza A sialidase (Aichi) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50118030 (4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Chymotrypsinogen using selectivity assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118029 (3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM4929 ((2R,3R,4S)-4-amino-2-(dipropylcarbamoyl)-3-acetami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza B sialidase (Victoria) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50097652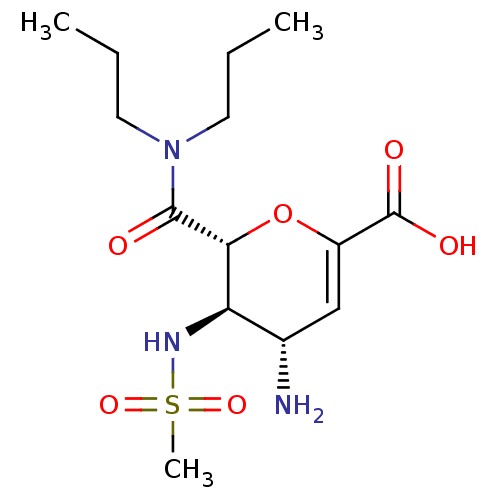 ((4S,5R,6R)-4-Amino-6-dipropylcarbamoyl-5-methanesu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza A sialidase (Aichi) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50118028 (3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Chymotrypsinogen using selectivity assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 91 total ) | Next | Last >> |