

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50365855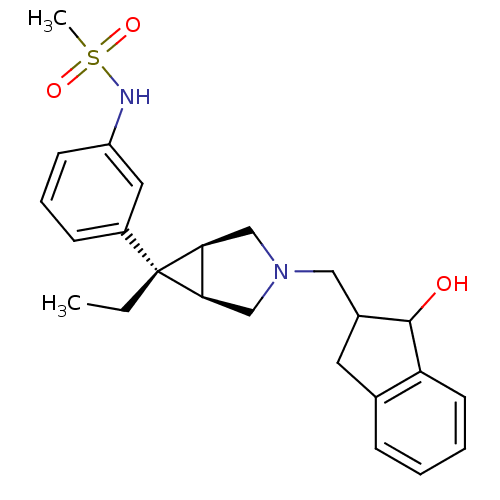 (CHEMBL1957843) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor by GTP-gamma S binding assay | Bioorg Med Chem Lett 22: 2200-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.099 BindingDB Entry DOI: 10.7270/Q2M90958 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50279775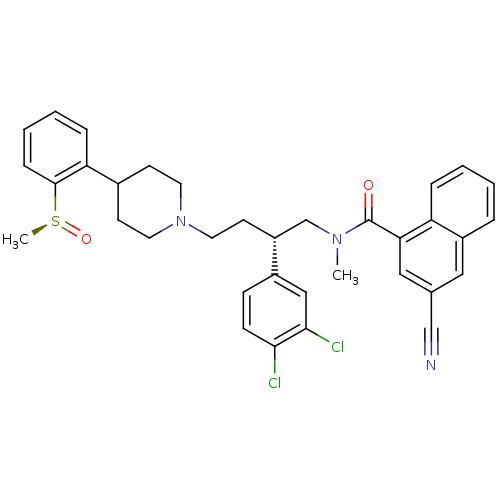 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Binding affinity against human cloned Tachykinin receptor 1 expressed in MEL cells | Bioorg Med Chem Lett 11: 2769-73 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1M4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50365855 (CHEMBL1957843) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor | Bioorg Med Chem Lett 22: 2200-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.099 BindingDB Entry DOI: 10.7270/Q2M90958 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50279775 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118098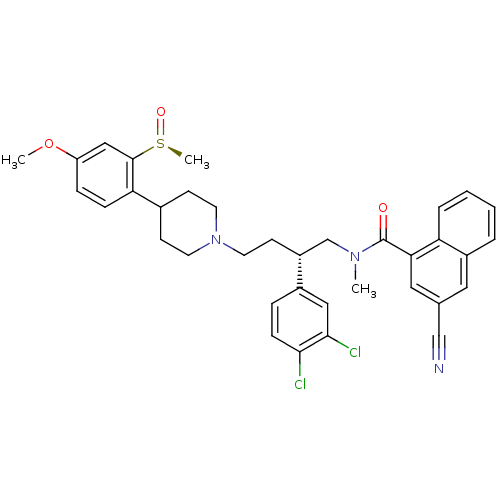 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398598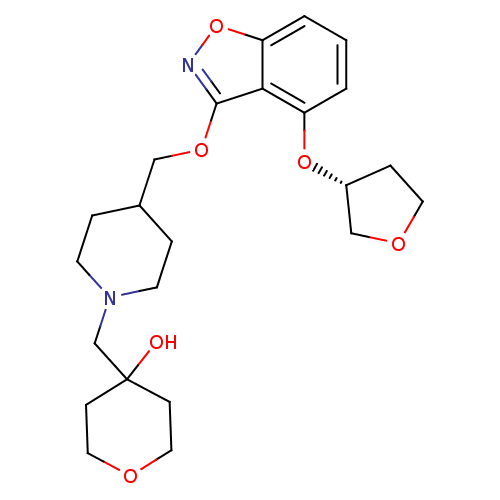 (CHEMBL2152922) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118100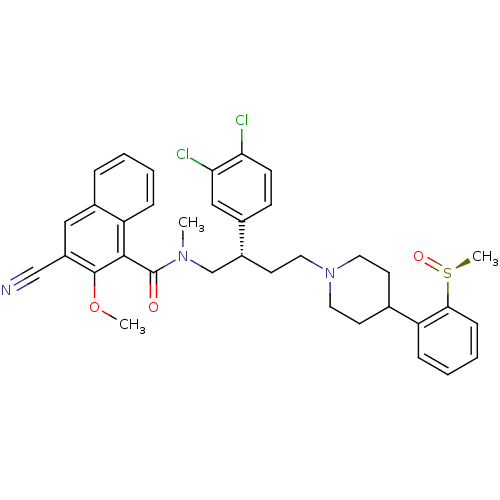 (3-Cyano-2-methoxy-naphthalene-1-carboxylic acid ((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027094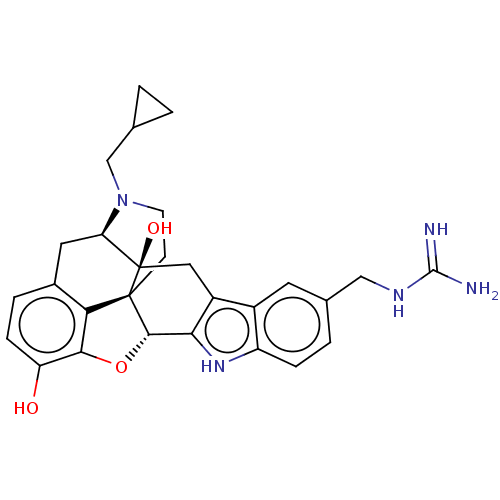 (CHEMBL2112374) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Binding affinity against cloned human Opioid receptor kappa 1 transfected into chinese hamster ovary cells using [3H]U-69593 as radioligand | J Med Chem 46: 5505-11 (2003) Article DOI: 10.1021/jm0309203 BindingDB Entry DOI: 10.7270/Q2V69K9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551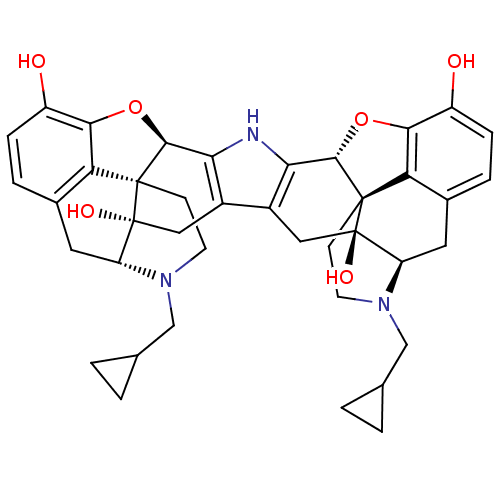 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Binding affinity against cloned human Opioid receptor kappa 1 transfected into chinese hamster ovary cells using [3H]U-69593 as radioligand | J Med Chem 46: 5505-11 (2003) Article DOI: 10.1021/jm0309203 BindingDB Entry DOI: 10.7270/Q2V69K9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354849 (CCI-18781 | Cutivate | FLUTICASONE PROPIONATE | Fl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.238 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by PDSP Ki Database | J Pharmacol Exp Ther 314: 568-74 (2005) Article DOI: 10.1124/jpet.105.085217 BindingDB Entry DOI: 10.7270/Q2CC0Z7J | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398597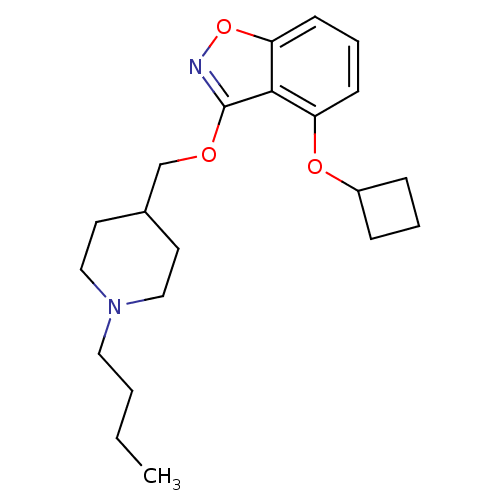 (CHEMBL2179584) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118099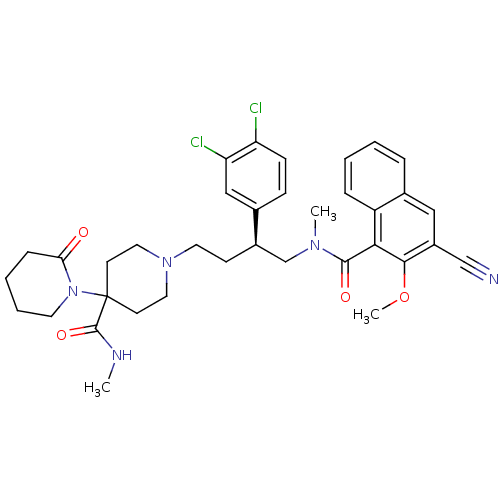 (1'-[4-[(3-Cyano-2-methoxy-naphthalene-1-carbonyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398593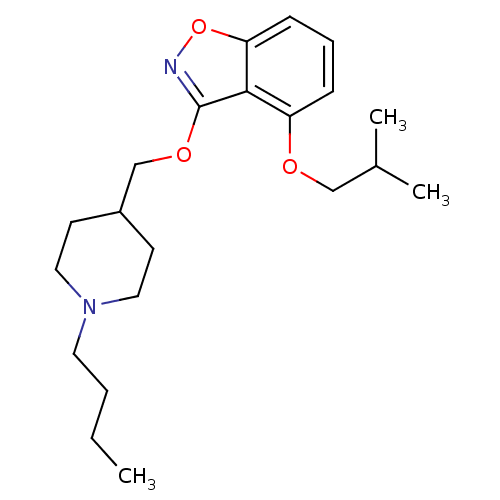 (CHEMBL2179587) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM50398598 (CHEMBL2152922) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5HT4 receptor in rat striatal membrane after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50175494 (1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Binding affinity against human cloned Tachykinin receptor 1 expressed in MEL cells | Bioorg Med Chem Lett 11: 2769-73 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1M4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50365853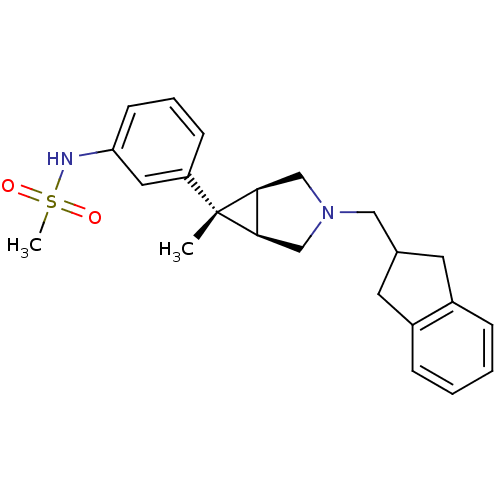 (CHEMBL1957717) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor by GTP-gamma S binding assay | Bioorg Med Chem Lett 22: 2200-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.099 BindingDB Entry DOI: 10.7270/Q2M90958 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM86696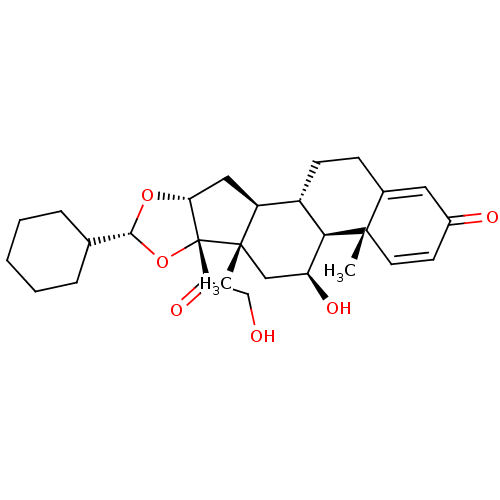 (des-CIC | desisobutyryl-ciclesonide) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by PDSP Ki Database | J Pharmacol Exp Ther 314: 568-74 (2005) Article DOI: 10.1124/jpet.105.085217 BindingDB Entry DOI: 10.7270/Q2CC0Z7J | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398598 (CHEMBL2152922) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4E receptor expressed in CHO cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50118098 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]NKA to human Tachykinin receptor 2 (NK2) in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398596 (CHEMBL2179589) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398598 (CHEMBL2152922) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4A receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50138823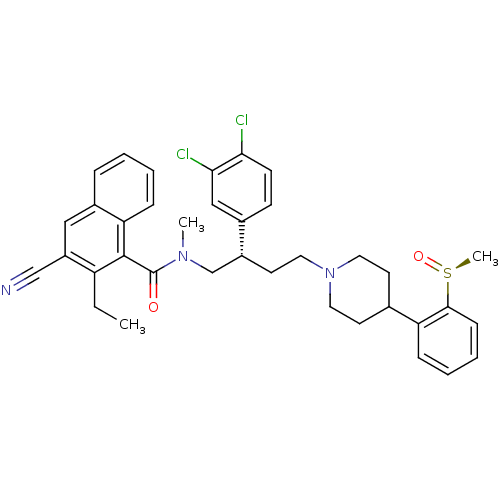 (3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description In vitro binding affinity towards human Tachykinin receptor 1 was determined by using [3H]-SP as a radioligand | J Med Chem 47: 519-29 (2004) Article DOI: 10.1021/jm030197g BindingDB Entry DOI: 10.7270/Q2FJ2G6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398598 (CHEMBL2152922) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4B receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-(+)-7-OH-DPAT from human dopamine D2 receptor expressed in HEK293 cell membranes after 90 mins by micro beta scintillation c... | J Med Chem 60: 2890-2907 (2017) Article DOI: 10.1021/acs.jmedchem.6b01875 BindingDB Entry DOI: 10.7270/Q28P62SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027093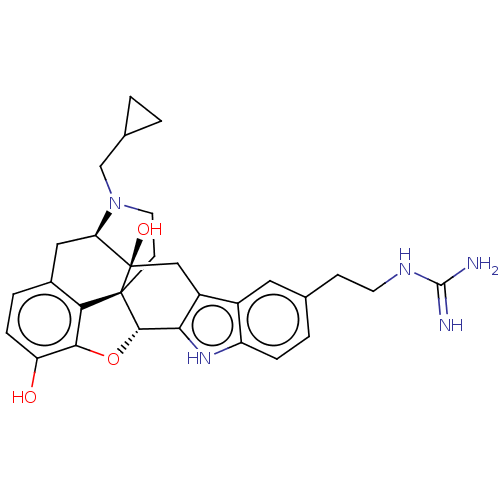 (CHEMBL2112373) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Binding affinity against cloned human Opioid receptor kappa 1 transfected into chinese hamster ovary cells using [3H]U-69593 as radioligand | J Med Chem 46: 5505-11 (2003) Article DOI: 10.1021/jm0309203 BindingDB Entry DOI: 10.7270/Q2V69K9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50350987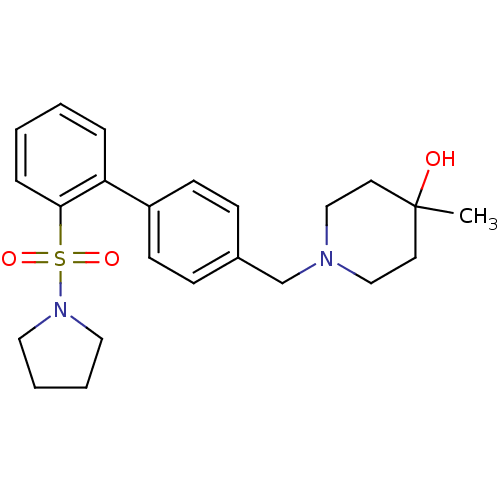 (CHEMBL1818233) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells after 1 hr by liquid scintillation counting | J Med Chem 54: 5868-77 (2011) Article DOI: 10.1021/jm2006035 BindingDB Entry DOI: 10.7270/Q2X63NBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50451786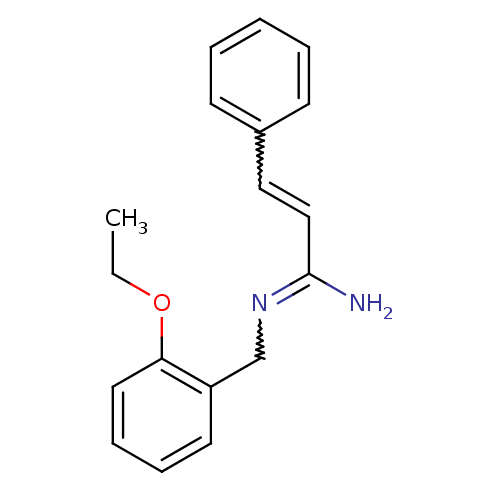 (CHEMBL423244) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]ifenprodil binding to recombinant human NR1a/NR2B receptors expressed in L(tk-) cells | Bioorg Med Chem Lett 13: 693-6 (2003) BindingDB Entry DOI: 10.7270/Q2KK9D95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398599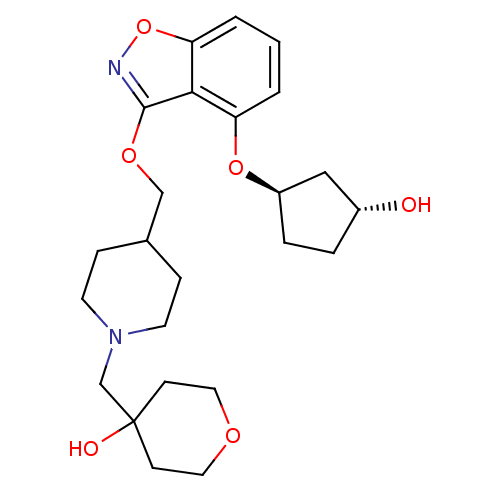 (CHEMBL2179580) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50279775 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Binding affinity against human cloned Tachykinin receptor 2 expressed in MEL cells | Bioorg Med Chem Lett 11: 2769-73 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1M4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50451785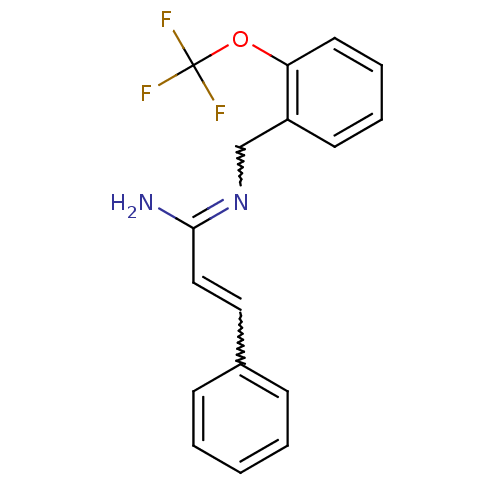 (CHEMBL159675) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]ifenprodil binding to recombinant human NR1a/NR2B receptors expressed in L(tk-) cells | Bioorg Med Chem Lett 13: 693-6 (2003) BindingDB Entry DOI: 10.7270/Q2KK9D95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50136119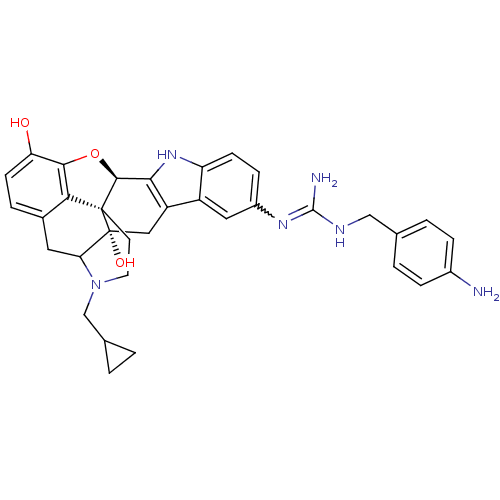 (7-[4-aminobenzylamino(imino)methylamino]-22-cyclop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Binding affinity against cloned human Opioid receptor kappa 1 transfected into chinese hamster ovary cells using [3H]U-69593 as radioligand | J Med Chem 46: 5505-11 (2003) Article DOI: 10.1021/jm0309203 BindingDB Entry DOI: 10.7270/Q2V69K9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50279775 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]NKA to human Tachykinin receptor 2 (NK2) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50136114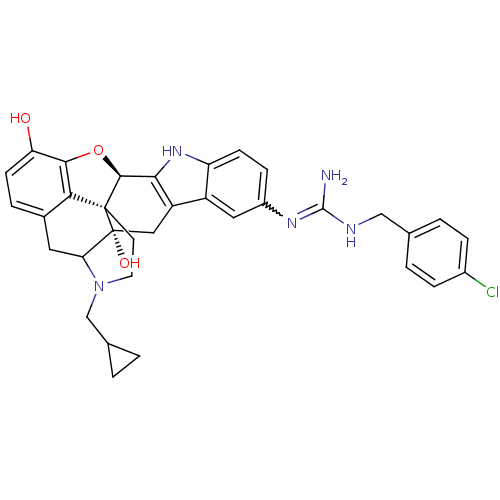 (7-[4-chlorobenzylamino(imino)methylamino]-22-cyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Binding affinity against cloned human Opioid receptor kappa 1 transfected into chinese hamster ovary cells using [3H]U-69593 as radioligand | J Med Chem 46: 5505-11 (2003) Article DOI: 10.1021/jm0309203 BindingDB Entry DOI: 10.7270/Q2V69K9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50237347 (CHEMBL4093477) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-(+)-7-OH-DPAT from human dopamine D3 receptor expressed in HEK293 cell membranes after 90 mins by micro beta scintillation c... | J Med Chem 60: 2890-2907 (2017) Article DOI: 10.1021/acs.jmedchem.6b01875 BindingDB Entry DOI: 10.7270/Q28P62SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50124885 ((E)-N-(2-Methoxy-benzyl)-3-phenyl-acrylamidine | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]ifenprodil binding to recombinant human NR1a/NR2B receptors expressed in L(tk-) cells | Bioorg Med Chem Lett 13: 693-6 (2003) BindingDB Entry DOI: 10.7270/Q2KK9D95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398594 (CHEMBL2179585) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50451789 (CHEMBL159762) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]ifenprodil binding to recombinant human NR1a/NR2B receptors expressed in L(tk-) cells | Bioorg Med Chem Lett 13: 693-6 (2003) BindingDB Entry DOI: 10.7270/Q2KK9D95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50365853 (CHEMBL1957717) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor | Bioorg Med Chem Lett 22: 2200-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.099 BindingDB Entry DOI: 10.7270/Q2M90958 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells after 1 hr by liquid scintillation counting | J Med Chem 54: 5868-77 (2011) Article DOI: 10.1021/jm2006035 BindingDB Entry DOI: 10.7270/Q2X63NBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50136122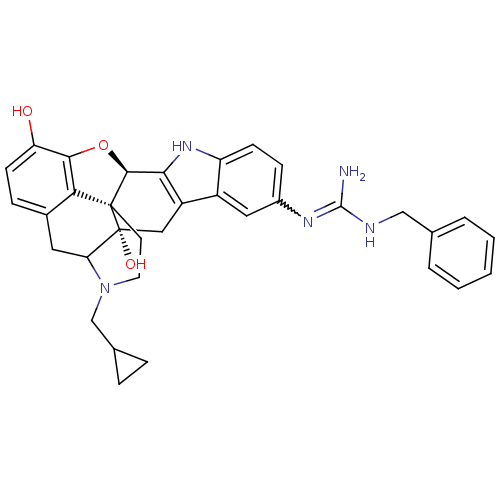 (7-benzylamino(imino)methylamino-22-cyclopropylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Binding affinity against cloned human Opioid receptor kappa 1 transfected into chinese hamster ovary cells using [3H]U-69593 as radioligand | J Med Chem 46: 5505-11 (2003) Article DOI: 10.1021/jm0309203 BindingDB Entry DOI: 10.7270/Q2V69K9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50448144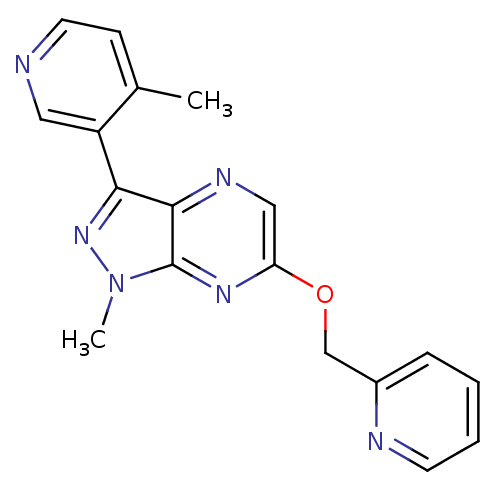 (CHEMBL3122212) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 861-77 (2014) Article DOI: 10.1021/jm401622k BindingDB Entry DOI: 10.7270/Q27P90WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50409575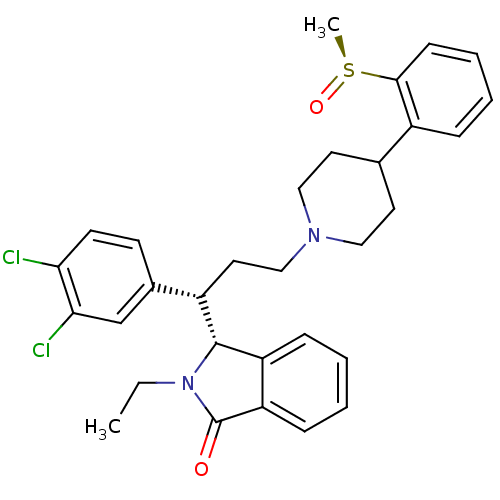 (CHEMBL339767 | ZD-7944) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]NKA to human Tachykinin receptor 2 (NK2) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50136111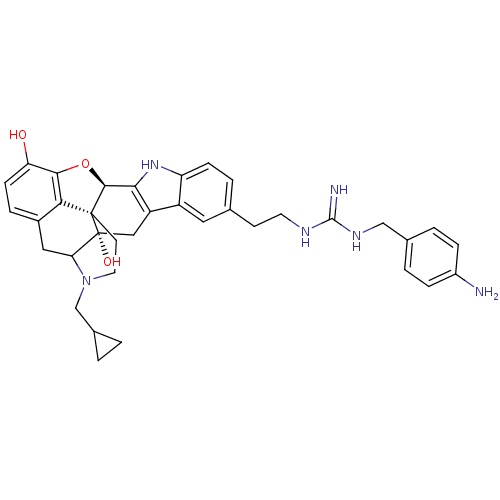 (7-{2-[4-aminobenzylamino(imino)methylamino]ethyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Binding affinity against cloned human Opioid receptor kappa 1 transfected into chinese hamster ovary cells using [3H]U-69593 as radioligand | J Med Chem 46: 5505-11 (2003) Article DOI: 10.1021/jm0309203 BindingDB Entry DOI: 10.7270/Q2V69K9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398595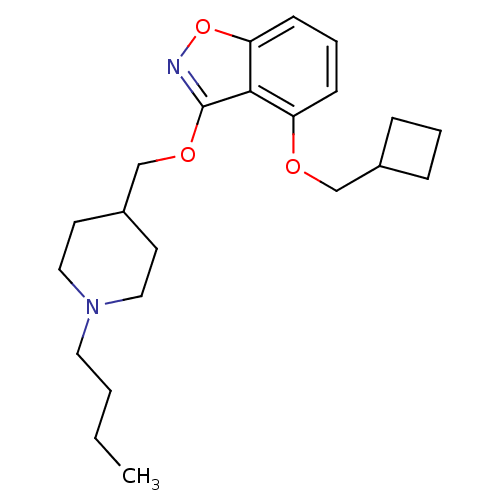 (CHEMBL2179586) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50451766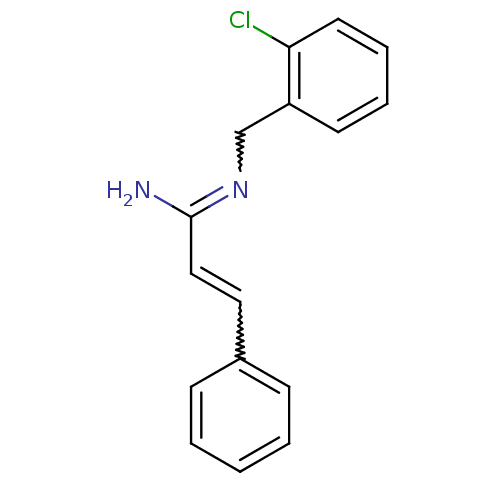 (CHEMBL159761) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]ifenprodil binding to recombinant human NR1a/NR2B receptors expressed in L(tk-) cells | Bioorg Med Chem Lett 13: 693-6 (2003) BindingDB Entry DOI: 10.7270/Q2KK9D95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50175494 (1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Binding affinity against human cloned Tachykinin receptor 2 expressed in MEL cells | Bioorg Med Chem Lett 11: 2769-73 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1M4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50350958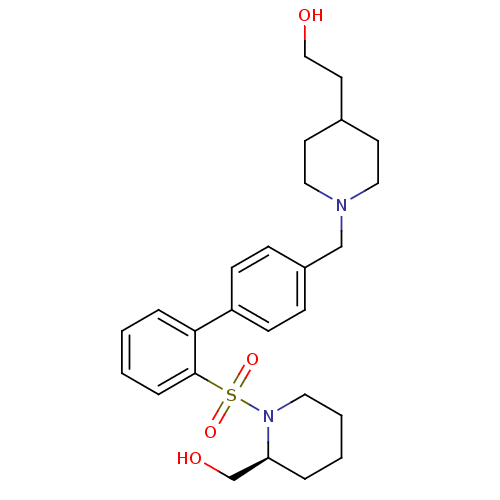 (CHEMBL1818236) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells after 1 hr by liquid scintillation counting | J Med Chem 54: 5868-77 (2011) Article DOI: 10.1021/jm2006035 BindingDB Entry DOI: 10.7270/Q2X63NBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50350976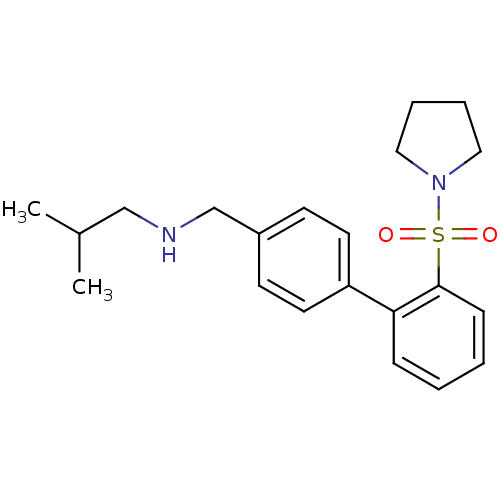 (CHEMBL1818341) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor by GTPgamma S binding assay | J Med Chem 54: 5868-77 (2011) Article DOI: 10.1021/jm2006035 BindingDB Entry DOI: 10.7270/Q2X63NBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50138823 (3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description In vitro binding affinity towards human Tachykinin receptor 1 was determined by using [3H]-SP as a radioligand | J Med Chem 47: 519-29 (2004) Article DOI: 10.1021/jm030197g BindingDB Entry DOI: 10.7270/Q2FJ2G6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50448157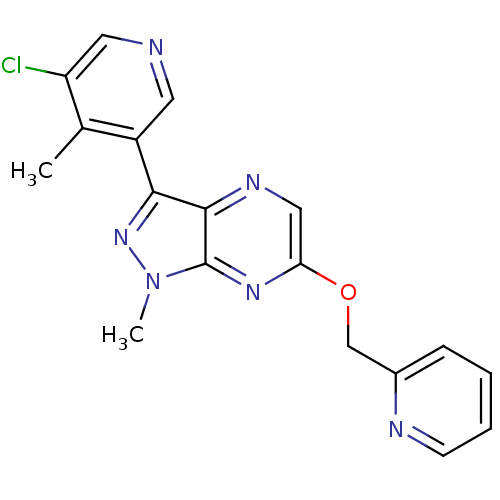 (CHEMBL3122215) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 861-77 (2014) Article DOI: 10.1021/jm401622k BindingDB Entry DOI: 10.7270/Q27P90WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4746 total ) | Next | Last >> |