 Found 162 hits with Last Name = 'woster' and Initial = 'pm'
Found 162 hits with Last Name = 'woster' and Initial = 'pm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Plasmepsin II
(Plasmodium falciparum) | BDBM7977

((3S,4S)-5-[(4-bromophenyl)methoxy]-N-[(1S)-1-{[(1S...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](COCc1ccc(Br)cc1)NC(=O)[C@@H](NC(=O)c1ccccn1)C(C)C)C(N)=O |r| Show InChI InChI=1S/C32H45BrN6O7/c1-18(2)14-24(29(34)42)37-30(43)20(5)36-27(41)15-26(40)25(17-46-16-21-9-11-22(33)12-10-21)38-32(45)28(19(3)4)39-31(44)23-8-6-7-13-35-23/h6-13,18-20,24-26,28,40H,14-17H2,1-5H3,(H2,34,42)(H,36,41)(H,37,43)(H,38,45)(H,39,44)/t20-,24-,25-,26-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
J Med Chem 53: 4234-47 (2010)
Article DOI: 10.1021/jm100233b
BindingDB Entry DOI: 10.7270/Q22807RR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasmepsin II
(Plasmodium falciparum) | BDBM7974
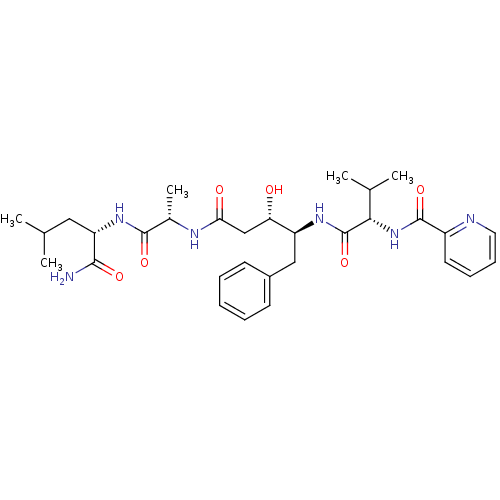
((3S,4S)-N-[(1S)-1-{[(1S)-1-carbamoyl-3-methylbutyl...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccccn1)C(C)C)C(N)=O |r| Show InChI InChI=1S/C31H44N6O6/c1-18(2)15-24(28(32)40)36-29(41)20(5)34-26(39)17-25(38)23(16-21-11-7-6-8-12-21)35-31(43)27(19(3)4)37-30(42)22-13-9-10-14-33-22/h6-14,18-20,23-25,27,38H,15-17H2,1-5H3,(H2,32,40)(H,34,39)(H,35,43)(H,36,41)(H,37,42)/t20-,23-,24-,25-,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant plasmepsin 2 expressed in Escherichia coli BL21 (DE3) |
J Med Chem 53: 4234-47 (2010)
Article DOI: 10.1021/jm100233b
BindingDB Entry DOI: 10.7270/Q22807RR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346873
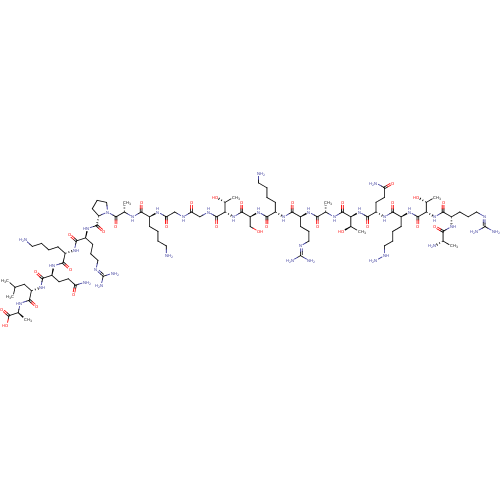
(CHEMBL1797652)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C94H173N37O28/c1-46(2)42-63(84(151)116-50(6)91(158)159)126-81(148)61(30-32-66(99)136)122-76(143)55(23-11-15-35-96)120-79(146)59(27-19-38-109-93(103)104)124-86(153)65-29-21-41-131(65)90(157)49(5)115-75(142)54(22-10-14-34-95)117-69(139)44-111-68(138)43-112-87(154)70(51(7)133)128-85(152)64(45-132)127-80(147)56(24-12-16-36-97)121-78(145)58(26-18-37-108-92(101)102)119-74(141)48(4)114-88(155)71(52(8)134)129-83(150)62(31-33-67(100)137)123-77(144)57(25-13-17-40-113-107)125-89(156)72(53(9)135)130-82(149)60(118-73(140)47(3)98)28-20-39-110-94(105)106/h46-65,70-72,113,132-135H,10-45,95-98,107H2,1-9H3,(H2,99,136)(H2,100,137)(H,111,138)(H,112,154)(H,114,155)(H,115,142)(H,116,151)(H,117,139)(H,118,140)(H,119,141)(H,120,146)(H,121,145)(H,122,143)(H,123,144)(H,124,153)(H,125,156)(H,126,148)(H,127,147)(H,128,152)(H,129,150)(H,130,149)(H,158,159)(H4,101,102,108)(H4,103,104,109)(H4,105,106,110)/t47-,48-,49-,50-,51+,52+,53+,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,70-,71-,72-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant LSD1 (178 to 831) (unknown origin) expressed in baculovirus infected insect Sf9 cells using diMeK4H3-21 as substrate by per... |
ACS Med Chem Lett 5: 29-33 (2014)
Article DOI: 10.1021/ml4002997
BindingDB Entry DOI: 10.7270/Q2HT2QS9 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50446141
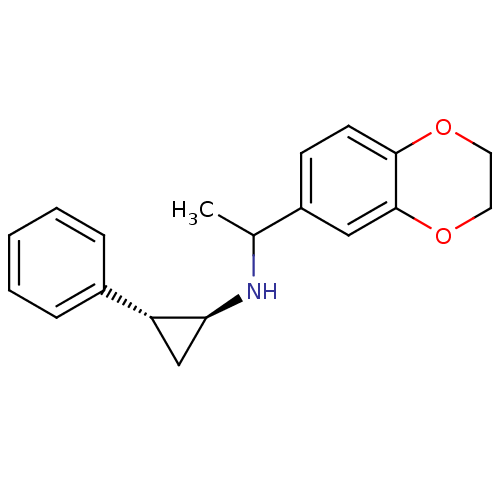
(CHEMBL3108901)Show SMILES CC(N[C@H]1C[C@@H]1c1ccccc1)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C19H21NO2/c1-13(15-7-8-18-19(11-15)22-10-9-21-18)20-17-12-16(17)14-5-3-2-4-6-14/h2-8,11,13,16-17,20H,9-10,12H2,1H3/t13?,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LSD1 using di-methylated H3-K4 peptide as substrate assessed as release of H2O2 preincubated for 15 mins followed by ... |
ACS Med Chem Lett 5: 29-33 (2014)
Article DOI: 10.1021/ml4002997
BindingDB Entry DOI: 10.7270/Q2HT2QS9 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM101302
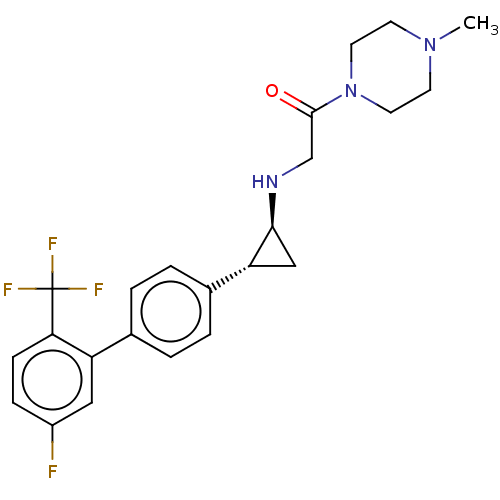
(CHEMBL3108900 | US8524717, 57)Show SMILES CN1CCN(CC1)C(=O)CN[C@H]1C[C@@H]1c1ccc(cc1)-c1cc(F)ccc1C(F)(F)F |r| Show InChI InChI=1S/C23H25F4N3O/c1-29-8-10-30(11-9-29)22(31)14-28-21-13-19(21)16-4-2-15(3-5-16)18-12-17(24)6-7-20(18)23(25,26)27/h2-7,12,19,21,28H,8-11,13-14H2,1H3/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LSD1 using di-methylated H3-K4 peptide as substrate assessed as release of H2O2 preincubated for 15 mins followed by ... |
ACS Med Chem Lett 5: 29-33 (2014)
Article DOI: 10.1021/ml4002997
BindingDB Entry DOI: 10.7270/Q2HT2QS9 |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50316596
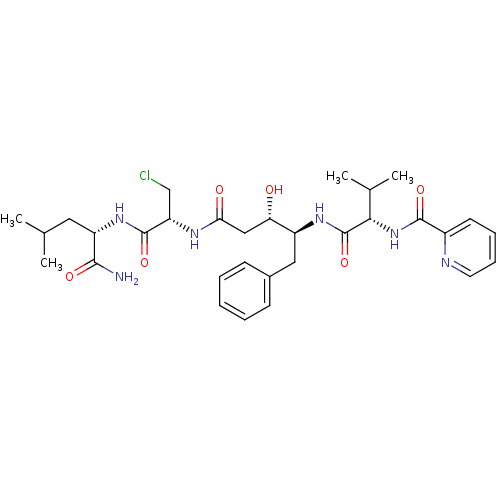
(CHEMBL1098668 | N-((S)-1-((2S,3S)-5-((R)-1-((S)-1-...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCl)NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccccn1)C(C)C)C(N)=O |r| Show InChI InChI=1S/C31H43ClN6O6/c1-18(2)14-23(28(33)41)37-30(43)24(17-32)35-26(40)16-25(39)22(15-20-10-6-5-7-11-20)36-31(44)27(19(3)4)38-29(42)21-12-8-9-13-34-21/h5-13,18-19,22-25,27,39H,14-17H2,1-4H3,(H2,33,41)(H,35,40)(H,36,44)(H,37,43)(H,38,42)/t22-,23-,24-,25-,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 expressed in Escherichia coli BL21 (DE3) after 40 mins by Kitz-Wilson plot analysis |
J Med Chem 53: 4234-47 (2010)
Article DOI: 10.1021/jm100233b
BindingDB Entry DOI: 10.7270/Q22807RR |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50458057

(CHEMBL4210908)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)N)[C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O |r| Show InChI InChI=1S/C94H173N35O27S/c1-47(2)43-64(84(148)114-51(6)91(155)156)124-80(144)62(31-32-67(100)134)121-76(140)56(24-12-16-35-96)118-78(142)60(28-20-39-108-93(103)104)122-86(150)66-30-22-41-129(66)90(154)50(5)113-75(139)55(23-11-15-34-95)115-69(136)45-110-68(135)44-111-87(151)70(52(7)131)126-85(149)65(46-130)125-79(143)57(25-13-17-36-97)119-77(141)59(27-19-38-107-92(101)102)117-74(138)49(4)112-88(152)71(53(8)132)127-82(146)58(26-14-18-37-98)120-81(145)63(33-42-157-10)123-89(153)72(54(9)133)128-83(147)61(116-73(137)48(3)99)29-21-40-109-94(105)106/h47-66,70-72,130-133H,11-46,95-99H2,1-10H3,(H2,100,134)(H,110,135)(H,111,151)(H,112,152)(H,113,139)(H,114,148)(H,115,136)(H,116,137)(H,117,138)(H,118,142)(H,119,141)(H,120,145)(H,121,140)(H,122,150)(H,123,153)(H,124,144)(H,125,143)(H,126,149)(H,127,146)(H,128,147)(H,155,156)(H4,101,102,107)(H4,103,104,108)(H4,105,106,109)/t48-,49-,50-,51-,52+,53+,54+,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,70-,71-,72-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of LSD1/CoREST (unknown origin) |
Eur J Med Chem 148: 210-220 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.098
BindingDB Entry DOI: 10.7270/Q2N0195V |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346870
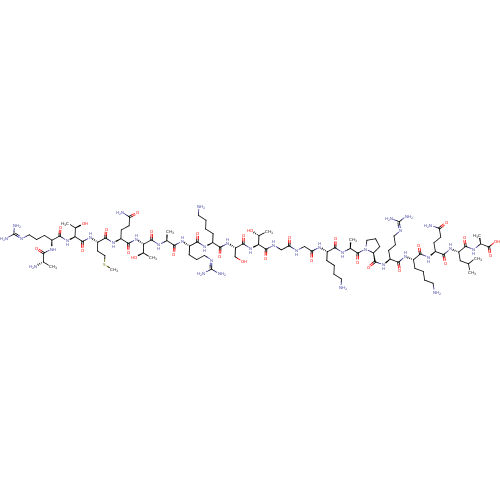
(CHEMBL1797647)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C93H169N35O28S/c1-45(2)41-62(83(148)113-49(6)90(155)156)123-79(144)59(28-30-65(98)133)119-75(140)54(22-12-15-34-95)117-77(142)57(25-18-37-107-92(102)103)121-85(150)64-27-20-39-128(64)89(154)48(5)112-74(139)53(21-11-14-33-94)114-68(136)43-109-67(135)42-110-86(151)69(50(7)130)125-84(149)63(44-129)124-78(143)55(23-13-16-35-96)118-76(141)56(24-17-36-106-91(100)101)116-73(138)47(4)111-87(152)70(51(8)131)126-82(147)60(29-31-66(99)134)120-80(145)61(32-40-157-10)122-88(153)71(52(9)132)127-81(146)58(115-72(137)46(3)97)26-19-38-108-93(104)105/h45-64,69-71,129-132H,11-44,94-97H2,1-10H3,(H2,98,133)(H2,99,134)(H,109,135)(H,110,151)(H,111,152)(H,112,139)(H,113,148)(H,114,136)(H,115,137)(H,116,138)(H,117,142)(H,118,141)(H,119,140)(H,120,145)(H,121,150)(H,122,153)(H,123,144)(H,124,143)(H,125,149)(H,126,147)(H,127,146)(H,155,156)(H4,100,101,106)(H4,102,103,107)(H4,104,105,108)/t46-,47-,48-,49-,50+,51+,52+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,69-,70-,71-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human LSD1 expressed in Escherichia coli using methylated H3-K4 peptide as substrate by peroxidase-coupled assay |
ACS Med Chem Lett 5: 29-33 (2014)
Article DOI: 10.1021/ml4002997
BindingDB Entry DOI: 10.7270/Q2HT2QS9 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A/REST corepressor 1 [4-485]
(Homo sapiens (Human)) | BDBM50346870

(CHEMBL1797647)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C93H169N35O28S/c1-45(2)41-62(83(148)113-49(6)90(155)156)123-79(144)59(28-30-65(98)133)119-75(140)54(22-12-15-34-95)117-77(142)57(25-18-37-107-92(102)103)121-85(150)64-27-20-39-128(64)89(154)48(5)112-74(139)53(21-11-14-33-94)114-68(136)43-109-67(135)42-110-86(151)69(50(7)130)125-84(149)63(44-129)124-78(143)55(23-13-16-35-96)118-76(141)56(24-17-36-106-91(100)101)116-73(138)47(4)111-87(152)70(51(8)131)126-82(147)60(29-31-66(99)134)120-80(145)61(32-40-157-10)122-88(153)71(52(9)132)127-81(146)58(115-72(137)46(3)97)26-19-38-108-93(104)105/h45-64,69-71,129-132H,11-44,94-97H2,1-10H3,(H2,98,133)(H2,99,134)(H,109,135)(H,110,151)(H,111,152)(H,112,139)(H,113,148)(H,114,136)(H,115,137)(H,116,138)(H,117,142)(H,118,141)(H,119,140)(H,120,145)(H,121,150)(H,122,153)(H,123,144)(H,124,143)(H,125,149)(H,126,147)(H,127,146)(H,155,156)(H4,100,101,106)(H4,102,103,107)(H4,104,105,108)/t46-,47-,48-,49-,50+,51+,52+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,69-,70-,71-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human LSD1/CoREST complex expressed in Escherichia coli using methylated H3-K4 peptide as substrate by peroxidase-coupled a... |
ACS Med Chem Lett 5: 29-33 (2014)
Article DOI: 10.1021/ml4002997
BindingDB Entry DOI: 10.7270/Q2HT2QS9 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346875
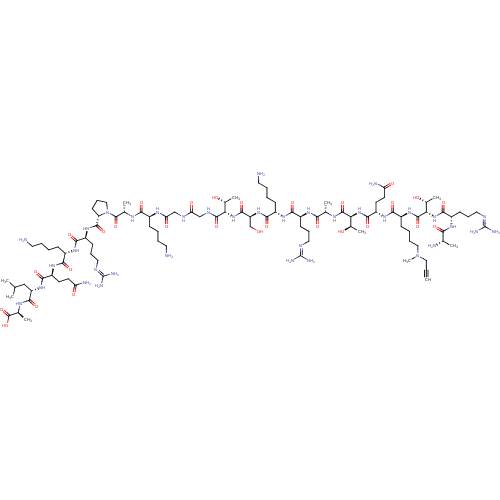
(CHEMBL1797648)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7](-[#6])-[#6]C#C)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C98H176N36O28/c1-12-43-133(11)44-20-16-28-61(127-93(159)76(57(10)138)132-86(152)64(120-77(143)51(4)102)31-23-42-113-98(109)110)81(147)125-66(34-36-71(104)140)87(153)131-75(56(9)137)92(158)116-52(5)78(144)121-62(29-21-40-111-96(105)106)82(148)123-60(27-15-19-39-101)84(150)129-68(49-135)89(155)130-74(55(8)136)91(157)115-47-72(141)114-48-73(142)119-58(25-13-17-37-99)79(145)117-53(6)94(160)134-45-24-32-69(134)90(156)126-63(30-22-41-112-97(107)108)83(149)122-59(26-14-18-38-100)80(146)124-65(33-35-70(103)139)85(151)128-67(46-50(2)3)88(154)118-54(7)95(161)162/h1,50-69,74-76,135-138H,13-49,99-102H2,2-11H3,(H2,103,139)(H2,104,140)(H,114,141)(H,115,157)(H,116,158)(H,117,145)(H,118,154)(H,119,142)(H,120,143)(H,121,144)(H,122,149)(H,123,148)(H,124,146)(H,125,147)(H,126,156)(H,127,159)(H,128,151)(H,129,150)(H,130,155)(H,131,153)(H,132,152)(H,161,162)(H4,105,106,111)(H4,107,108,112)(H4,109,110,113)/t51-,52-,53-,54-,55+,56+,57+,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,74-,75-,76-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant LSD1 (178 to 831) (unknown origin) expressed in baculovirus infected insect Sf9 cells using diMeK4H3-21 as substrate by per... |
ACS Med Chem Lett 5: 29-33 (2014)
Article DOI: 10.1021/ml4002997
BindingDB Entry DOI: 10.7270/Q2HT2QS9 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50446142
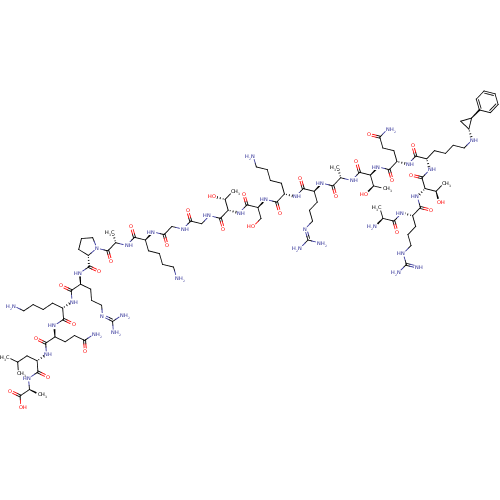
(CHEMBL3108892)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6@@H]-1-[#6]-[#6@H]-1-c1ccccc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C103H180N36O28/c1-52(2)47-72(93(159)124-56(6)100(166)167)134-90(156)69(35-37-75(108)144)130-85(151)63(28-14-18-40-105)128-88(154)67(32-22-44-118-102(112)113)132-95(161)74-34-24-46-139(74)99(165)55(5)123-84(150)62(27-13-17-39-104)125-78(147)50-120-77(146)49-121-96(162)79(57(7)141)136-94(160)73(51-140)135-89(155)64(29-15-19-41-106)129-87(153)66(31-21-43-117-101(110)111)127-83(149)54(4)122-97(163)80(58(8)142)137-92(158)70(36-38-76(109)145)131-86(152)65(30-16-20-42-116-71-48-61(71)60-25-11-10-12-26-60)133-98(164)81(59(9)143)138-91(157)68(126-82(148)53(3)107)33-23-45-119-103(114)115/h10-12,25-26,52-59,61-74,79-81,116,140-143H,13-24,27-51,104-107H2,1-9H3,(H2,108,144)(H2,109,145)(H,120,146)(H,121,162)(H,122,163)(H,123,150)(H,124,159)(H,125,147)(H,126,148)(H,127,149)(H,128,154)(H,129,153)(H,130,151)(H,131,152)(H,132,161)(H,133,164)(H,134,156)(H,135,155)(H,136,160)(H,137,158)(H,138,157)(H,166,167)(H4,110,111,117)(H4,112,113,118)(H4,114,115,119)/t53-,54-,55-,56-,57+,58+,59+,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71+,72-,73-,74-,79-,80-,81-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant LSD1 (unknown origin) using di-methylated H3-K4 peptide as substrate incubated for 5 mins prior to substrate addition by fl... |
ACS Med Chem Lett 5: 29-33 (2014)
Article DOI: 10.1021/ml4002997
BindingDB Entry DOI: 10.7270/Q2HT2QS9 |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM8027

((2S)-N-[(2S,3S)-4-{[(1S)-1-carbamoyl-2-[4-(4-methy...)Show SMILES CC(C)[C@H](NC(=O)c1ccccn1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN[C@@H](Cc1ccc(cc1)-c1ccc(C)cc1)C(N)=O |r| Show InChI InChI=1S/C37H43N5O4/c1-24(2)34(42-36(45)30-11-7-8-20-39-30)37(46)41-31(21-26-9-5-4-6-10-26)33(43)23-40-32(35(38)44)22-27-14-18-29(19-15-27)28-16-12-25(3)13-17-28/h4-20,24,31-34,40,43H,21-23H2,1-3H3,(H2,38,44)(H,41,46)(H,42,45)/t31-,32-,33-,34-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
J Med Chem 53: 4234-47 (2010)
Article DOI: 10.1021/jm100233b
BindingDB Entry DOI: 10.7270/Q22807RR |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50316598

(CHEMBL1098670 | N-((S)-1-((2S,3S)-5-((S)-1-((S)-1-...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC#C)NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccccn1)C(C)C)C(N)=O |r| Show InChI InChI=1S/C33H44N6O6/c1-6-12-24(32(44)38-26(30(34)42)17-20(2)3)36-28(41)19-27(40)25(18-22-13-8-7-9-14-22)37-33(45)29(21(4)5)39-31(43)23-15-10-11-16-35-23/h1,7-11,13-16,20-21,24-27,29,40H,12,17-19H2,2-5H3,(H2,34,42)(H,36,41)(H,37,45)(H,38,44)(H,39,43)/t24-,25-,26-,27-,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 333 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 expressed in Escherichia coli BL21 (DE3) after 40 mins by Kitz-Wilson plot analysis |
J Med Chem 53: 4234-47 (2010)
Article DOI: 10.1021/jm100233b
BindingDB Entry DOI: 10.7270/Q22807RR |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50119879

((S)-N-((4S,6S,7S)-7-(2-(2,6-dimethylphenoxy)acetam...)Show SMILES CC(C)C[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)NC(=O)[C@H](C(C)C)N1CCCNC1=O Show InChI InChI=1S/C34H50N4O5/c1-22(2)18-27(36-33(41)31(23(3)4)38-17-11-16-35-34(38)42)20-29(39)28(19-26-14-8-7-9-15-26)37-30(40)21-43-32-24(5)12-10-13-25(32)6/h7-10,12-15,22-23,27-29,31,39H,11,16-21H2,1-6H3,(H,35,42)(H,36,41)(H,37,40)/t27-,28-,29-,31-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 357 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
J Med Chem 53: 4234-47 (2010)
Article DOI: 10.1021/jm100233b
BindingDB Entry DOI: 10.7270/Q22807RR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50446143
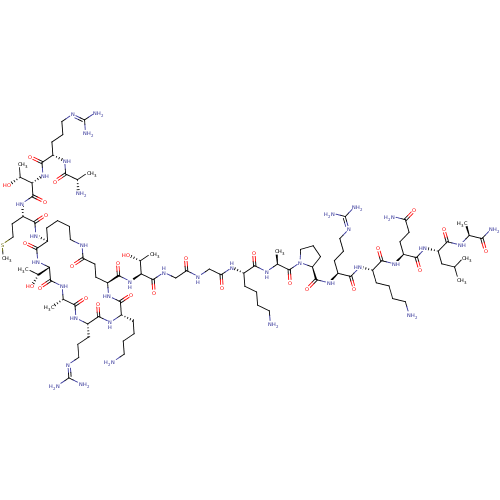
(CHEMBL3108897)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@H]-1-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6]-1=O)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#7])=O |r| Show InChI InChI=1S/C96H174N36O26S/c1-48(2)45-66(88(153)115-50(4)75(102)140)128-83(148)63(31-33-68(101)136)124-79(144)57(24-12-16-37-98)122-82(147)61(28-20-41-111-95(105)106)126-89(154)67-30-22-43-132(67)93(158)52(6)117-78(143)56(23-11-15-36-97)118-71(139)47-113-70(138)46-114-90(155)72(53(7)133)129-87(152)64-32-34-69(137)109-39-18-14-26-59(123-84(149)65(35-44-159-10)127-92(157)74(55(9)135)131-86(151)62(119-76(141)49(3)100)29-21-42-112-96(107)108)85(150)130-73(54(8)134)91(156)116-51(5)77(142)120-60(27-19-40-110-94(103)104)81(146)121-58(80(145)125-64)25-13-17-38-99/h48-67,72-74,133-135H,11-47,97-100H2,1-10H3,(H2,101,136)(H2,102,140)(H,109,137)(H,113,138)(H,114,155)(H,115,153)(H,116,156)(H,117,143)(H,118,139)(H,119,141)(H,120,142)(H,121,146)(H,122,147)(H,123,149)(H,124,144)(H,125,145)(H,126,154)(H,127,157)(H,128,148)(H,129,152)(H,130,150)(H,131,151)(H4,103,104,110)(H4,105,106,111)(H4,107,108,112)/t49-,50-,51-,52-,53+,54+,55+,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,72-,73-,74-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 385 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant LSD1 (unknown origin) using H3K4Me2 peptide as substrate preincubated for 30 mins followed by substrate additio... |
ACS Med Chem Lett 5: 29-33 (2014)
Article DOI: 10.1021/ml4002997
BindingDB Entry DOI: 10.7270/Q2HT2QS9 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Rattus norvegicus) | BDBM50369827

(CHEMBL611536)Show SMILES NCCC=NC[C@@H]1OC([C@@H](O)[C@H]1O)n1cnc2c(N)ncnc12 |r,w:3.2| Show InChI InChI=1S/C13H19N7O3/c14-2-1-3-16-4-7-9(21)10(22)13(23-7)20-6-19-8-11(15)17-5-18-12(8)20/h3,5-7,9-10,13,21-22H,1-2,4,14H2,(H2,15,17,18)/t7-,9-,10-,13?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver form of S-adenosyl-methionine decarboxylase enzyme |
J Med Chem 44: 1-26 (2001)
BindingDB Entry DOI: 10.7270/Q28S4QNR |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50046201

((2E)-2-((2E)-2-{[(E)-amino(imino)methyl]hydrazono}...)Show SMILES [#6]-[#6](-[#6]=[#7]-[#7]-[#6](-[#7])=[#7])=[#7]\[#7]=[#6](\[#7])-[#7] |w:3.3,8.8| Show InChI InChI=1S/C5H12N8/c1-3(11-13-5(8)9)2-10-12-4(6)7/h2H,1H3,(H4,6,7,12)(H4,8,9,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver form of S-adenosyl-methionine decarboxylase enzyme |
J Med Chem 44: 1-26 (2001)
BindingDB Entry DOI: 10.7270/Q28S4QNR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346869
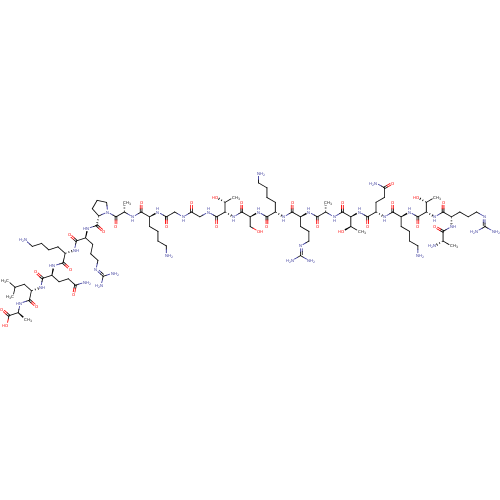
(CHEMBL1797646)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C94H172N36O28/c1-46(2)42-63(84(150)115-50(6)91(157)158)125-81(147)61(30-32-66(100)135)121-76(142)55(23-11-15-35-96)119-79(145)59(27-19-39-109-93(104)105)123-86(152)65-29-21-41-130(65)90(156)49(5)114-75(141)54(22-10-14-34-95)116-69(138)44-111-68(137)43-112-87(153)70(51(7)132)127-85(151)64(45-131)126-80(146)56(24-12-16-36-97)120-78(144)58(26-18-38-108-92(102)103)118-74(140)48(4)113-88(154)71(52(8)133)128-83(149)62(31-33-67(101)136)122-77(143)57(25-13-17-37-98)124-89(155)72(53(9)134)129-82(148)60(117-73(139)47(3)99)28-20-40-110-94(106)107/h46-65,70-72,131-134H,10-45,95-99H2,1-9H3,(H2,100,135)(H2,101,136)(H,111,137)(H,112,153)(H,113,154)(H,114,141)(H,115,150)(H,116,138)(H,117,139)(H,118,140)(H,119,145)(H,120,144)(H,121,142)(H,122,143)(H,123,152)(H,124,155)(H,125,147)(H,126,146)(H,127,151)(H,128,149)(H,129,148)(H,157,158)(H4,102,103,108)(H4,104,105,109)(H4,106,107,110)/t47-,48-,49-,50-,51+,52+,53+,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,70-,71-,72-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant LSD1 (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using H3K4 peptide as substrate by Dixon p... |
ACS Med Chem Lett 5: 29-33 (2014)
Article DOI: 10.1021/ml4002997
BindingDB Entry DOI: 10.7270/Q2HT2QS9 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50464671

(CHEMBL3621615)Show InChI InChI=1S/C15H14ClN5O/c16-12-7-4-8-13(22-10-5-2-1-3-6-10)11(12)9-18-15-19-14(17)20-21-15/h1-8H,9H2,(H4,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Competitive inhibition of LSD1 (unknown origin) incubated for 30 mins using H3K4me2 substrate by fluorescence based assay |
Medchemcomm 5: 1863-1870 (2014)
Article DOI: 10.1039/c4md00283k
BindingDB Entry DOI: 10.7270/Q2Q81H3T |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50065386

(CHEMBL3401327)Show SMILES Cl.S=C(NCCCNCCCCCNCCCNC(=S)NCCC(c1ccccc1)c1ccccc1)NCCC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C43H58N6S2.ClH/c50-42(48-34-26-40(36-18-6-1-7-19-36)37-20-8-2-9-21-37)46-32-16-30-44-28-14-5-15-29-45-31-17-33-47-43(51)49-35-27-41(38-22-10-3-11-23-38)39-24-12-4-13-25-39;/h1-4,6-13,18-25,40-41,44-45H,5,14-17,26-35H2,(H2,46,48,50)(H2,47,49,51);1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
John Hopkins University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant LSD1 by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 1601-12 (2015)
Article DOI: 10.1016/j.bmc.2015.01.049
BindingDB Entry DOI: 10.7270/Q25Q4XSD |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50403124

(CHEMBL2115571)Show SMILES C[S+](CCC(N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C15H22N6O5S/c1-27(3-2-7(16)15(24)25)4-8-10(22)11(23)14(26-8)21-6-20-9-12(17)18-5-19-13(9)21/h5-8,10-11,14,22-23H,2-4,16H2,1H3,(H2-,17,18,19,24,25)/p+1/t7?,8-,10-,11-,14-,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated to inactivate the bacterial AdoMet-DC; value ranges from 3.8 to 39.6 uM |
Bioorg Med Chem Lett 3: 2811-2816 (1993)
Article DOI: 10.1016/S0960-894X(01)80770-8
BindingDB Entry DOI: 10.7270/Q2TH8N6T |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | CHEMBL5287761

| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 5.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50281293
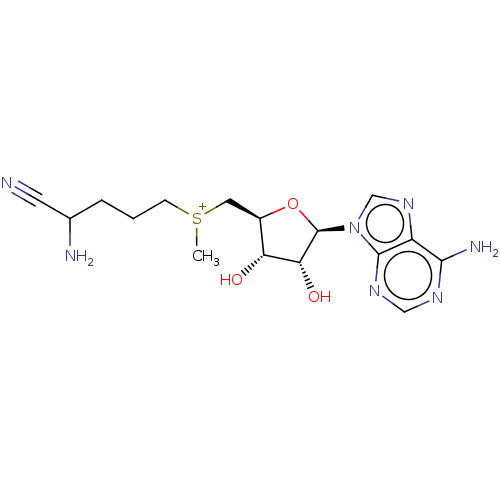
(2-amino-5-({[(2S,3S,4R)-5-(6-amino-9H-purin-9-yl)-...)Show SMILES Br.[Br-].C[S+](CCCC(N)C#N)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C16H24N7O3S/c1-27(4-2-3-9(18)5-17)6-10-12(24)13(25)16(26-10)23-8-22-11-14(19)20-7-21-15(11)23/h7-10,12-13,16,24-25H,2-4,6,18H2,1H3,(H2,19,20,21)/q+1/p+1/t9?,10-,12-,13-,16?,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated to inactivate the human AdoMet-DC |
Bioorg Med Chem Lett 3: 2811-2816 (1993)
Article DOI: 10.1016/S0960-894X(01)80770-8
BindingDB Entry DOI: 10.7270/Q2TH8N6T |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50403124

(CHEMBL2115571)Show SMILES C[S+](CCC(N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C15H22N6O5S/c1-27(3-2-7(16)15(24)25)4-8-10(22)11(23)14(26-8)21-6-20-9-12(17)18-5-19-13(9)21/h5-8,10-11,14,22-23H,2-4,16H2,1H3,(H2-,17,18,19,24,25)/p+1/t7?,8-,10-,11-,14-,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated to inactivate the bacterial AdoMet-DC; value ranges from 3.8 to 39.6 uM |
Bioorg Med Chem Lett 3: 2811-2816 (1993)
Article DOI: 10.1016/S0960-894X(01)80770-8
BindingDB Entry DOI: 10.7270/Q2TH8N6T |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50403124

(CHEMBL2115571)Show SMILES C[S+](CCC(N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C15H22N6O5S/c1-27(3-2-7(16)15(24)25)4-8-10(22)11(23)14(26-8)21-6-20-9-12(17)18-5-19-13(9)21/h5-8,10-11,14,22-23H,2-4,16H2,1H3,(H2-,17,18,19,24,25)/p+1/t7?,8-,10-,11-,14-,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated to inactivate the human AdoMet-DC; value ranges from 10.7 to 62.7 uM |
Bioorg Med Chem Lett 3: 2811-2816 (1993)
Article DOI: 10.1016/S0960-894X(01)80770-8
BindingDB Entry DOI: 10.7270/Q2TH8N6T |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50318565
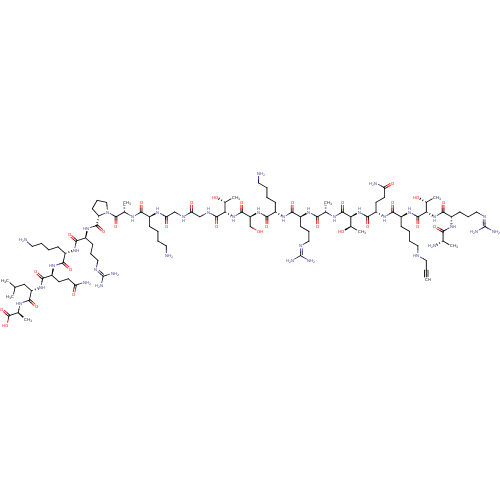
(CHEMBL1086217)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6]C#C)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C97H174N36O28/c1-11-39-110-40-19-15-27-60(127-92(158)75(56(10)137)132-85(151)63(120-76(142)50(4)101)30-22-43-113-97(108)109)80(146)125-65(33-35-70(103)139)86(152)131-74(55(9)136)91(157)116-51(5)77(143)121-61(28-20-41-111-95(104)105)81(147)123-59(26-14-18-38-100)83(149)129-67(48-134)88(154)130-73(54(8)135)90(156)115-46-71(140)114-47-72(141)119-57(24-12-16-36-98)78(144)117-52(6)93(159)133-44-23-31-68(133)89(155)126-62(29-21-42-112-96(106)107)82(148)122-58(25-13-17-37-99)79(145)124-64(32-34-69(102)138)84(150)128-66(45-49(2)3)87(153)118-53(7)94(160)161/h1,49-68,73-75,110,134-137H,12-48,98-101H2,2-10H3,(H2,102,138)(H2,103,139)(H,114,140)(H,115,156)(H,116,157)(H,117,144)(H,118,153)(H,119,141)(H,120,142)(H,121,143)(H,122,148)(H,123,147)(H,124,145)(H,125,146)(H,126,155)(H,127,158)(H,128,150)(H,129,149)(H,130,154)(H,131,152)(H,132,151)(H,160,161)(H4,104,105,111)(H4,106,107,112)(H4,108,109,113)/t50-,51-,52-,53-,54+,55+,56+,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,73-,74-,75-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Time-dependent inhibition of recombinant LSD1 catalytic domain (178 to 831 amino acids) (unknown origin) expressed in baculovirus infected insect Sf9... |
Medchemcomm 3: 14-21 (2012)
Article DOI: 10.1039/c1md00220a
BindingDB Entry DOI: 10.7270/Q2SX6H67 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50318565

(CHEMBL1086217)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6]C#C)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C97H174N36O28/c1-11-39-110-40-19-15-27-60(127-92(158)75(56(10)137)132-85(151)63(120-76(142)50(4)101)30-22-43-113-97(108)109)80(146)125-65(33-35-70(103)139)86(152)131-74(55(9)136)91(157)116-51(5)77(143)121-61(28-20-41-111-95(104)105)81(147)123-59(26-14-18-38-100)83(149)129-67(48-134)88(154)130-73(54(8)135)90(156)115-46-71(140)114-47-72(141)119-57(24-12-16-36-98)78(144)117-52(6)93(159)133-44-23-31-68(133)89(155)126-62(29-21-42-112-96(106)107)82(148)122-58(25-13-17-37-99)79(145)124-64(32-34-69(102)138)84(150)128-66(45-49(2)3)87(153)118-53(7)94(160)161/h1,49-68,73-75,110,134-137H,12-48,98-101H2,2-10H3,(H2,102,138)(H2,103,139)(H,114,140)(H,115,156)(H,116,157)(H,117,144)(H,118,153)(H,119,141)(H,120,142)(H,121,143)(H,122,148)(H,123,147)(H,124,145)(H,125,146)(H,126,155)(H,127,158)(H,128,150)(H,129,149)(H,130,154)(H,131,152)(H,132,151)(H,160,161)(H4,104,105,111)(H4,106,107,112)(H4,108,109,113)/t50-,51-,52-,53-,54+,55+,56+,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,73-,74-,75-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 53: 5197-212 (2010)
Article DOI: 10.1021/jm100217a
BindingDB Entry DOI: 10.7270/Q2QR4Z35 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50366321

(CHEMBL3392203 | CHEMBL606101)Show SMILES OS(O)(=O)=O.OS([O-])(=O)=O.C[S+](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C1C[C@@H](N)C=C1 |r,c:35| Show InChI InChI=1S/C16H23N6O3S/c1-26(9-3-2-8(17)4-9)5-10-12(23)13(24)16(25-10)22-7-21-11-14(18)19-6-20-15(11)22/h2-3,6-10,12-13,16,23-24H,4-5,17H2,1H3,(H2,18,19,20)/q+1/p+1/t8-,9?,10+,12+,13+,16?,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coli |
Bioorg Med Chem Lett 3: 147-152 (1993)
Article DOI: 10.1016/S0960-894X(01)80865-9
BindingDB Entry DOI: 10.7270/Q2PR7WG3 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50368997

(CHEMBL1791422)Show SMILES C[S+](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@@H]1C[C@@H](O[NH3+])C=C1 |r,c:28| Show InChI InChI=1S/C16H24N6O4S/c1-27(9-3-2-8(4-9)26-18)5-10-12(23)13(24)16(25-10)22-7-21-11-14(17)19-6-20-15(11)22/h2-3,6-10,12-13,16,23-24H,4-5H2,1,18H3,(H2,17,19,20)/q+2/t8-,9-,10+,12+,13+,16+,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
compound was evaluated for the inhibitor constant against human S-adenosyl-L-methionine decarboxylase |
J Med Chem 38: 1770-7 (1995)
BindingDB Entry DOI: 10.7270/Q2X92BX3 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50034925

(CHEMBL299398 | O-{(1S,4S)-4-[6-(6-Amino-purin-9-yl...)Show SMILES CC1(C)OC2C(CS[C@H]3C[C@H](ON)C=C3)OC(C2O1)n1cnc2c(N)ncnc12 |c:13| Show InChI InChI=1S/C18H24N6O4S/c1-18(2)26-13-11(6-29-10-4-3-9(5-10)28-20)25-17(14(13)27-18)24-8-23-12-15(19)21-7-22-16(12)24/h3-4,7-11,13-14,17H,5-6,20H2,1-2H3,(H2,19,21,22)/t9-,10-,11?,13?,14?,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
compound was evaluated for the inhibitor constant against Escherichia coli S-adenosyl-L-methionine decarboxylase |
J Med Chem 38: 1770-7 (1995)
BindingDB Entry DOI: 10.7270/Q2X92BX3 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50034925

(CHEMBL299398 | O-{(1S,4S)-4-[6-(6-Amino-purin-9-yl...)Show SMILES CC1(C)OC2C(CS[C@H]3C[C@H](ON)C=C3)OC(C2O1)n1cnc2c(N)ncnc12 |c:13| Show InChI InChI=1S/C18H24N6O4S/c1-18(2)26-13-11(6-29-10-4-3-9(5-10)28-20)25-17(14(13)27-18)24-8-23-12-15(19)21-7-22-16(12)24/h3-4,7-11,13-14,17H,5-6,20H2,1-2H3,(H2,19,21,22)/t9-,10-,11?,13?,14?,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
compound was evaluated for the inhibitor constant against Escherichia coli S-adenosyl-L-methionine decarboxylase |
J Med Chem 38: 1770-7 (1995)
BindingDB Entry DOI: 10.7270/Q2X92BX3 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50368997

(CHEMBL1791422)Show SMILES C[S+](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@@H]1C[C@@H](O[NH3+])C=C1 |r,c:28| Show InChI InChI=1S/C16H24N6O4S/c1-27(9-3-2-8(4-9)26-18)5-10-12(23)13(24)16(25-10)22-7-21-11-14(17)19-6-20-15(11)22/h2-3,6-10,12-13,16,23-24H,4-5H2,1,18H3,(H2,17,19,20)/q+2/t8-,9-,10+,12+,13+,16+,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
compound was evaluated for the inhibitor constant against Escherichia coli S-adenosyl-L-methionine decarboxylase |
J Med Chem 38: 1770-7 (1995)
BindingDB Entry DOI: 10.7270/Q2X92BX3 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50368996
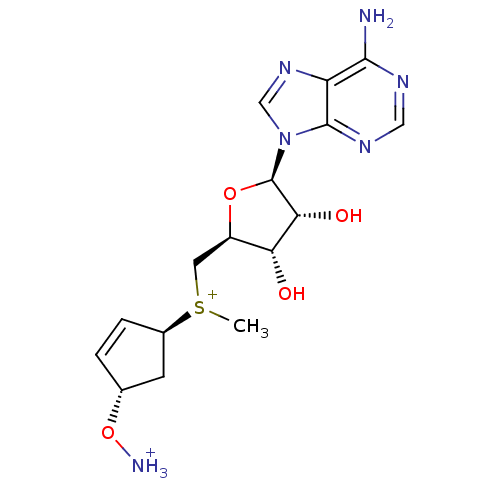
(CHEMBL1791421)Show SMILES C[S+](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H]1C[C@H](O[NH3+])C=C1 |r,c:28| Show InChI InChI=1S/C16H24N6O4S/c1-27(9-3-2-8(4-9)26-18)5-10-12(23)13(24)16(25-10)22-7-21-11-14(17)19-6-20-15(11)22/h2-3,6-10,12-13,16,23-24H,4-5H2,1,18H3,(H2,17,19,20)/q+2/t8-,9-,10-,12-,13-,16-,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitor constant against Escherichia coli S-adenosyl-L-methionine decarboxylase |
J Med Chem 38: 1770-7 (1995)
BindingDB Entry DOI: 10.7270/Q2X92BX3 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50368996

(CHEMBL1791421)Show SMILES C[S+](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H]1C[C@H](O[NH3+])C=C1 |r,c:28| Show InChI InChI=1S/C16H24N6O4S/c1-27(9-3-2-8(4-9)26-18)5-10-12(23)13(24)16(25-10)22-7-21-11-14(17)19-6-20-15(11)22/h2-3,6-10,12-13,16,23-24H,4-5H2,1,18H3,(H2,17,19,20)/q+2/t8-,9-,10-,12-,13-,16-,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitor constant against Escherichia coli S-adenosyl-L-methionine decarboxylase |
J Med Chem 38: 1770-7 (1995)
BindingDB Entry DOI: 10.7270/Q2X92BX3 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50281292
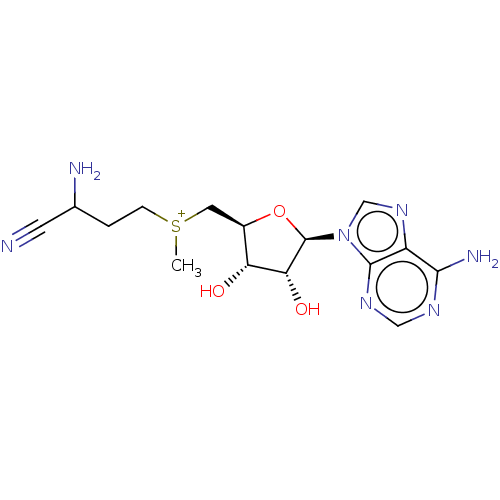
(2-amino-4-({[(2S,3S,4R)-5-(6-amino-9H-purin-9-yl)-...)Show SMILES Br.[Br-].C[S+](CCC(N)C#N)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C15H22N7O3S/c1-26(3-2-8(17)4-16)5-9-11(23)12(24)15(25-9)22-7-21-10-13(18)19-6-20-14(10)22/h6-9,11-12,15,23-24H,2-3,5,17H2,1H3,(H2,18,19,20)/q+1/p+1/t8?,9-,11-,12-,15?,26?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated to inactivate the bacterial AdoMet-DC |
Bioorg Med Chem Lett 3: 2811-2816 (1993)
Article DOI: 10.1016/S0960-894X(01)80770-8
BindingDB Entry DOI: 10.7270/Q2TH8N6T |
More data for this
Ligand-Target Pair | |
Diamine acetyltransferase 1
(Homo sapiens (Human)) | BDBM50095444
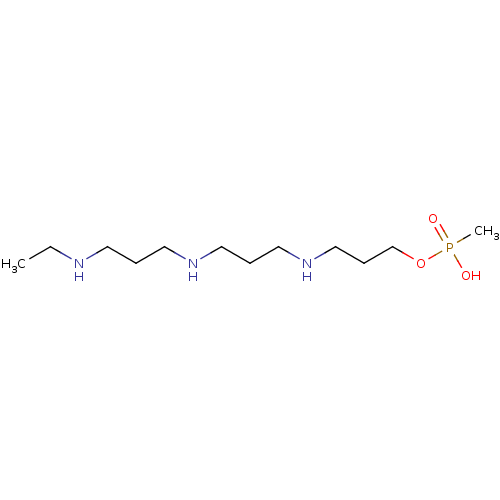
(CHEMBL315330 | Methyl-phosphonic acid mono-{3-[3-(...)Show InChI InChI=1S/C12H30N3O3P/c1-3-13-7-4-8-14-9-5-10-15-11-6-12-18-19(2,16)17/h13-15H,3-12H2,1-2H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against human SSAT |
J Med Chem 44: 1-26 (2001)
BindingDB Entry DOI: 10.7270/Q28S4QNR |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50034927

(CHEMBL48973 | O-{(1R,4R)-4-[6-(6-Amino-purin-9-yl)...)Show SMILES CC1(C)OC2C(CS[C@@H]3C[C@@H](ON)C=C3)OC(C2O1)n1cnc2c(N)ncnc12 |c:13| Show InChI InChI=1S/C18H24N6O4S/c1-18(2)26-13-11(6-29-10-4-3-9(5-10)28-20)25-17(14(13)27-18)24-8-23-12-15(19)21-7-22-16(12)24/h3-4,7-11,13-14,17H,5-6,20H2,1-2H3,(H2,19,21,22)/t9-,10-,11?,13?,14?,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
compound was evaluated for the inhibitory constant against human S-adenosyl-L-methionine decarboxylase |
J Med Chem 38: 1770-7 (1995)
BindingDB Entry DOI: 10.7270/Q2X92BX3 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50034927

(CHEMBL48973 | O-{(1R,4R)-4-[6-(6-Amino-purin-9-yl)...)Show SMILES CC1(C)OC2C(CS[C@@H]3C[C@@H](ON)C=C3)OC(C2O1)n1cnc2c(N)ncnc12 |c:13| Show InChI InChI=1S/C18H24N6O4S/c1-18(2)26-13-11(6-29-10-4-3-9(5-10)28-20)25-17(14(13)27-18)24-8-23-12-15(19)21-7-22-16(12)24/h3-4,7-11,13-14,17H,5-6,20H2,1-2H3,(H2,19,21,22)/t9-,10-,11?,13?,14?,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
compound was evaluated for the inhibitory constant against human S-adenosyl-L-methionine decarboxylase |
J Med Chem 38: 1770-7 (1995)
BindingDB Entry DOI: 10.7270/Q2X92BX3 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50281292

(2-amino-4-({[(2S,3S,4R)-5-(6-amino-9H-purin-9-yl)-...)Show SMILES Br.[Br-].C[S+](CCC(N)C#N)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C15H22N7O3S/c1-26(3-2-8(17)4-16)5-9-11(23)12(24)15(25-9)22-7-21-10-13(18)19-6-20-14(10)22/h6-9,11-12,15,23-24H,2-3,5,17H2,1H3,(H2,18,19,20)/q+1/p+1/t8?,9-,11-,12-,15?,26?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated to inactivate the human AdoMet-DC |
Bioorg Med Chem Lett 3: 2811-2816 (1993)
Article DOI: 10.1016/S0960-894X(01)80770-8
BindingDB Entry DOI: 10.7270/Q2TH8N6T |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50366318
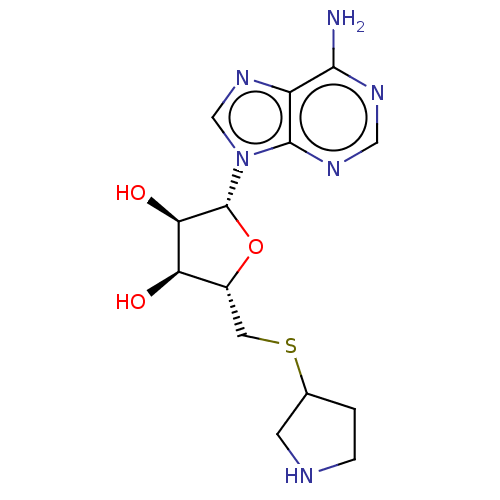
(CHEMBL3392208 | CHEMBL605698)Show SMILES OS(O)(=O)=O.Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSC2CCNC2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H20N6O3S/c15-12-9-13(18-5-17-12)20(6-19-9)14-11(22)10(21)8(23-14)4-24-7-1-2-16-3-7/h5-8,10-11,14,16,21-22H,1-4H2,(H2,15,17,18)/t7?,8-,10-,11-,14?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coli |
Bioorg Med Chem Lett 3: 147-152 (1993)
Article DOI: 10.1016/S0960-894X(01)80865-9
BindingDB Entry DOI: 10.7270/Q2PR7WG3 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50366320

(CHEMBL3392206 | CHEMBL605899)Show SMILES OS(O)(=O)=O.Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CS[C@@H]2CN[C@@H](C2)C(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H20N6O5S/c16-12-9-13(19-4-18-12)21(5-20-9)14-11(23)10(22)8(26-14)3-27-6-1-7(15(24)25)17-2-6/h4-8,10-11,14,17,22-23H,1-3H2,(H,24,25)(H2,16,18,19)/t6-,7+,8-,10-,11-,14?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coli |
Bioorg Med Chem Lett 3: 147-152 (1993)
Article DOI: 10.1016/S0960-894X(01)80865-9
BindingDB Entry DOI: 10.7270/Q2PR7WG3 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50366317

(CHEMBL3392204 | CHEMBL606102)Show SMILES OS(O)(=O)=O.OS([O-])(=O)=O.C[S+](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C1CCNC1 |r| Show InChI InChI=1S/C15H23N6O3S/c1-25(8-2-3-17-4-8)5-9-11(22)12(23)15(24-9)21-7-20-10-13(16)18-6-19-14(10)21/h6-9,11-12,15,17,22-23H,2-5H2,1H3,(H2,16,18,19)/q+1/p+1/t8?,9-,11-,12-,15?,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.69E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coli |
Bioorg Med Chem Lett 3: 147-152 (1993)
Article DOI: 10.1016/S0960-894X(01)80865-9
BindingDB Entry DOI: 10.7270/Q2PR7WG3 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50366316

(CHEMBL3392209 | CHEMBL605267)Show SMILES OS(O)(=O)=O.Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CS[C@H]2CN[C@@H](C2)C(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H20N6O5S/c16-12-9-13(19-4-18-12)21(5-20-9)14-11(23)10(22)8(26-14)3-27-6-1-7(15(24)25)17-2-6/h4-8,10-11,14,17,22-23H,1-3H2,(H,24,25)(H2,16,18,19)/t6-,7-,8+,10+,11+,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 9.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coli |
Bioorg Med Chem Lett 3: 147-152 (1993)
Article DOI: 10.1016/S0960-894X(01)80865-9
BindingDB Entry DOI: 10.7270/Q2PR7WG3 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50366319

(CHEMBL1791405 | CHEMBL3392205)Show SMILES OS(O)(=O)=O.OS([O-])(=O)=O.C[S+](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C1CN[C@@H](C1)C(O)=O |r| Show InChI InChI=1S/C16H22N6O5S/c1-28(7-2-8(16(25)26)18-3-7)4-9-11(23)12(24)15(27-9)22-6-21-10-13(17)19-5-20-14(10)22/h5-9,11-12,15,18,23-24H,2-4H2,1H3,(H2-,17,19,20,25,26)/p+2/t7-,8+,9-,11-,12-,15-,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.07E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coli |
Bioorg Med Chem Lett 3: 147-152 (1993)
Article DOI: 10.1016/S0960-894X(01)80865-9
BindingDB Entry DOI: 10.7270/Q2PR7WG3 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50366319

(CHEMBL1791405 | CHEMBL3392205)Show SMILES OS(O)(=O)=O.OS([O-])(=O)=O.C[S+](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C1CN[C@@H](C1)C(O)=O |r| Show InChI InChI=1S/C16H22N6O5S/c1-28(7-2-8(16(25)26)18-3-7)4-9-11(23)12(24)15(27-9)22-6-21-10-13(17)19-5-20-14(10)22/h5-9,11-12,15,18,23-24H,2-4H2,1H3,(H2-,17,19,20,25,26)/p+2/t7-,8+,9-,11-,12-,15-,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.07E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coli |
Bioorg Med Chem Lett 3: 147-152 (1993)
Article DOI: 10.1016/S0960-894X(01)80865-9
BindingDB Entry DOI: 10.7270/Q2PR7WG3 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50119879

((S)-N-((4S,6S,7S)-7-(2-(2,6-dimethylphenoxy)acetam...)Show SMILES CC(C)C[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)NC(=O)[C@H](C(C)C)N1CCCNC1=O Show InChI InChI=1S/C34H50N4O5/c1-22(2)18-27(36-33(41)31(23(3)4)38-17-11-16-35-34(38)42)20-29(39)28(19-26-14-8-7-9-15-26)37-30(40)21-43-32-24(5)12-10-13-25(32)6/h7-10,12-15,22-23,27-29,31,39H,11,16-21H2,1-6H3,(H,35,42)(H,36,41)(H,37,40)/t27-,28-,29-,31-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 NL4-3 wild type protease expressed in Escherichia coli preincubated for 20 mins by FRET analysis |
Bioorg Med Chem 21: 7430-4 (2013)
Article DOI: 10.1016/j.bmc.2013.09.045
BindingDB Entry DOI: 10.7270/Q23B633V |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50180655

(A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...)Show SMILES CC(C)[C@H](N1CCCNC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 Show InChI InChI=1S/C37H48N4O5/c1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t30-,31-,32-,34-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 NL4-3 wild type protease expressed in Escherichia coli preincubated for 20 mins by FRET analysis |
Bioorg Med Chem 21: 7430-4 (2013)
Article DOI: 10.1016/j.bmc.2013.09.045
BindingDB Entry DOI: 10.7270/Q23B633V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50494545

(CHEMBL3093183)Show SMILES CCCC[C@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](N)CCCNC(N)=N)C(C)C)C(O)=O |r| Show InChI InChI=1S/C39H66N10O9/c1-6-7-15-29(38(57)58)47-31(50)22-45-35(54)28(16-17-32(51)52)48-36(55)30(20-25-12-9-8-10-13-25)44-21-26(19-23(2)3)46-37(56)33(24(4)5)49-34(53)27(40)14-11-18-43-39(41)42/h8-10,12-13,23-24,26-30,33,44H,6-7,11,14-22,40H2,1-5H3,(H,45,54)(H,46,56)(H,47,50)(H,48,55)(H,49,53)(H,51,52)(H,57,58)(H4,41,42,43)/t26-,27-,28-,29-,30-,33-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 NL4-3 wild type protease expressed in Escherichia coli preincubated for 20 mins by FRET analysis |
Bioorg Med Chem 21: 7430-4 (2013)
Article DOI: 10.1016/j.bmc.2013.09.045
BindingDB Entry DOI: 10.7270/Q23B633V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 by microplate reader assay |
Medchemcomm 6: 613-618 (2015)
BindingDB Entry DOI: 10.7270/Q2H70HPV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Spermine synthase
(Homo sapiens (Human)) | BDBM50095449

(2-[1-(2-Amino-ethyl)-6-(3-amino-propylamino)-hexyl...)Show SMILES NCCCNCCCCCC(CCN)SC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C21H38N8O3S/c22-7-4-10-25-9-3-1-2-5-14(6-8-23)33-11-15-17(30)18(31)21(32-15)29-13-28-16-19(24)26-12-27-20(16)29/h12-15,17-18,21,25,30-31H,1-11,22-23H2,(H2,24,26,27)/t14?,15-,17-,18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against spermine synthase |
J Med Chem 44: 1-26 (2001)
BindingDB Entry DOI: 10.7270/Q28S4QNR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data