

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227529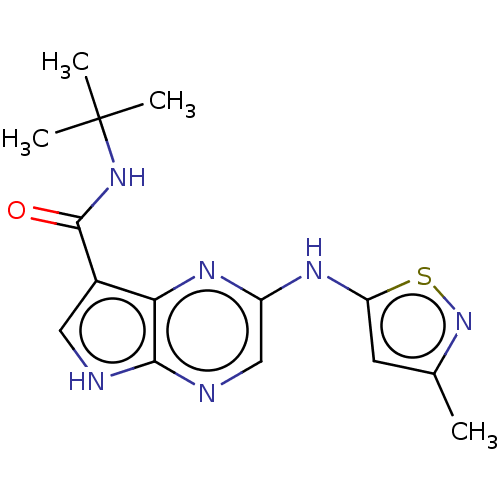 (US9334278, I-18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227523 (US9334278, I-12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227549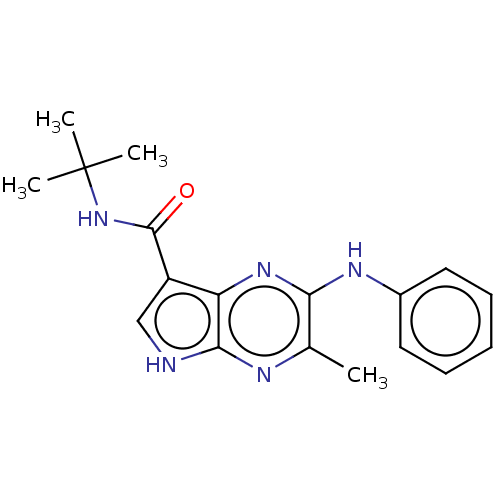 (US9334278, I-37) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227537 (US9334278, I-26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227535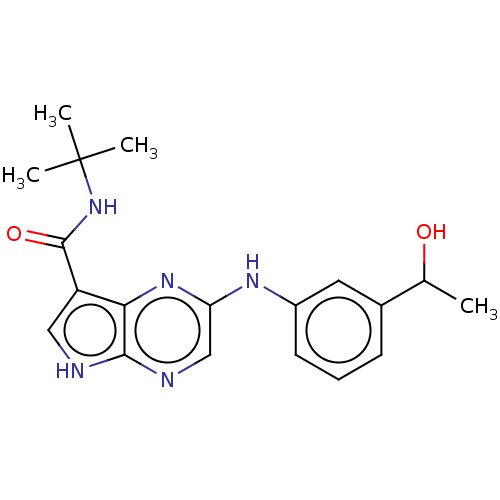 (US9334278, I-24) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227512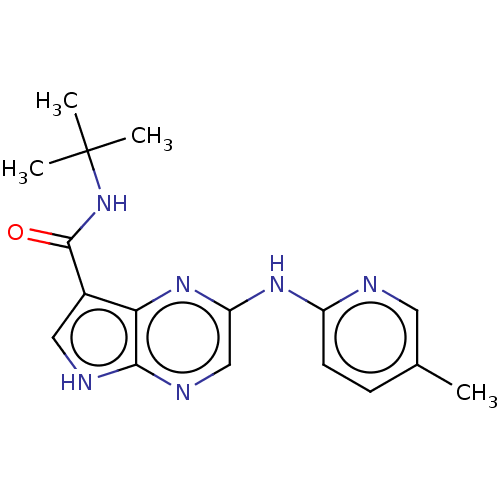 (US9334278, I-1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM291171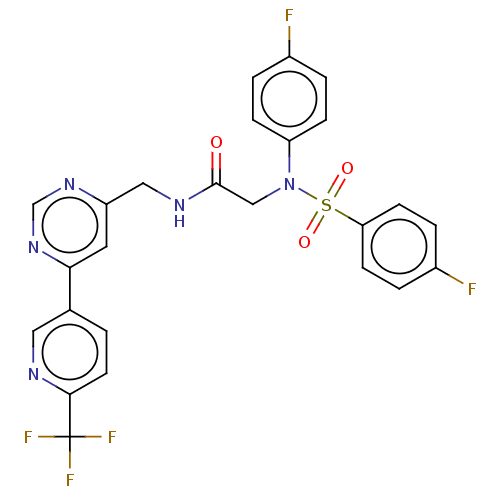 (US9580411, Example 86) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e... | US Patent US9580411 (2017) BindingDB Entry DOI: 10.7270/Q25T3NJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2 (Homo sapiens (Human)) | BDBM50328264 (5-(2-chlorophenyl)-3-isopropyl-7-nitro-2,10-dihydr...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CDK2-cyclin E | Bioorg Med Chem Lett 20: 5984-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.079 BindingDB Entry DOI: 10.7270/Q21C1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50426474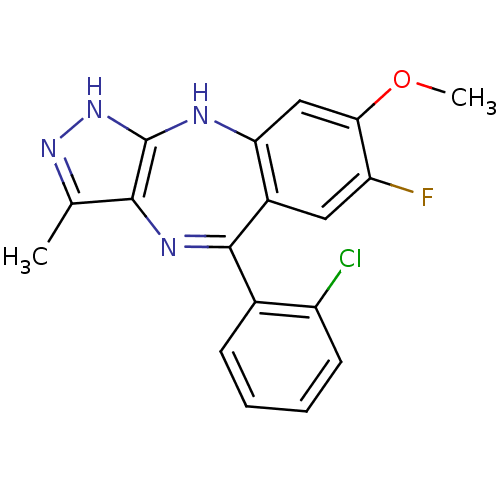 (CHEMBL1980391) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of KDR (unknown origin) | ACS Med Chem Lett 4: 259-63 (2013) Article DOI: 10.1021/ml300351e BindingDB Entry DOI: 10.7270/Q2902541 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227522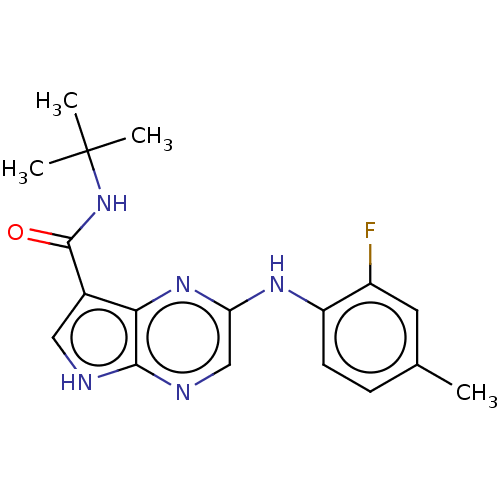 (US9334278, I-11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.9 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227524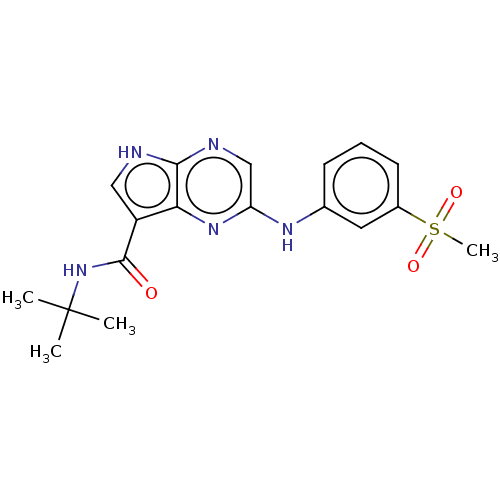 (US9334278, I-13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.8 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM291170 (2-[N-(Propan-2-yl)(4-fluorobenzene)sulfonamido]-N-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e... | US Patent US9580411 (2017) BindingDB Entry DOI: 10.7270/Q25T3NJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227516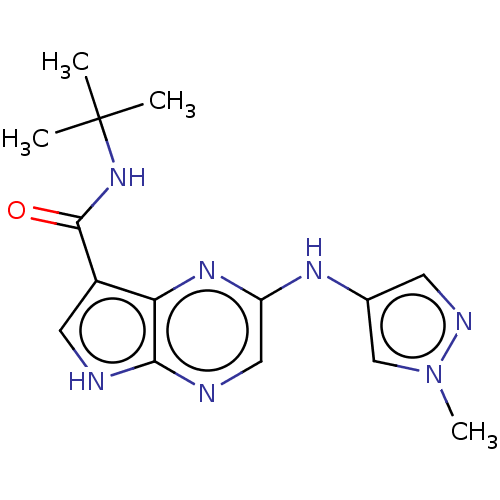 (US9334278, I-5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.7 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227520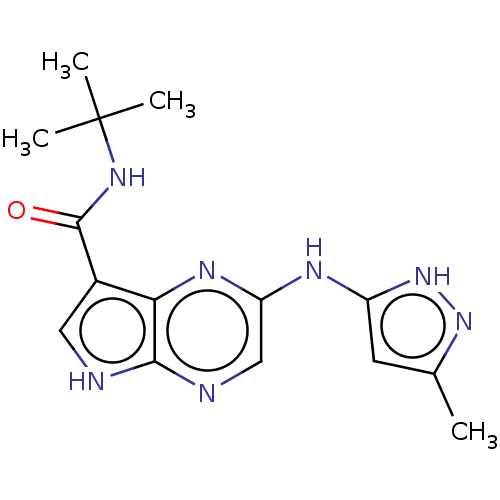 (US9334278, I-9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15.5 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2 (Homo sapiens (Human)) | BDBM50328256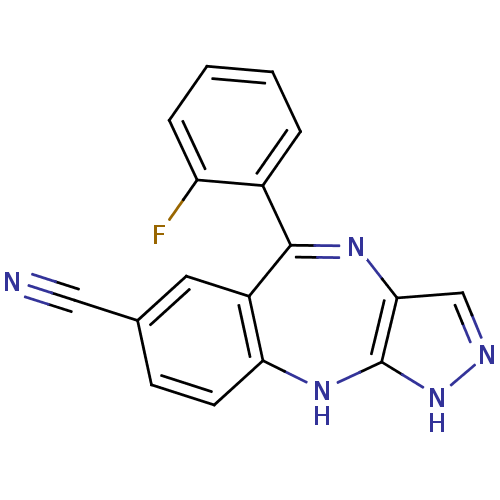 (5-(2-fluorophenyl)-2,10-dihydrobenzo[e]pyrazolo[4,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CDK2-cyclin E | Bioorg Med Chem Lett 20: 5984-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.079 BindingDB Entry DOI: 10.7270/Q21C1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227521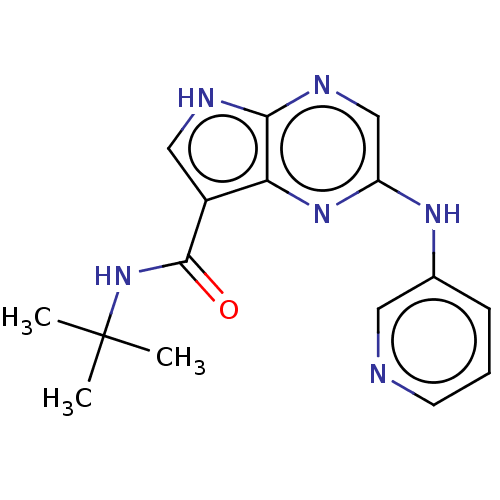 (US9334278, I-10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16.3 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2 (Homo sapiens (Human)) | BDBM50328279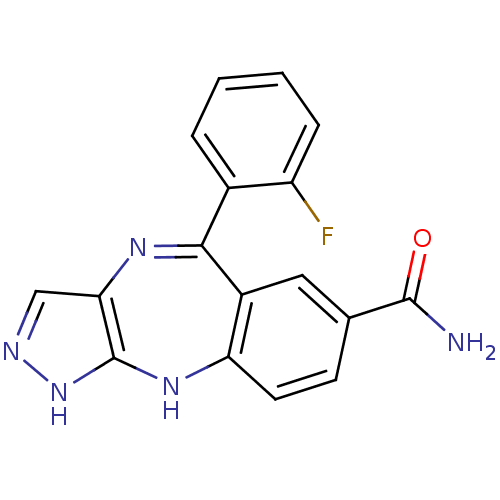 (5-(2-fluorophenyl)-2,10-dihydrobenzo[e]pyrazolo[4,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CDK2-cyclin E | Bioorg Med Chem Lett 20: 5984-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.079 BindingDB Entry DOI: 10.7270/Q21C1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227517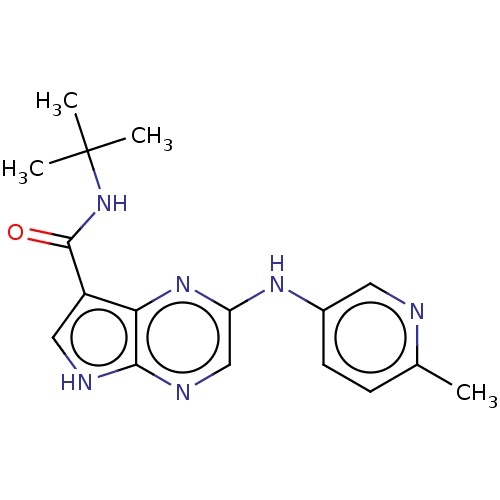 (US9334278, I-6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17.1 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227542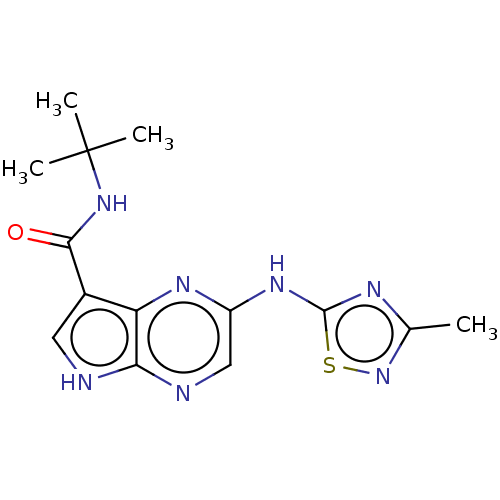 (US9334278, I-31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17.8 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227538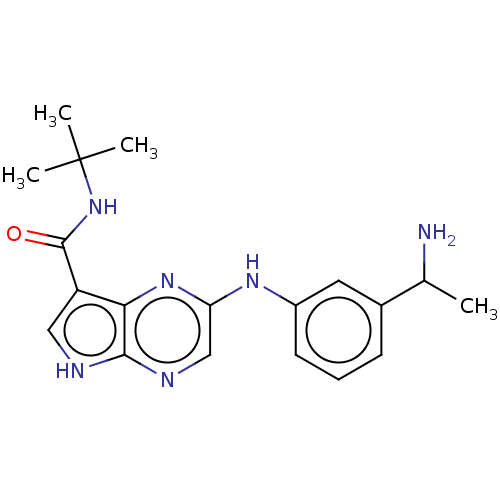 (US9334278, I-27) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18.8 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2 (Homo sapiens (Human)) | BDBM50328267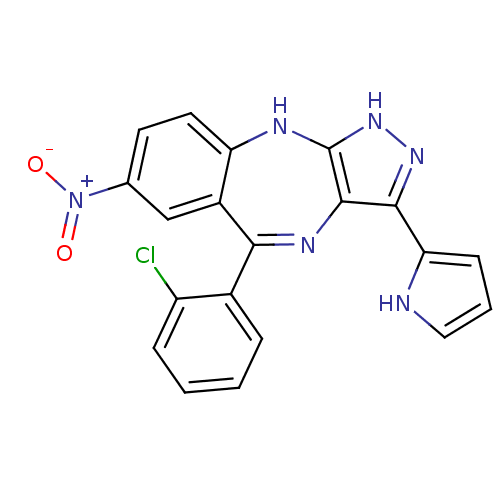 (5-(2-chlorophenyl)-7-nitro-3-(1H-pyrrol-2-yl)-2,10...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CDK2-cyclin E | Bioorg Med Chem Lett 20: 5984-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.079 BindingDB Entry DOI: 10.7270/Q21C1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227514 (US9334278, I-3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21.1 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50426474 (CHEMBL1980391) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CHK2 (unknown origin) | ACS Med Chem Lett 4: 259-63 (2013) Article DOI: 10.1021/ml300351e BindingDB Entry DOI: 10.7270/Q2902541 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2 (Homo sapiens (Human)) | BDBM50328280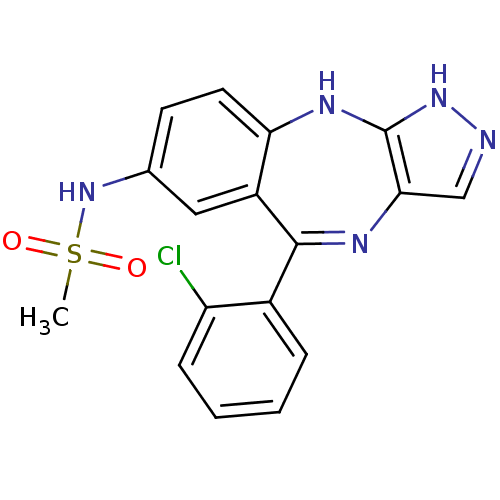 (CHEMBL1258022 | N-(5-(2-chlorophenyl)-2,10-dihydro...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CDK2-cyclin E | Bioorg Med Chem Lett 20: 5984-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.079 BindingDB Entry DOI: 10.7270/Q21C1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227533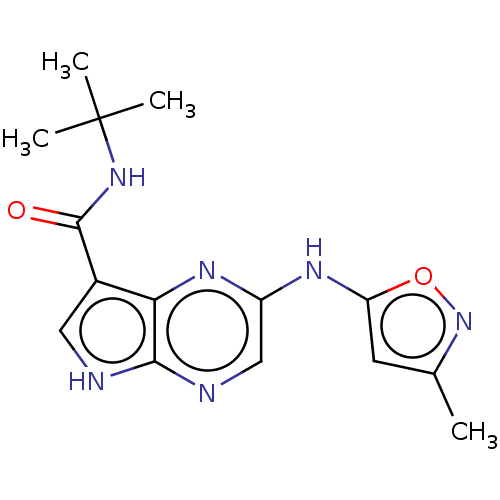 (US9334278, I-22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227513 (US9334278, I-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27.4 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50426474 (CHEMBL1980391) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of FGFR1 (unknown origin) | ACS Med Chem Lett 4: 259-63 (2013) Article DOI: 10.1021/ml300351e BindingDB Entry DOI: 10.7270/Q2902541 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM291169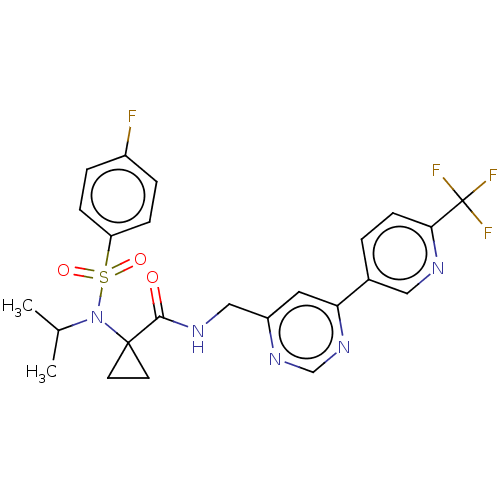 (1-(4-Fluoro-N-isopropylphenylsulfonamido)-N-((6-(6...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e... | US Patent US9580411 (2017) BindingDB Entry DOI: 10.7270/Q25T3NJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227531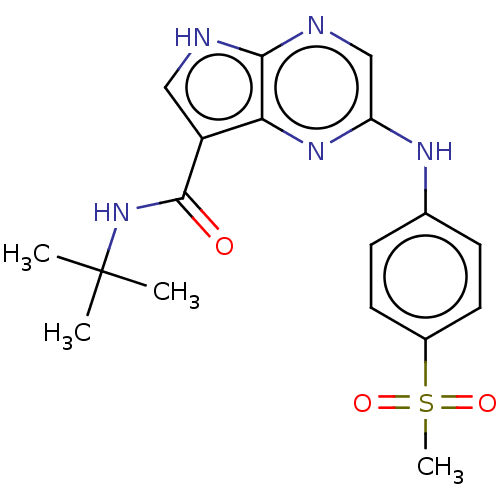 (US9334278, I-20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50328258 (CHEMBL1257912 | N-(5-(2-chlorophenyl)-2,10-dihydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of KDR | Bioorg Med Chem Lett 20: 5984-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.079 BindingDB Entry DOI: 10.7270/Q21C1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50426473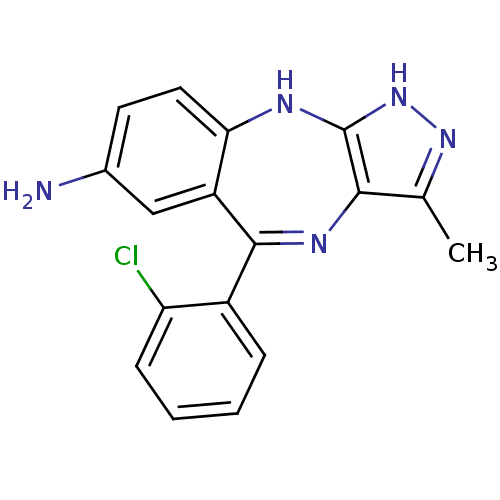 (CHEMBL2322701) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of KDR (unknown origin) | ACS Med Chem Lett 4: 259-63 (2013) Article DOI: 10.1021/ml300351e BindingDB Entry DOI: 10.7270/Q2902541 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50328257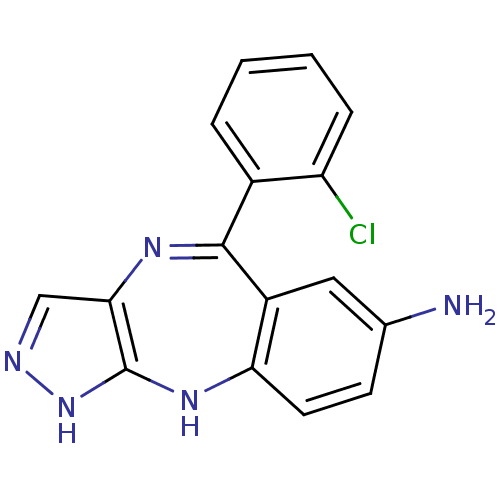 (5-(2-chlorophenyl)-2,10-dihydrobenzo[e]pyrazolo[4,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of KDR | Bioorg Med Chem Lett 20: 5984-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.079 BindingDB Entry DOI: 10.7270/Q21C1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2 (Homo sapiens (Human)) | BDBM50328255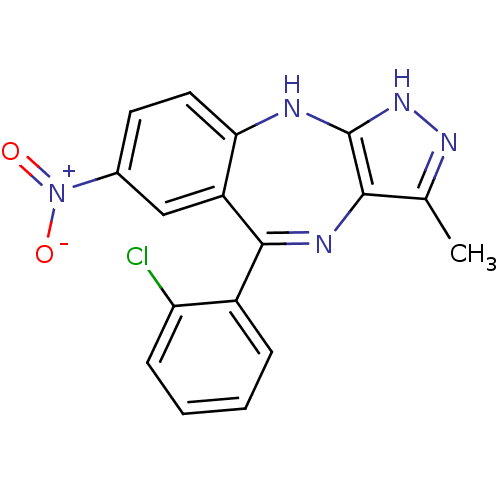 (5-(2-chlorophenyl)-3-methyl-7-nitro-2,10-dihydrobe...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CDK2-cyclin E | Bioorg Med Chem Lett 20: 5984-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.079 BindingDB Entry DOI: 10.7270/Q21C1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227525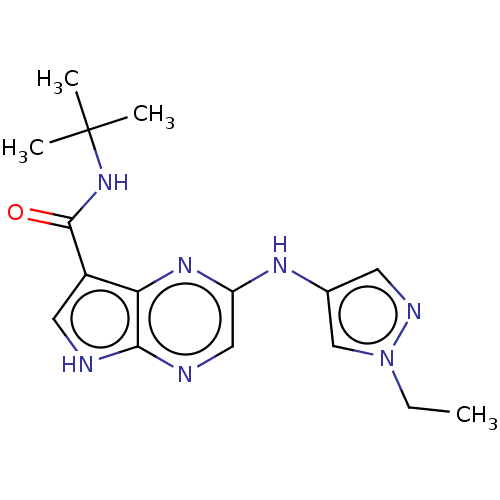 (US9334278, I-14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 35.9 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2 (Homo sapiens (Human)) | BDBM50328258 (CHEMBL1257912 | N-(5-(2-chlorophenyl)-2,10-dihydro...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CDK2-cyclin E | Bioorg Med Chem Lett 20: 5984-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.079 BindingDB Entry DOI: 10.7270/Q21C1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM291163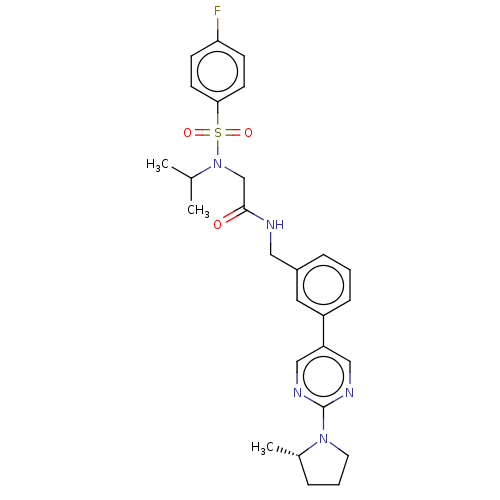 (US9580411, Example 79-(R) | US9580411, Example 79-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e... | US Patent US9580411 (2017) BindingDB Entry DOI: 10.7270/Q25T3NJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50328256 (5-(2-fluorophenyl)-2,10-dihydrobenzo[e]pyrazolo[4,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of KDR | Bioorg Med Chem Lett 20: 5984-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.079 BindingDB Entry DOI: 10.7270/Q21C1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2 (Homo sapiens (Human)) | BDBM50328274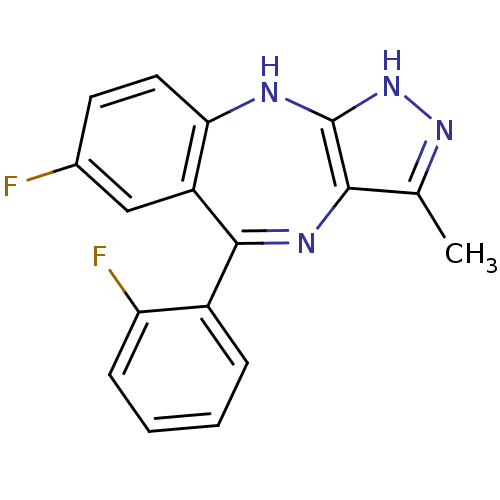 (7-fluoro-5-(2-fluorophenyl)-3-methyl-2,10-dihydrob...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CDK2-cyclin E | Bioorg Med Chem Lett 20: 5984-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.079 BindingDB Entry DOI: 10.7270/Q21C1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227515 (US9334278, I-4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 42.8 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227543 (US9334278, I-32) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 43.7 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2 (Homo sapiens (Human)) | BDBM50328281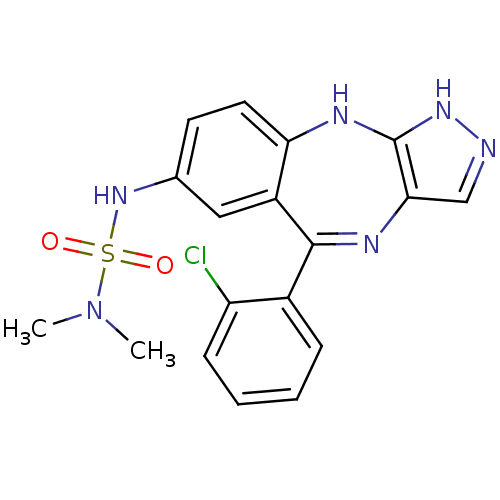 (CHEMBL1258141 | N'-[5-(2-chlorophenyl)-2,10-dihydr...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CDK2-cyclin E | Bioorg Med Chem Lett 20: 5984-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.079 BindingDB Entry DOI: 10.7270/Q21C1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227528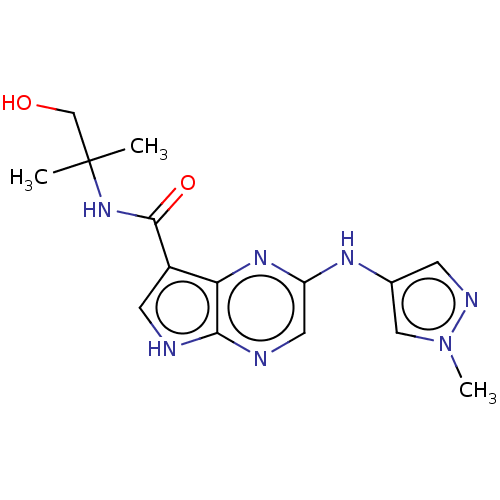 (US9334278, I-17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 49.9 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50426473 (CHEMBL2322701) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of FGFR1 (unknown origin) | ACS Med Chem Lett 4: 259-63 (2013) Article DOI: 10.1021/ml300351e BindingDB Entry DOI: 10.7270/Q2902541 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2 (Homo sapiens (Human)) | BDBM50328269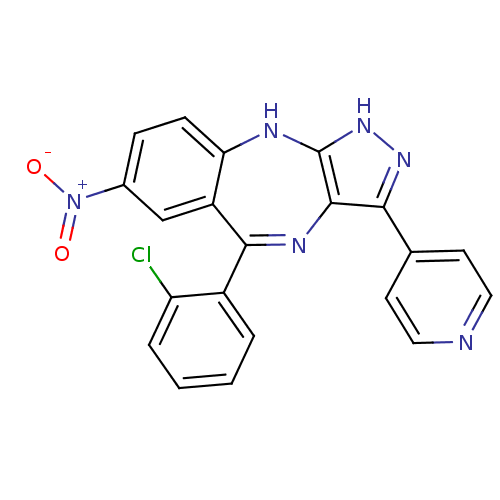 (5-(2-chlorophenyl)-7-nitro-3-(pyridin-4-yl)-2,10-d...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CDK2-cyclin E | Bioorg Med Chem Lett 20: 5984-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.079 BindingDB Entry DOI: 10.7270/Q21C1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM227519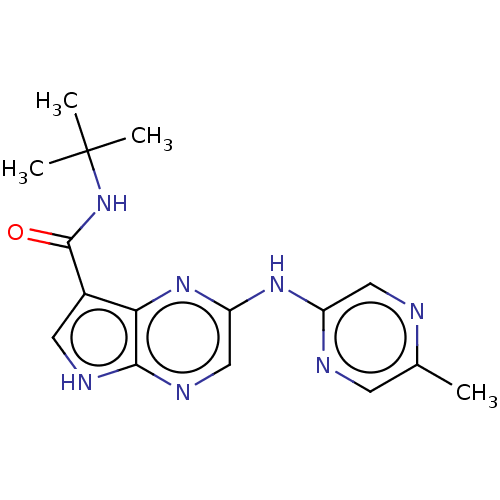 (US9334278, I-8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 51.3 | n/a | n/a | n/a | n/a | n/a | 30 |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay plates: 96-well MultiScreen 0.65 μm filter plates (Millipore Cat. No.: MADVNOB 10) Streptavidin coated beads: Streptavidin Sepharose™,... | US Patent US9334278 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM291163 (US9580411, Example 79-(R) | US9580411, Example 79-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e... | US Patent US9580411 (2017) BindingDB Entry DOI: 10.7270/Q25T3NJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM291168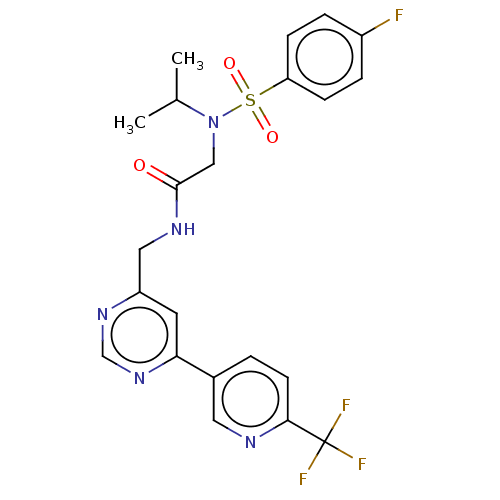 (2,2-Dideuterio-2-(4-fluoro-N-isopropylphenylsulfon...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e... | US Patent US9580411 (2017) BindingDB Entry DOI: 10.7270/Q25T3NJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50426474 (CHEMBL1980391) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of aurora-A (unknown origin) | ACS Med Chem Lett 4: 259-63 (2013) Article DOI: 10.1021/ml300351e BindingDB Entry DOI: 10.7270/Q2902541 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2 (Homo sapiens (Human)) | BDBM50328272 (5-(2-fluorophenyl)-3-methyl-2,10-dihydrobenzo[e]py...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CDK2-cyclin E | Bioorg Med Chem Lett 20: 5984-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.079 BindingDB Entry DOI: 10.7270/Q21C1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2 (Homo sapiens (Human)) | BDBM50328260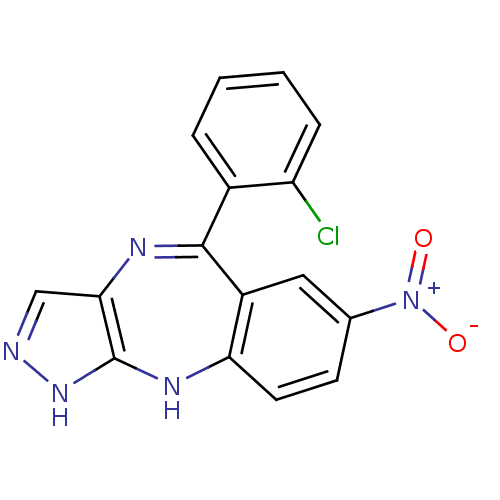 (5-(2-chlorophenyl)-7-nitro-2,10-dihydrobenzo[e]pyr...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CDK2-cyclin E | Bioorg Med Chem Lett 20: 5984-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.079 BindingDB Entry DOI: 10.7270/Q21C1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 102 total ) | Next | Last >> |