 Found 8227 hits with Last Name = 'wu' and Initial = 'd'
Found 8227 hits with Last Name = 'wu' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526620
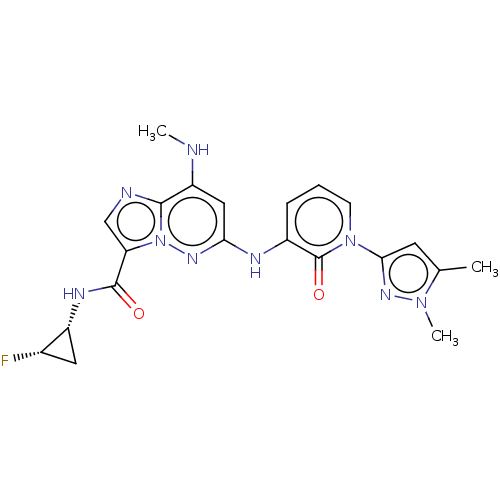
(CHEMBL4460368)Show SMILES CNc1cc(Nc2cccn(-c3cc(C)n(C)n3)c2=O)nn2c(cnc12)C(=O)N[C@@H]1C[C@@H]1F |r| Show InChI InChI=1S/C21H22FN9O2/c1-11-7-18(28-29(11)3)30-6-4-5-13(21(30)33)25-17-9-15(23-2)19-24-10-16(31(19)27-17)20(32)26-14-8-12(14)22/h4-7,9-10,12,14,23H,8H2,1-3H3,(H,25,27)(H,26,32)/t12-,14+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526622
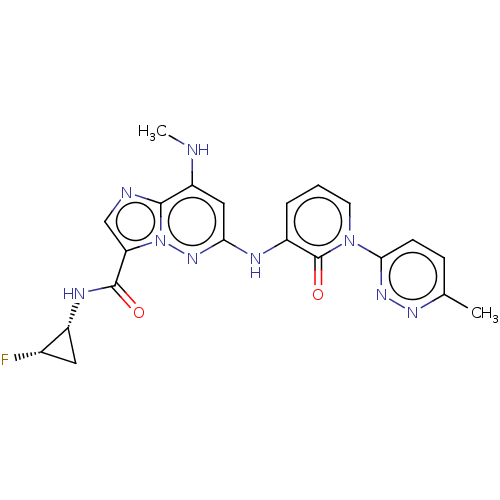
(CHEMBL4442827)Show SMILES CNc1cc(Nc2cccn(-c3ccc(C)nn3)c2=O)nn2c(cnc12)C(=O)N[C@@H]1C[C@@H]1F |r| Show InChI InChI=1S/C21H20FN9O2/c1-11-5-6-18(28-27-11)30-7-3-4-13(21(30)33)25-17-9-15(23-2)19-24-10-16(31(19)29-17)20(32)26-14-8-12(14)22/h3-7,9-10,12,14,23H,8H2,1-2H3,(H,25,29)(H,26,32)/t12-,14+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50063292
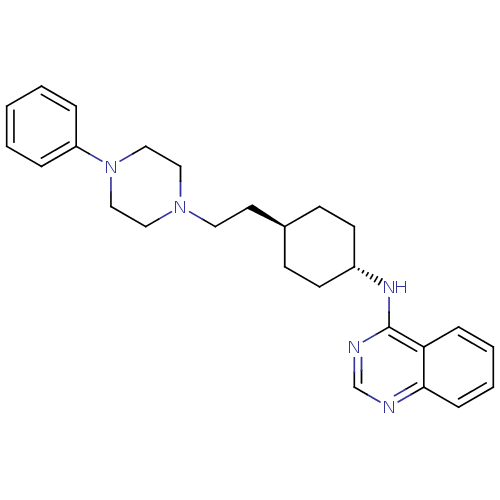
(CHEMBL349426 | {4-[2-(4-Phenyl-piperazin-1-yl)-eth...)Show SMILES C(CN1CCN(CC1)c1ccccc1)[C@H]1CC[C@@H](CC1)Nc1ncnc2ccccc12 |wU:17.22,wD:14.15,(10.31,-6.02,;11.15,-7.32,;12.69,-7.24,;13.39,-5.86,;14.93,-5.79,;15.76,-7.07,;15.07,-8.45,;13.53,-8.53,;17.3,-7,;17.99,-5.63,;19.5,-5.53,;20.36,-6.83,;19.66,-8.19,;18.13,-8.28,;8.79,-6.11,;7.95,-4.81,;6.42,-4.88,;5.72,-6.26,;6.55,-7.54,;8.09,-7.47,;4.18,-6.33,;3.34,-5.04,;4.04,-3.66,;3.23,-2.36,;1.69,-2.43,;.99,-3.81,;-.55,-3.87,;-1.26,-5.23,;-.44,-6.54,;1.1,-6.47,;1.8,-5.11,)| Show InChI InChI=1S/C26H33N5/c1-2-6-23(7-3-1)31-18-16-30(17-19-31)15-14-21-10-12-22(13-11-21)29-26-24-8-4-5-9-25(24)27-20-28-26/h1-9,20-22H,10-19H2,(H,27,28,29)/t21-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity determined by measuring displacement of [3H]-spiperone from cloned Human Dopamine receptor D3 in CHO-K1 cells |
J Med Chem 41: 760-71 (1998)
Article DOI: 10.1021/jm9707378
BindingDB Entry DOI: 10.7270/Q20G3J97 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50290221
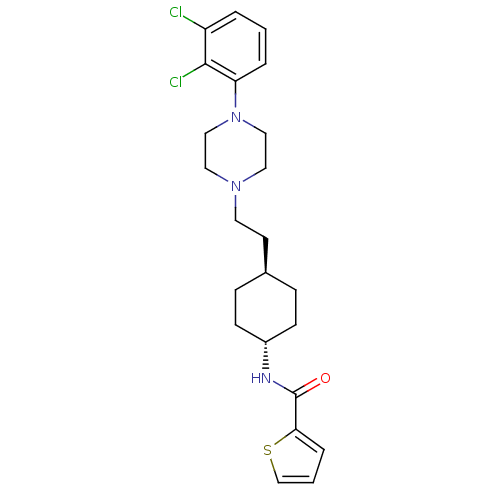
(CHEMBL80919 | Thiophene-2-carboxylic acid (4-{2-[4...)Show SMILES Clc1cccc(N2CCN(CC[C@H]3CC[C@@H](CC3)NC(=O)c3cccs3)CC2)c1Cl |wU:12.11,wD:15.18,(20.53,-4.53,;19.79,-5.88,;20.6,-7.19,;19.85,-8.55,;18.31,-8.59,;17.52,-7.26,;15.98,-7.29,;15.24,-8.63,;13.7,-8.66,;12.91,-7.36,;11.37,-7.38,;10.6,-8.73,;9.06,-8.73,;8.29,-10.06,;6.75,-10.06,;5.98,-8.73,;6.74,-7.4,;8.28,-7.4,;4.44,-8.75,;3.67,-10.08,;2.13,-10.09,;4.44,-11.41,;5.98,-11.49,;5.83,-14.15,;4.3,-14.05,;3.59,-12.7,;13.66,-6,;15.2,-5.98,;18.25,-5.91,;17.46,-4.6,)| Show InChI InChI=1S/C23H29Cl2N3OS/c24-19-3-1-4-20(22(19)25)28-14-12-27(13-15-28)11-10-17-6-8-18(9-7-17)26-23(29)21-5-2-16-30-21/h1-5,16-18H,6-15H2,(H,26,29)/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. |
Bioorg Med Chem Lett 7: 2403-2408 (1997)
Article DOI: 10.1016/S0960-894X(97)00443-5
BindingDB Entry DOI: 10.7270/Q27W6CQ0 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526613
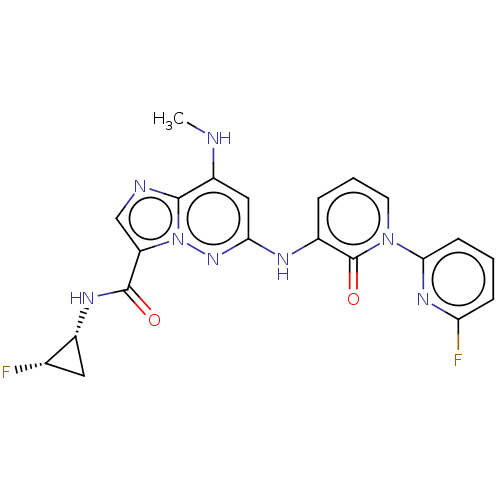
(CHEMBL4571920)Show SMILES CNc1cc(Nc2cccn(-c3cccc(F)n3)c2=O)nn2c(cnc12)C(=O)N[C@@H]1C[C@@H]1F |r| Show InChI InChI=1S/C21H18F2N8O2/c1-24-14-9-17(29-31-15(10-25-19(14)31)20(32)27-13-8-11(13)22)26-12-4-3-7-30(21(12)33)18-6-2-5-16(23)28-18/h2-7,9-11,13,24H,8H2,1H3,(H,26,29)(H,27,32)/t11-,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526619
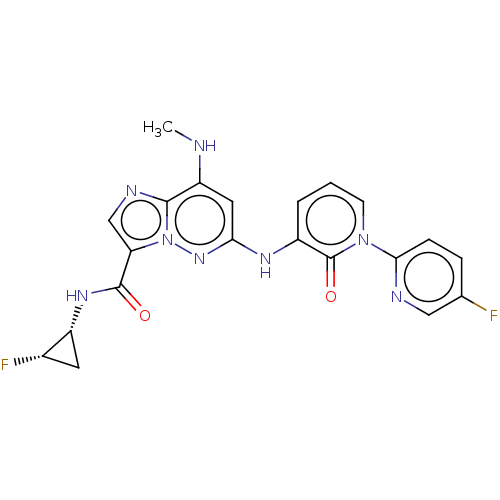
(CHEMBL4439957)Show SMILES CNc1cc(Nc2cccn(-c3ccc(F)cn3)c2=O)nn2c(cnc12)C(=O)N[C@@H]1C[C@@H]1F |r| Show InChI InChI=1S/C21H18F2N8O2/c1-24-15-8-17(29-31-16(10-26-19(15)31)20(32)28-14-7-12(14)23)27-13-3-2-6-30(21(13)33)18-5-4-11(22)9-25-18/h2-6,8-10,12,14,24H,7H2,1H3,(H,27,29)(H,28,32)/t12-,14+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50286859
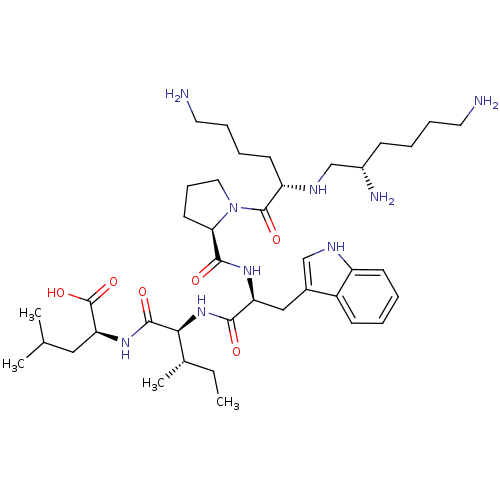
(2-{2-[(S)-2-({1-[(S)-6-Amino-2-((S)-(S)-2,6-diamin...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H]1CCCN1C(=O)[C@H](CCCCN)NC[C@@H](N)CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C40H67N9O6/c1-5-26(4)35(38(52)47-33(40(54)55)21-25(2)3)48-36(50)32(22-27-23-44-30-15-7-6-14-29(27)30)46-37(51)34-17-12-20-49(34)39(53)31(16-9-11-19-42)45-24-28(43)13-8-10-18-41/h6-7,14-15,23,25-26,28,31-35,44-45H,5,8-13,16-22,24,41-43H2,1-4H3,(H,46,51)(H,47,52)(H,48,50)(H,54,55)/t26-,28-,31-,32-,33-,34+,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Neurotensin Receptor |
Bioorg Med Chem Lett 5: 997-1002 (1995)
Article DOI: 10.1016/0960-894X(95)00155-M
BindingDB Entry DOI: 10.7270/Q2GQ6Z76 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50240339
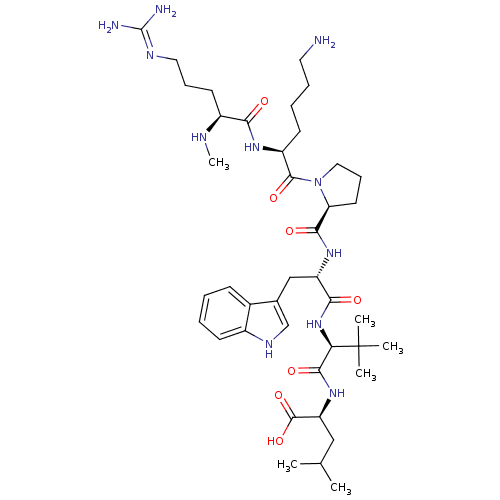
((S)-2-((S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)-5-g...)Show SMILES CN[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)C(C)(C)C |r,wU:2.1,43.45,wD:25.26,29.29,47.49,13.12,(-9.76,-17.57,;-8.43,-18.35,;-7.09,-17.6,;-7.09,-16.06,;-8.41,-15.28,;-8.4,-13.73,;-9.73,-12.95,;-9.72,-11.41,;-11.06,-10.63,;-8.38,-10.64,;-5.76,-18.38,;-5.77,-19.91,;-4.42,-17.61,;-3.09,-18.39,;-3.1,-19.93,;-4.44,-20.7,;-4.45,-22.24,;-5.78,-23,;-5.8,-24.54,;-1.74,-17.63,;-1.73,-16.1,;-.42,-18.41,;-0,-19.94,;1.55,-19.94,;2.08,-18.51,;.87,-17.56,;.94,-16,;-.36,-15.18,;2.3,-15.29,;3.6,-16.11,;3.53,-17.65,;4.83,-18.49,;6.26,-17.92,;7.23,-19.11,;6.4,-20.41,;6.81,-21.89,;5.72,-22.98,;4.24,-22.59,;3.84,-21.1,;4.92,-20.02,;4.97,-15.4,;5.05,-13.86,;6.27,-16.23,;7.64,-15.53,;8.93,-16.36,;8.86,-17.9,;10.3,-15.65,;11.59,-16.49,;11.52,-18.02,;12.82,-18.86,;12.82,-20.4,;14.19,-18.15,;12.96,-15.78,;14.25,-16.61,;13.04,-14.25,;7.72,-13.99,;7.71,-12.44,;9.26,-14.02,;6.18,-13.96,)| Show InChI InChI=1S/C41H67N11O7/c1-24(2)21-31(39(58)59)50-37(56)33(41(3,4)5)51-35(54)30(22-25-23-47-27-14-8-7-13-26(25)27)49-36(55)32-17-12-20-52(32)38(57)29(15-9-10-18-42)48-34(53)28(45-6)16-11-19-46-40(43)44/h7-8,13-14,23-24,28-33,45,47H,9-12,15-22,42H2,1-6H3,(H,48,53)(H,49,55)(H,50,56)(H,51,54)(H,58,59)(H4,43,44,46)/t28-,29-,30-,31-,32-,33+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for its binding affinity against Neurotensin Receptor after peripheral administration |
Bioorg Med Chem Lett 5: 997-1002 (1995)
Article DOI: 10.1016/0960-894X(95)00155-M
BindingDB Entry DOI: 10.7270/Q2GQ6Z76 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50582801
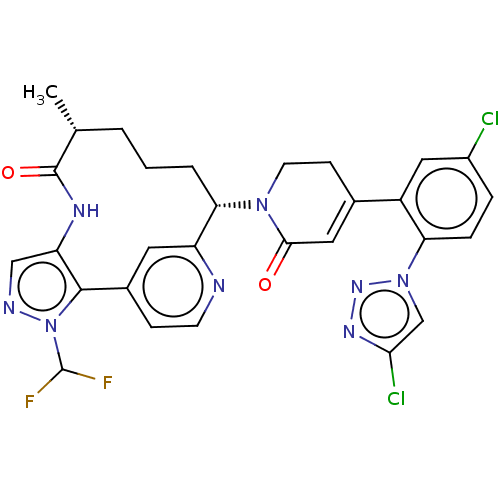
(CHEMBL5076656)Show SMILES C[C@@H]1CCC[C@H](N2CCC(=CC2=O)c2cc(Cl)ccc2-n2cc(Cl)nn2)c2cc(ccn2)-c2c(NC1=O)cnn2C(F)F |r,c:9| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00613
BindingDB Entry DOI: 10.7270/Q20005Z7 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526615
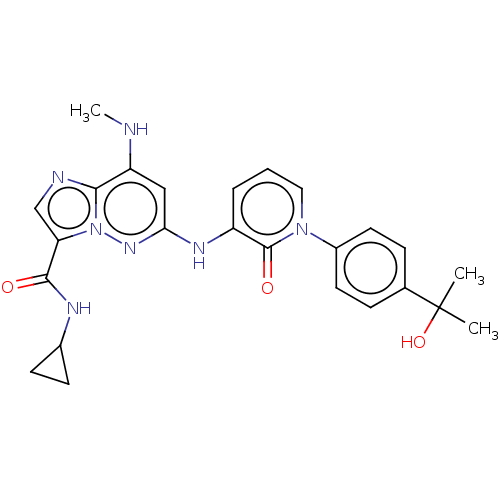
(CHEMBL4434711)Show SMILES CNc1cc(Nc2cccn(-c3ccc(cc3)C(C)(C)O)c2=O)nn2c(cnc12)C(=O)NC1CC1 Show InChI InChI=1S/C25H27N7O3/c1-25(2,35)15-6-10-17(11-7-15)31-12-4-5-18(24(31)34)29-21-13-19(26-3)22-27-14-20(32(22)30-21)23(33)28-16-8-9-16/h4-7,10-14,16,26,35H,8-9H2,1-3H3,(H,28,33)(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- prazosin binding against Alpha-1A adrenergic receptor from rat submaxillary gland |
J Med Chem 42: 5181-7 (2000)
BindingDB Entry DOI: 10.7270/Q23T9HZ0 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526606

(CHEMBL4532948 | US11174264, Compound I-3)Show SMILES CNc1cc(Nc2cccn(-c3ccccn3)c2=O)nn2c(cnc12)C(=O)N[C@@H]1C[C@@H]1F |r| Show InChI InChI=1S/C21H19FN8O2/c1-23-15-10-17(26-13-5-4-8-29(21(13)32)18-6-2-3-7-24-18)28-30-16(11-25-19(15)30)20(31)27-14-9-12(14)22/h2-8,10-12,14,23H,9H2,1H3,(H,26,28)(H,27,31)/t12-,14+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526624
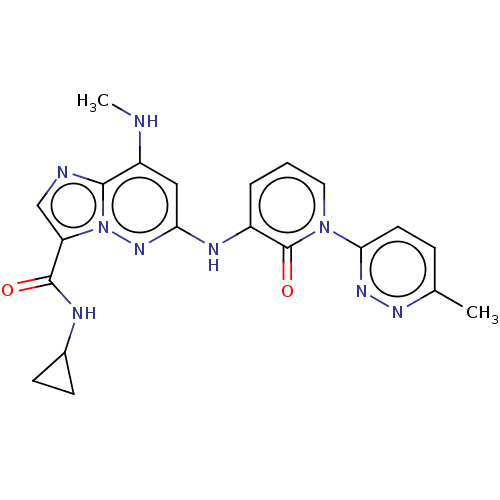
(CHEMBL4443010)Show SMILES CNc1cc(Nc2cccn(-c3ccc(C)nn3)c2=O)nn2c(cnc12)C(=O)NC1CC1 Show InChI InChI=1S/C21H21N9O2/c1-12-5-8-18(27-26-12)29-9-3-4-14(21(29)32)25-17-10-15(22-2)19-23-11-16(30(19)28-17)20(31)24-13-6-7-13/h3-5,8-11,13,22H,6-7H2,1-2H3,(H,24,31)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50286864

((S)-2-{(S)-2-[(S)-2-({(R)-1-[5-Guanidino-2-(3-guan...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H]1CCCN1C(=O)C(CCCNC(N)=N)CCCNC(N)=N)C(C)(C)C)C(O)=O Show InChI InChI=1S/C38H61N11O6/c1-22(2)19-28(35(54)55)47-33(52)30(38(3,4)5)48-31(50)27(20-24-21-45-26-14-7-6-13-25(24)26)46-32(51)29-15-10-18-49(29)34(53)23(11-8-16-43-36(39)40)12-9-17-44-37(41)42/h6-7,13-14,21-23,27-30,45H,8-12,15-20H2,1-5H3,(H,46,51)(H,47,52)(H,48,50)(H,54,55)(H4,39,40,43)(H4,41,42,44)/t27-,28-,29+,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Neurotensin Receptor |
Bioorg Med Chem Lett 5: 997-1002 (1995)
Article DOI: 10.1016/0960-894X(95)00155-M
BindingDB Entry DOI: 10.7270/Q2GQ6Z76 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM247411
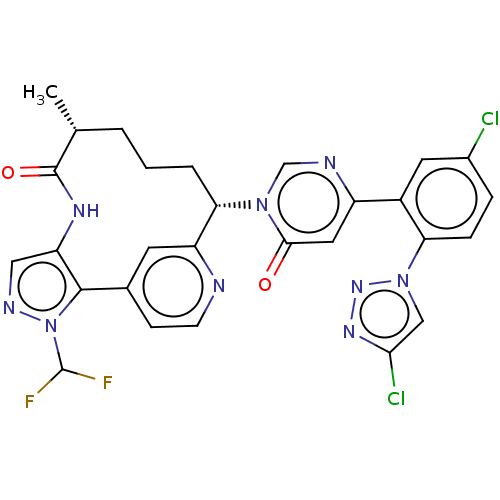
(US10336754, Example 353 | US11053247, Example 353 ...)Show SMILES C[C@@H]1CCC[C@@H](c2cc(ccn2)-c2c(NC1=O)cnn2C(F)F)n1cnc(cc1=O)-c1cc(Cl)ccc1-n1cc(Cl)nn1 |r| Show InChI InChI=1S/C28H23Cl2F2N9O2/c1-15-3-2-4-23(20-9-16(7-8-33-20)26-21(36-27(15)43)12-35-41(26)28(31)32)39-14-34-19(11-25(39)42)18-10-17(29)5-6-22(18)40-13-24(30)37-38-40/h5-15,23,28H,2-4H2,1H3,(H,36,43)/t15-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00613
BindingDB Entry DOI: 10.7270/Q20005Z7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50582799
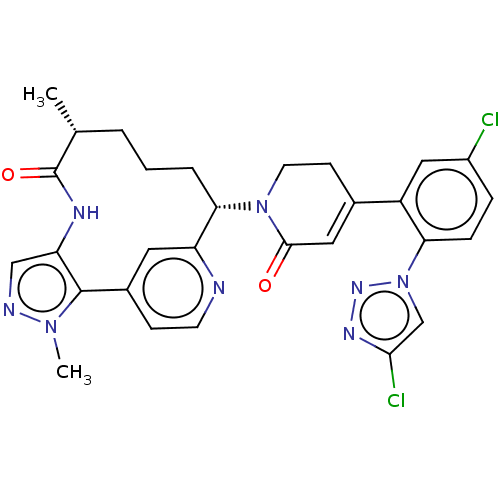
(CHEMBL5094166)Show SMILES C[C@@H]1CCC[C@H](N2CCC(=CC2=O)c2cc(Cl)ccc2-n2cc(Cl)nn2)c2cc(ccn2)-c2c(NC1=O)cnn2C |r,c:9| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00613
BindingDB Entry DOI: 10.7270/Q20005Z7 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526617
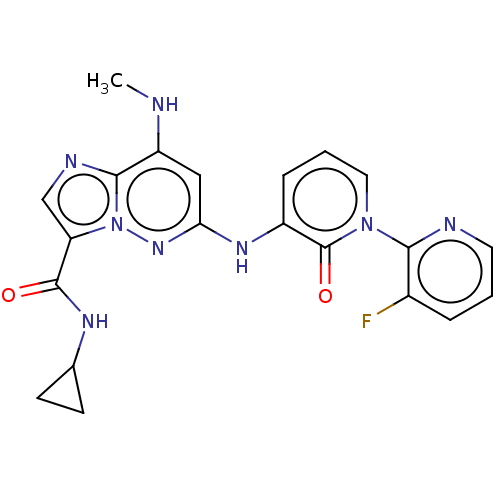
(CHEMBL4435047)Show SMILES CNc1cc(Nc2cccn(-c3ncccc3F)c2=O)nn2c(cnc12)C(=O)NC1CC1 Show InChI InChI=1S/C21H19FN8O2/c1-23-15-10-17(28-30-16(11-25-19(15)30)20(31)26-12-6-7-12)27-14-5-3-9-29(21(14)32)18-13(22)4-2-8-24-18/h2-5,8-12,23H,6-7H2,1H3,(H,26,31)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526621
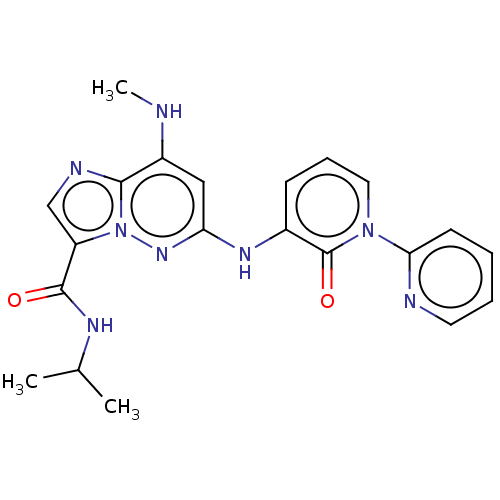
(CHEMBL4438107)Show SMILES CNc1cc(Nc2cccn(-c3ccccn3)c2=O)nn2c(cnc12)C(=O)NC(C)C Show InChI InChI=1S/C21H22N8O2/c1-13(2)25-20(30)16-12-24-19-15(22-3)11-17(27-29(16)19)26-14-7-6-10-28(21(14)31)18-8-4-5-9-23-18/h4-13,22H,1-3H3,(H,25,30)(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526603
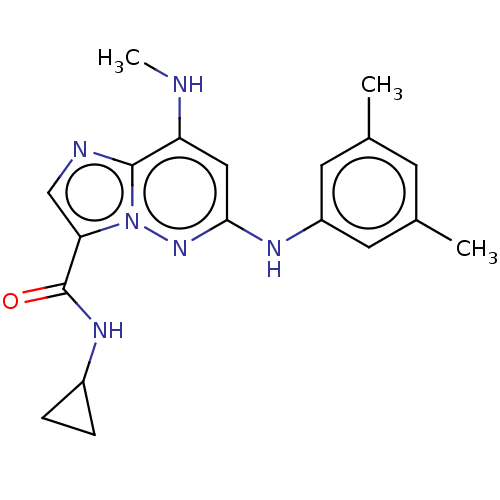
(CHEMBL4293907)Show SMILES CNc1cc(Nc2cc(C)cc(C)c2)nn2c(cnc12)C(=O)NC1CC1 Show InChI InChI=1S/C19H22N6O/c1-11-6-12(2)8-14(7-11)22-17-9-15(20-3)18-21-10-16(25(18)24-17)19(26)23-13-4-5-13/h6-10,13,20H,4-5H2,1-3H3,(H,22,24)(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526607
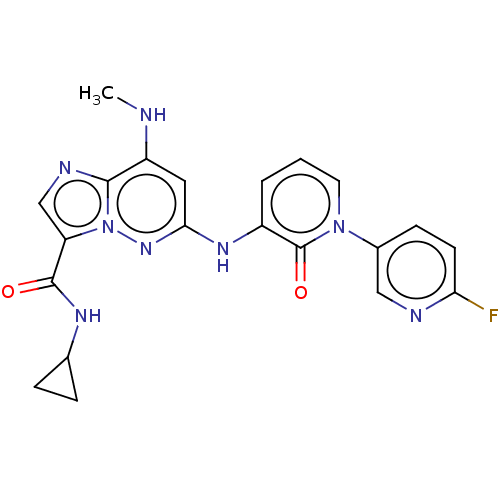
(CHEMBL4474801)Show SMILES CNc1cc(Nc2cccn(-c3ccc(F)nc3)c2=O)nn2c(cnc12)C(=O)NC1CC1 Show InChI InChI=1S/C21H19FN8O2/c1-23-15-9-18(28-30-16(11-25-19(15)30)20(31)26-12-4-5-12)27-14-3-2-8-29(21(14)32)13-6-7-17(22)24-10-13/h2-3,6-12,23H,4-5H2,1H3,(H,26,31)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50063279
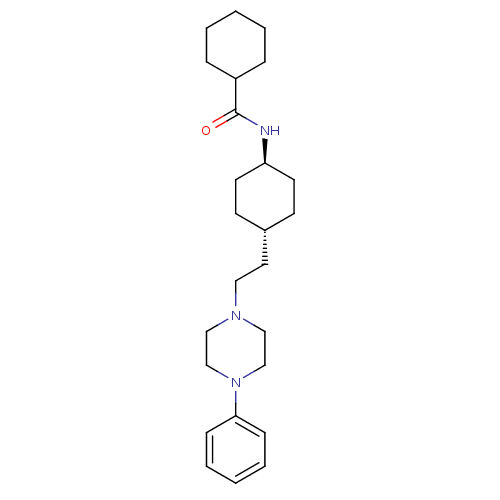
(CHEMBL309623 | Cyclohexanecarboxylic acid {4-[2-(4...)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2ccccc2)CC1)C1CCCCC1 |wU:6.6,wD:3.2,(1.34,-9.45,;2.88,-9.43,;3.66,-8.1,;5.2,-8.1,;5.97,-9.43,;7.51,-9.42,;8.29,-8.08,;9.83,-8.08,;10.6,-6.74,;12.14,-6.71,;12.89,-5.36,;14.44,-5.33,;15.22,-6.65,;14.48,-7.98,;12.94,-8.02,;16.77,-6.62,;17.56,-7.94,;19.09,-7.91,;19.85,-6.56,;19.04,-5.22,;17.5,-5.27,;7.51,-6.76,;5.96,-6.77,;3.66,-10.76,;5.19,-10.76,;5.95,-12.1,;5.18,-13.42,;3.66,-13.42,;2.88,-12.1,)| Show InChI InChI=1S/C25H39N3O/c29-25(22-7-3-1-4-8-22)26-23-13-11-21(12-14-23)15-16-27-17-19-28(20-18-27)24-9-5-2-6-10-24/h2,5-6,9-10,21-23H,1,3-4,7-8,11-20H2,(H,26,29)/t21-,23- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. |
Bioorg Med Chem Lett 7: 2403-2408 (1997)
Article DOI: 10.1016/S0960-894X(97)00443-5
BindingDB Entry DOI: 10.7270/Q27W6CQ0 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50063279

(CHEMBL309623 | Cyclohexanecarboxylic acid {4-[2-(4...)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2ccccc2)CC1)C1CCCCC1 |wU:6.6,wD:3.2,(1.34,-9.45,;2.88,-9.43,;3.66,-8.1,;5.2,-8.1,;5.97,-9.43,;7.51,-9.42,;8.29,-8.08,;9.83,-8.08,;10.6,-6.74,;12.14,-6.71,;12.89,-5.36,;14.44,-5.33,;15.22,-6.65,;14.48,-7.98,;12.94,-8.02,;16.77,-6.62,;17.56,-7.94,;19.09,-7.91,;19.85,-6.56,;19.04,-5.22,;17.5,-5.27,;7.51,-6.76,;5.96,-6.77,;3.66,-10.76,;5.19,-10.76,;5.95,-12.1,;5.18,-13.42,;3.66,-13.42,;2.88,-12.1,)| Show InChI InChI=1S/C25H39N3O/c29-25(22-7-3-1-4-8-22)26-23-13-11-21(12-14-23)15-16-27-17-19-28(20-18-27)24-9-5-2-6-10-24/h2,5-6,9-10,21-23H,1,3-4,7-8,11-20H2,(H,26,29)/t21-,23- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity determined by measuring displacement of [3H]-spiperone from cloned Human Dopamine receptor D3 in CHO-K1 cells |
J Med Chem 41: 760-71 (1998)
Article DOI: 10.1021/jm9707378
BindingDB Entry DOI: 10.7270/Q20G3J97 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50366427
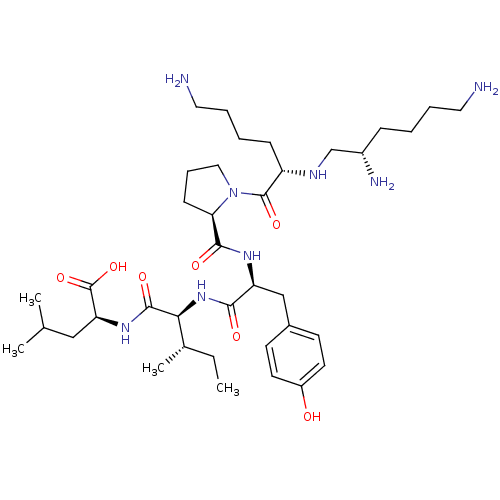
(CHEMBL1793865)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](CCCCN)NC[C@@H](N)CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C38H66N8O7/c1-5-25(4)33(36(50)44-31(38(52)53)21-24(2)3)45-34(48)30(22-26-14-16-28(47)17-15-26)43-35(49)32-13-10-20-46(32)37(51)29(12-7-9-19-40)42-23-27(41)11-6-8-18-39/h14-17,24-25,27,29-33,42,47H,5-13,18-23,39-41H2,1-4H3,(H,43,49)(H,44,50)(H,45,48)(H,52,53)/t25-,27-,29-,30-,31-,32+,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Neurotensin Receptor |
Bioorg Med Chem Lett 5: 997-1002 (1995)
Article DOI: 10.1016/0960-894X(95)00155-M
BindingDB Entry DOI: 10.7270/Q2GQ6Z76 |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Rattus norvegicus (rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- prazosin binding against Alpha-1B adrenergic receptor from rat liver |
J Med Chem 42: 5181-7 (2000)
BindingDB Entry DOI: 10.7270/Q23T9HZ0 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526604
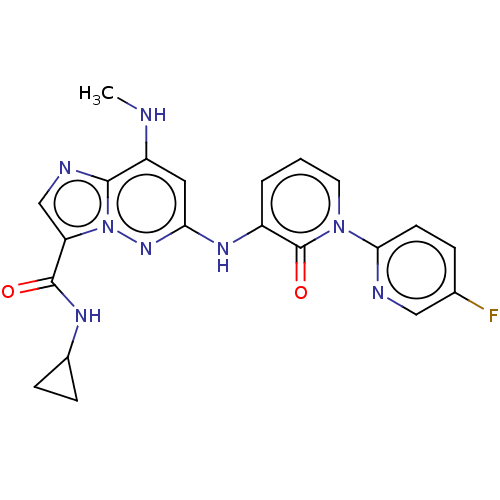
(CHEMBL4561123)Show SMILES CNc1cc(Nc2cccn(-c3ccc(F)cn3)c2=O)nn2c(cnc12)C(=O)NC1CC1 Show InChI InChI=1S/C21H19FN8O2/c1-23-15-9-17(28-30-16(11-25-19(15)30)20(31)26-13-5-6-13)27-14-3-2-8-29(21(14)32)18-7-4-12(22)10-24-18/h2-4,7-11,13,23H,5-6H2,1H3,(H,26,31)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50582800
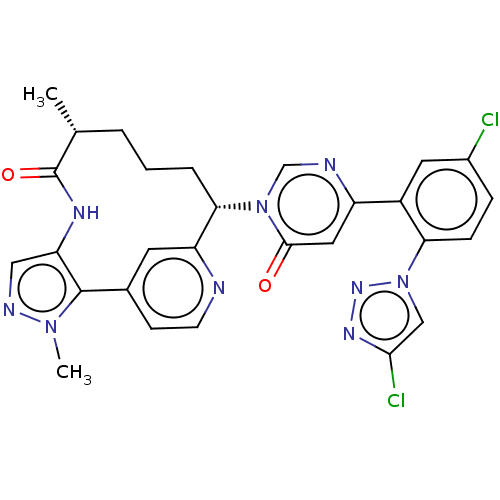
(CHEMBL5093567)Show SMILES C[C@@H]1CCC[C@@H](c2cc(ccn2)-c2c(NC1=O)cnn2C)n1cnc(cc1=O)-c1cc(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00613
BindingDB Entry DOI: 10.7270/Q20005Z7 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50067485
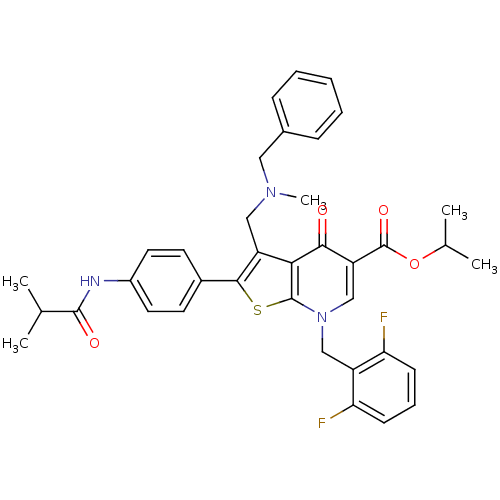
(3-[(Benzyl-methyl-amino)-methyl]-7-(2,6-difluoro-b...)Show SMILES CC(C)OC(=O)c1cn(Cc2c(F)cccc2F)c2sc(c(CN(C)Cc3ccccc3)c2c1=O)-c1ccc(NC(=O)C(C)C)cc1 Show InChI InChI=1S/C37H37F2N3O4S/c1-22(2)35(44)40-26-16-14-25(15-17-26)34-28(19-41(5)18-24-10-7-6-8-11-24)32-33(43)29(37(45)46-23(3)4)21-42(36(32)47-34)20-27-30(38)12-9-13-31(27)39/h6-17,21-23H,18-20H2,1-5H3,(H,40,44) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards human gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 13: 3617-22 (2003)
BindingDB Entry DOI: 10.7270/Q24X576N |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122654

(1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...)Show SMILES CONC(=O)Nc1ccc(cc1)-c1sc2n(Cc3c(F)cccc3F)c(=O)n(-c3ccccc3)c(=O)c2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C36H31F2N5O4S/c1-41(20-23-10-5-3-6-11-23)21-28-31-33(44)43(26-12-7-4-8-13-26)36(46)42(22-27-29(37)14-9-15-30(27)38)34(31)48-32(28)24-16-18-25(19-17-24)39-35(45)40-47-2/h3-19H,20-22H2,1-2H3,(H2,39,40,45) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards human gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 13: 3617-22 (2003)
BindingDB Entry DOI: 10.7270/Q24X576N |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526610
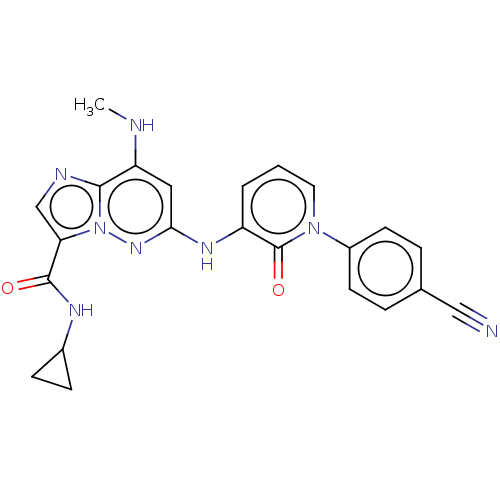
(CHEMBL4453441)Show SMILES CNc1cc(Nc2cccn(-c3ccc(cc3)C#N)c2=O)nn2c(cnc12)C(=O)NC1CC1 Show InChI InChI=1S/C23H20N8O2/c1-25-18-11-20(29-31-19(13-26-21(18)31)22(32)27-15-6-7-15)28-17-3-2-10-30(23(17)33)16-8-4-14(12-24)5-9-16/h2-5,8-11,13,15,25H,6-7H2,1H3,(H,27,32)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526616

(CHEMBL4579439)Show SMILES CNc1cc(Nc2cccn(-c3cccc(F)n3)c2=O)nn2c(cnc12)C(=O)NC1CC1 Show InChI InChI=1S/C21H19FN8O2/c1-23-14-10-17(28-30-15(11-24-19(14)30)20(31)25-12-7-8-12)26-13-4-3-9-29(21(13)32)18-6-2-5-16(22)27-18/h2-6,9-12,23H,7-8H2,1H3,(H,25,31)(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260806
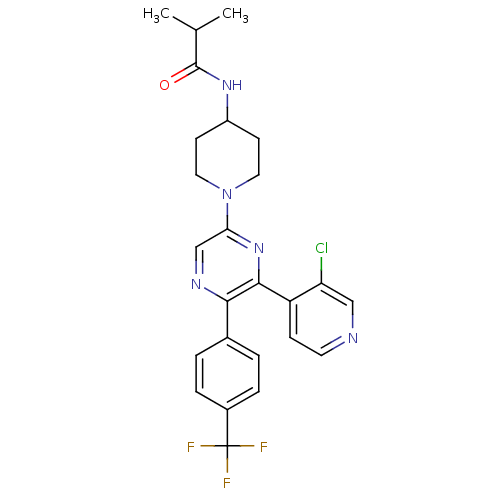
(CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...)Show SMILES CC(C)C(=O)NC1CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccncc1Cl Show InChI InChI=1S/C25H25ClF3N5O/c1-15(2)24(35)32-18-8-11-34(12-9-18)21-14-31-22(16-3-5-17(6-4-16)25(27,28)29)23(33-21)19-7-10-30-13-20(19)26/h3-7,10,13-15,18H,8-9,11-12H2,1-2H3,(H,32,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50541586
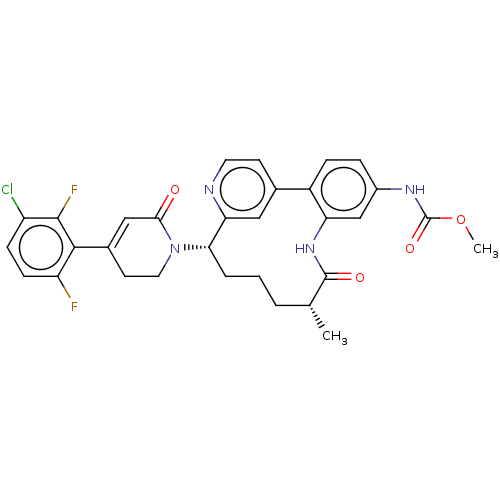
(CHEMBL4638245)Show SMILES COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](N3CCC(=CC3=O)c3c(F)ccc(Cl)c3F)c3cc-2ccn3)c1 |r,c:22| Show InChI InChI=1S/C31H29ClF2N4O4/c1-17-4-3-5-26(38-13-11-19(15-27(38)39)28-23(33)9-8-22(32)29(28)34)25-14-18(10-12-35-25)21-7-6-20(36-31(41)42-2)16-24(21)37-30(17)40/h6-10,12,14-17,26H,3-5,11,13H2,1-2H3,(H,36,41)(H,37,40)/t17-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00613
BindingDB Entry DOI: 10.7270/Q20005Z7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526605
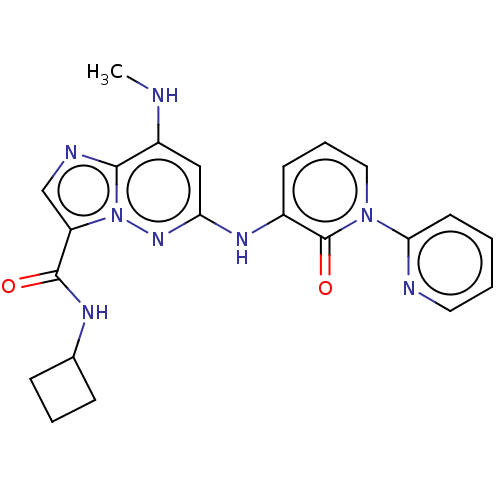
(CHEMBL4438296)Show SMILES CNc1cc(Nc2cccn(-c3ccccn3)c2=O)nn2c(cnc12)C(=O)NC1CCC1 Show InChI InChI=1S/C22H22N8O2/c1-23-16-12-18(27-15-8-5-11-29(22(15)32)19-9-2-3-10-24-19)28-30-17(13-25-20(16)30)21(31)26-14-6-4-7-14/h2-3,5,8-14,23H,4,6-7H2,1H3,(H,26,31)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526612

(CHEMBL4543066)Show SMILES CNc1cc(Nc2cccn(-c3ccccn3)c2=O)nn2c(cnc12)C(=O)NCC(C)(C)CO Show InChI InChI=1S/C23H26N8O3/c1-23(2,14-32)13-27-21(33)17-12-26-20-16(24-3)11-18(29-31(17)20)28-15-7-6-10-30(22(15)34)19-8-4-5-9-25-19/h4-12,24,32H,13-14H2,1-3H3,(H,27,33)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526611
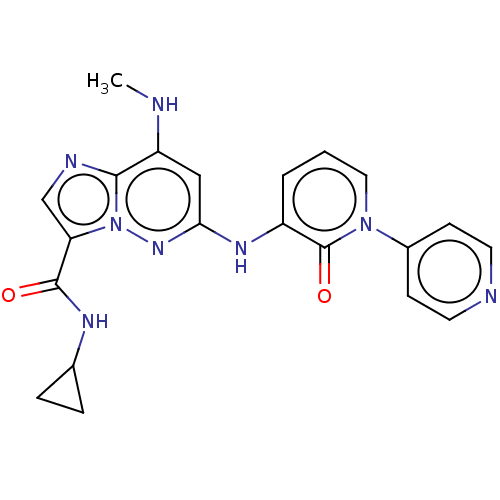
(CHEMBL4585272)Show SMILES CNc1cc(Nc2cccn(-c3ccncc3)c2=O)nn2c(cnc12)C(=O)NC1CC1 Show InChI InChI=1S/C21H20N8O2/c1-22-16-11-18(27-29-17(12-24-19(16)29)20(30)25-13-4-5-13)26-15-3-2-10-28(21(15)31)14-6-8-23-9-7-14/h2-3,6-13,22H,4-5H2,1H3,(H,25,30)(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50377898

(CHEMBL256109)Show SMILES COc1cccc(c1Cl)-c1c(C)n(Cc2c(F)cccc2C(F)(F)F)c(=O)n(C[C@H](C)NC2CCCC2)c1=O |wD:29.31,(31.26,-23.84,;29.93,-24.61,;29.93,-26.15,;31.27,-26.92,;31.27,-28.47,;29.93,-29.23,;28.61,-28.46,;28.6,-26.92,;27.27,-26.16,;27.27,-29.22,;27.27,-30.77,;28.6,-31.54,;25.93,-31.53,;25.93,-33.07,;25.11,-34.38,;23.57,-34.34,;22.84,-32.98,;22.77,-35.65,;23.51,-37,;25.05,-37.04,;25.85,-35.73,;27.39,-35.76,;28.93,-35.75,;27.38,-37.3,;27.41,-34.22,;24.61,-30.76,;23.27,-31.53,;24.61,-29.22,;23.28,-28.45,;21.95,-29.22,;21.95,-30.76,;20.61,-28.45,;20.61,-26.91,;21.85,-26,;21.37,-24.54,;19.83,-24.54,;19.36,-26.01,;25.94,-28.45,;25.94,-26.91,)| Show InChI InChI=1S/C28H30ClF4N3O3/c1-16(34-18-8-4-5-9-18)14-36-26(37)24(19-10-6-13-23(39-3)25(19)29)17(2)35(27(36)38)15-20-21(28(31,32)33)11-7-12-22(20)30/h6-7,10-13,16,18,34H,4-5,8-9,14-15H2,1-3H3/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GnRHR |
Bioorg Med Chem Lett 18: 3344-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.029
BindingDB Entry DOI: 10.7270/Q2CJ8FBF |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50377898

(CHEMBL256109)Show SMILES COc1cccc(c1Cl)-c1c(C)n(Cc2c(F)cccc2C(F)(F)F)c(=O)n(C[C@H](C)NC2CCCC2)c1=O |wD:29.31,(31.26,-23.84,;29.93,-24.61,;29.93,-26.15,;31.27,-26.92,;31.27,-28.47,;29.93,-29.23,;28.61,-28.46,;28.6,-26.92,;27.27,-26.16,;27.27,-29.22,;27.27,-30.77,;28.6,-31.54,;25.93,-31.53,;25.93,-33.07,;25.11,-34.38,;23.57,-34.34,;22.84,-32.98,;22.77,-35.65,;23.51,-37,;25.05,-37.04,;25.85,-35.73,;27.39,-35.76,;28.93,-35.75,;27.38,-37.3,;27.41,-34.22,;24.61,-30.76,;23.27,-31.53,;24.61,-29.22,;23.28,-28.45,;21.95,-29.22,;21.95,-30.76,;20.61,-28.45,;20.61,-26.91,;21.85,-26,;21.37,-24.54,;19.83,-24.54,;19.36,-26.01,;25.94,-28.45,;25.94,-26.91,)| Show InChI InChI=1S/C28H30ClF4N3O3/c1-16(34-18-8-4-5-9-18)14-36-26(37)24(19-10-6-13-23(39-3)25(19)29)17(2)35(27(36)38)15-20-21(28(31,32)33)11-7-12-22(20)30/h6-7,10-13,16,18,34H,4-5,8-9,14-15H2,1-3H3/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GnRHR |
Bioorg Med Chem Lett 18: 3344-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.029
BindingDB Entry DOI: 10.7270/Q2CJ8FBF |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Melatonin receptor type 1A stably expressed in NIH3T3 cells using 2-[125I]-iodomelatonin |
Bioorg Med Chem Lett 15: 1345-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.015
BindingDB Entry DOI: 10.7270/Q2KS6R1N |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526608

(CHEMBL4547009)Show SMILES CNc1cc(Nc2cccn(C3CC3)c2=O)nn2c(cnc12)C(=O)NC1CC1 Show InChI InChI=1S/C19H21N7O2/c1-20-14-9-16(23-13-3-2-8-25(19(13)28)12-6-7-12)24-26-15(10-21-17(14)26)18(27)22-11-4-5-11/h2-3,8-12,20H,4-7H2,1H3,(H,22,27)(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526623

(CHEMBL4466139 | US11174264, Compound I-4)Show SMILES CNc1cc(Nc2cccn(-c3ccccn3)c2=O)nn2c(cnc12)C(=O)NC1CC1 Show InChI InChI=1S/C21H20N8O2/c1-22-15-11-17(27-29-16(12-24-19(15)29)20(30)25-13-7-8-13)26-14-5-4-10-28(21(14)31)18-6-2-3-9-23-18/h2-6,9-13,22H,7-8H2,1H3,(H,25,30)(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50377895
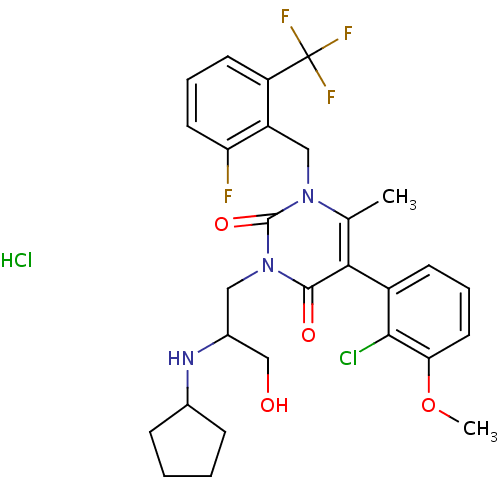
(CHEMBL556355)Show SMILES COc1cccc(c1Cl)-c1c(C)n(Cc2c(F)cccc2C(F)(F)F)c(=O)n(CC(CO)NC2CCCC2)c1=O Show InChI InChI=1S/C28H30ClF4N3O4.ClH/c1-16-24(19-9-5-12-23(40-2)25(19)29)26(38)36(13-18(15-37)34-17-7-3-4-8-17)27(39)35(16)14-20-21(28(31,32)33)10-6-11-22(20)30;/h5-6,9-12,17-18,34,37H,3-4,7-8,13-15H2,1-2H3;1H/t18-;/m1./s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GnRHR |
Bioorg Med Chem Lett 18: 3344-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.029
BindingDB Entry DOI: 10.7270/Q2CJ8FBF |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50526618
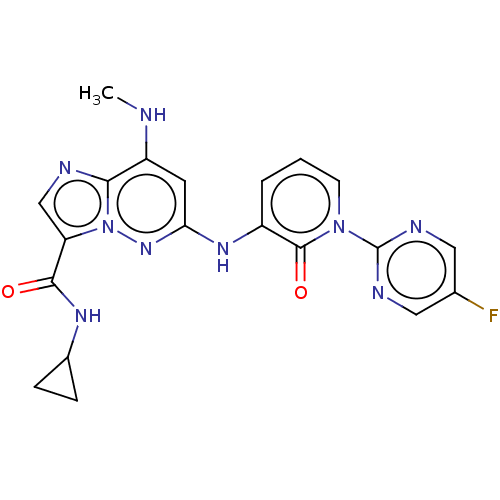
(CHEMBL4437714)Show SMILES CNc1cc(Nc2cccn(-c3ncc(F)cn3)c2=O)nn2c(cnc12)C(=O)NC1CC1 Show InChI InChI=1S/C20H18FN9O2/c1-22-14-7-16(28-30-15(10-23-17(14)30)18(31)26-12-4-5-12)27-13-3-2-6-29(19(13)32)20-24-8-11(21)9-25-20/h2-3,6-10,12,22H,4-5H2,1H3,(H,26,31)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
ACS Med Chem Lett 10: 383-388 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00035
BindingDB Entry DOI: 10.7270/Q2TM7FKX |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50098391

((S)-4-(2-(azetidin-2-yl)ethoxy)-7-chloro-2-oxo-N-(...)Show SMILES Cc1cc(cc(C)c1C)-c1c(OCC[C@@H]2CCN2)c2cc(C(=O)Nc3ccncn3)c(Cl)cc2[nH]c1=O |r| Show InChI InChI=1S/C28H28ClN5O3/c1-15-10-18(11-16(2)17(15)3)25-26(37-9-6-19-4-8-31-19)21-12-20(22(29)13-23(21)33-28(25)36)27(35)34-24-5-7-30-14-32-24/h5,7,10-14,19,31H,4,6,8-9H2,1-3H3,(H,33,36)(H,30,32,34,35)/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards human gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 13: 3617-22 (2003)
BindingDB Entry DOI: 10.7270/Q24X576N |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122652
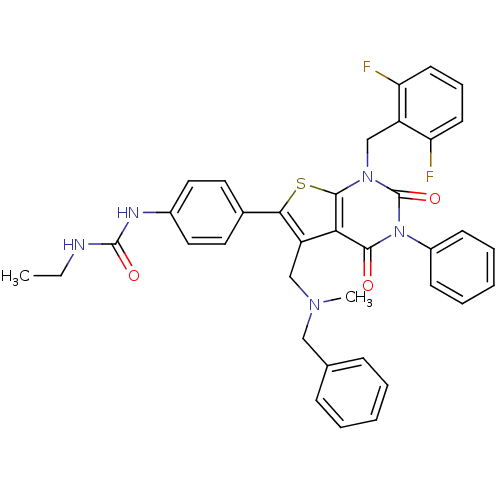
(1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1sc2n(Cc3c(F)cccc3F)c(=O)n(-c3ccccc3)c(=O)c2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C37H33F2N5O3S/c1-3-40-36(46)41-26-19-17-25(18-20-26)33-29(22-42(2)21-24-11-6-4-7-12-24)32-34(45)44(27-13-8-5-9-14-27)37(47)43(35(32)48-33)23-28-30(38)15-10-16-31(28)39/h4-20H,3,21-23H2,1-2H3,(H2,40,41,46) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards human gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 13: 3617-22 (2003)
BindingDB Entry DOI: 10.7270/Q24X576N |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50286868
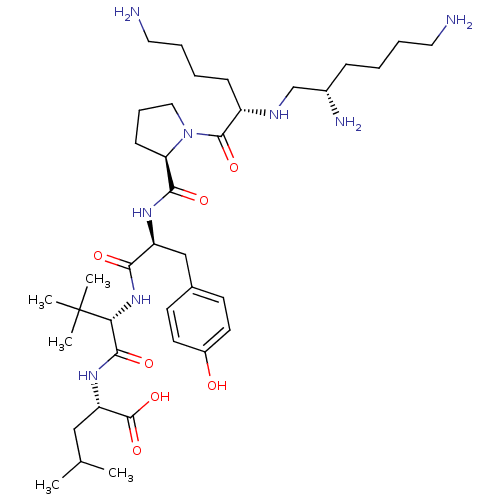
((1S,2S,4S)-2-{2-[(S)-2-({1-[6-Amino-2-(2,6-diamino...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](CCCCN)NC[C@@H](N)CCCCN)C(C)(C)C)C(O)=O Show InChI InChI=1S/C38H66N8O7/c1-24(2)21-30(37(52)53)44-35(50)32(38(3,4)5)45-33(48)29(22-25-14-16-27(47)17-15-25)43-34(49)31-13-10-20-46(31)36(51)28(12-7-9-19-40)42-23-26(41)11-6-8-18-39/h14-17,24,26,28-32,42,47H,6-13,18-23,39-41H2,1-5H3,(H,43,49)(H,44,50)(H,45,48)(H,52,53)/t26-,28-,29-,30-,31+,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Neurotensin Receptor |
Bioorg Med Chem Lett 5: 997-1002 (1995)
Article DOI: 10.1016/0960-894X(95)00155-M
BindingDB Entry DOI: 10.7270/Q2GQ6Z76 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50260806

(CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...)Show SMILES CC(C)C(=O)NC1CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccncc1Cl Show InChI InChI=1S/C25H25ClF3N5O/c1-15(2)24(35)32-18-8-11-34(12-9-18)21-14-31-22(16-3-5-17(6-4-16)25(27,28)29)23(33-21)19-7-10-30-13-20(19)26/h3-7,10,13-15,18H,8-9,11-12H2,1-2H3,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50202412
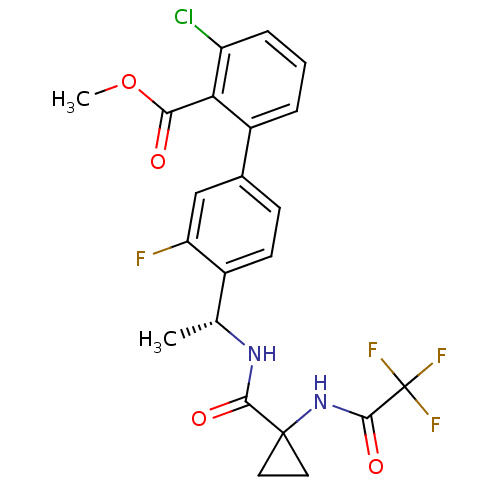
(3-Chloro-3'-fluoro-4'-((R)-1-{[1-(2,2,2-trifluoro-...)Show SMILES COC(=O)c1c(Cl)cccc1-c1ccc([C@@H](C)NC(=O)C2(CC2)NC(=O)C(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C22H19ClF4N2O4/c1-11(28-19(31)21(8-9-21)29-20(32)22(25,26)27)13-7-6-12(10-16(13)24)14-4-3-5-15(23)17(14)18(30)33-2/h3-7,10-11H,8-9H2,1-2H3,(H,28,31)(H,29,32)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human bradykinin B1 receptor |
Bioorg Med Chem Lett 18: 5027-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.014
BindingDB Entry DOI: 10.7270/Q2NG4QDX |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50281503
(4-{4-[4-(4-Methoxy-phenyl)-3,6-dihydro-2H-pyridin-...)Show InChI InChI=1S/C21H26N2O/c1-24-21-7-5-19(6-8-21)20-11-16-23(17-12-20)15-3-2-4-18-9-13-22-14-10-18/h5-11,13-14H,2-4,12,15-17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against sigma receptor in guinea pig brain preparation was estimated using [3H]-3-PPP as radioligand |
Bioorg Med Chem Lett 3: 277-280 (1993)
Article DOI: 10.1016/S0960-894X(01)80892-1
BindingDB Entry DOI: 10.7270/Q2HH6K0R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data