

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM23334 (3-(pyridin-3-yl)propyl (2S)-1-(3,3-dimethyl-2-oxop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 | Bioorg Med Chem Lett 12: 1429-33 (2002) BindingDB Entry DOI: 10.7270/Q23J3C9S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50095105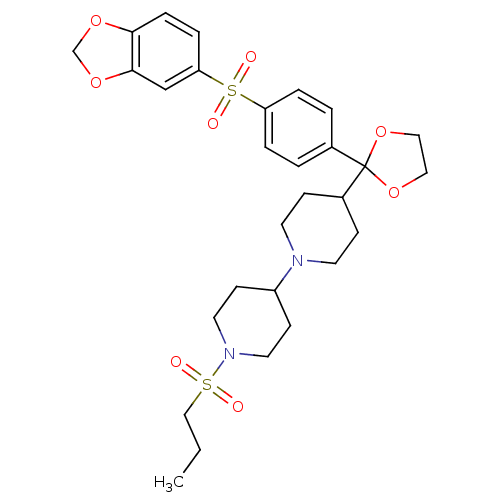 (4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Tested for binding affinity against human Coagulation factor Xa (trypsin-like serine protease) | Bioorg Med Chem Lett 12: 1651-5 (2002) BindingDB Entry DOI: 10.7270/Q2VT1RFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Coagulation factor Xa (serine protease) was determined | Bioorg Med Chem Lett 12: 1511-5 (2002) BindingDB Entry DOI: 10.7270/Q2P84B6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565828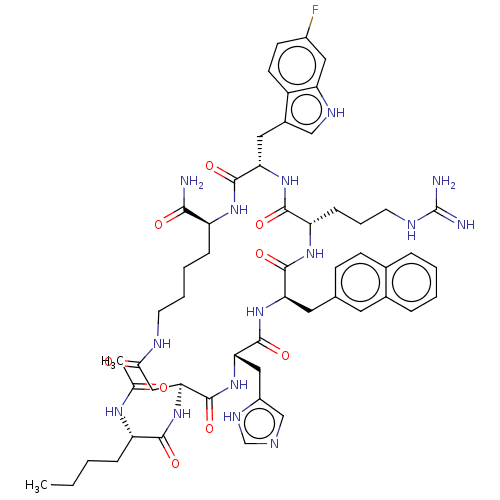 (CHEMBL4798971) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565828 (CHEMBL4798971) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565819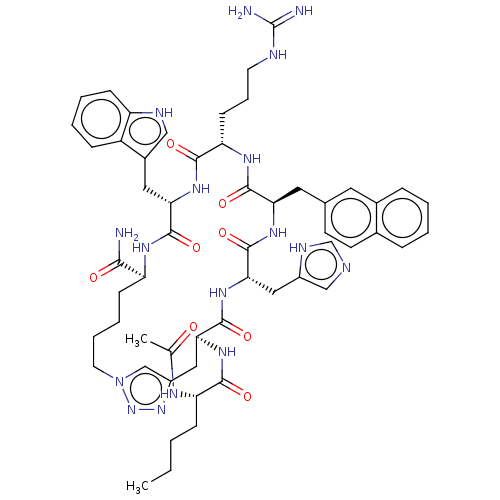 (CHEMBL4794168) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565819 (CHEMBL4794168) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565826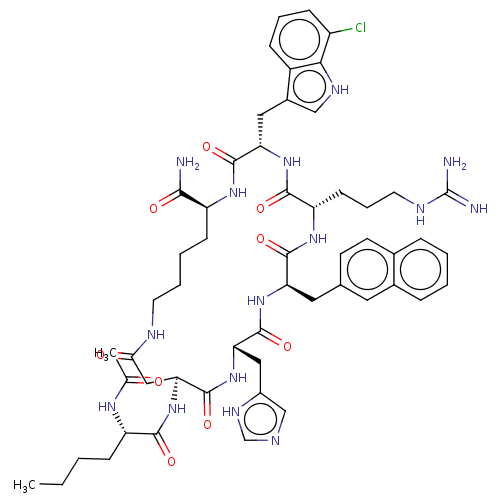 (CHEMBL4794121) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565826 (CHEMBL4794121) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565825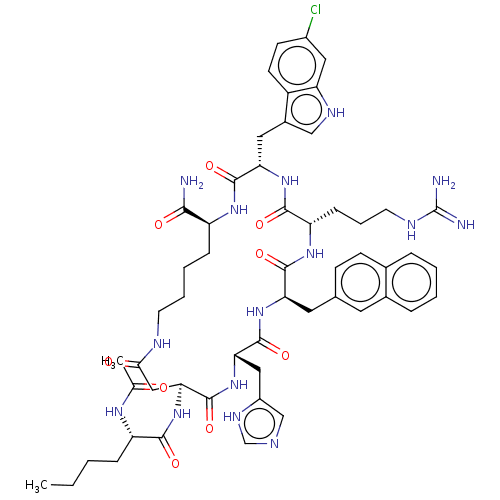 (CHEMBL4777048) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565825 (CHEMBL4777048) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50260646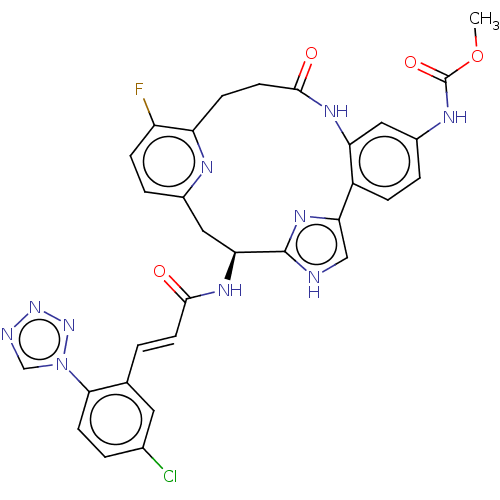 (CHEMBL4096251) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company, Research and Development, 350 Carter Road, Hopewell, NJ 08540 United States. Curated by ChEMBL | Assay Description Inhibition of human F11a using peptide substrate by spectrophotometry | Bioorg Med Chem Lett 27: 4056-4060 (2017) Article DOI: 10.1016/j.bmcl.2017.07.048 BindingDB Entry DOI: 10.7270/Q2TB19B3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565830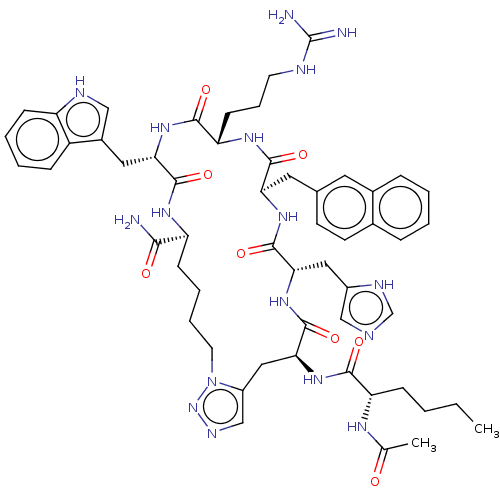 (CHEMBL4793821) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565830 (CHEMBL4793821) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565829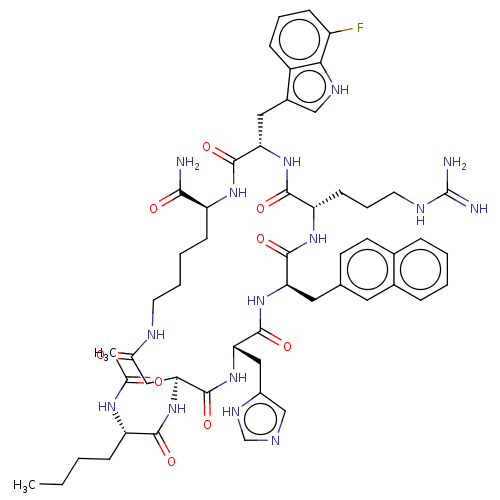 (CHEMBL4788071) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565829 (CHEMBL4788071) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50565825 (CHEMBL4777048) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0219 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC3R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50565825 (CHEMBL4777048) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC3R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565827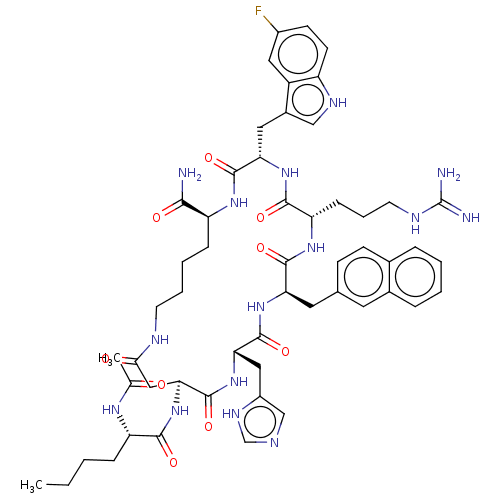 (CHEMBL4794990) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0257 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565827 (CHEMBL4794990) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565831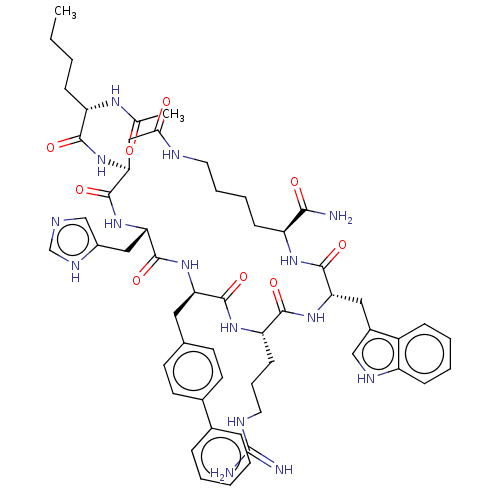 (CHEMBL4791788) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565831 (CHEMBL4791788) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50080576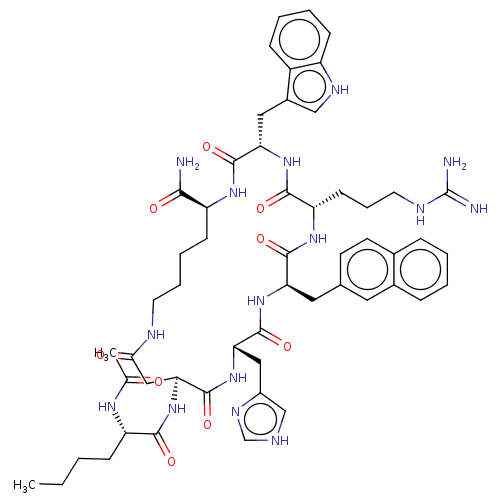 (CHEMBL3422426) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50080576 (CHEMBL3422426) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230326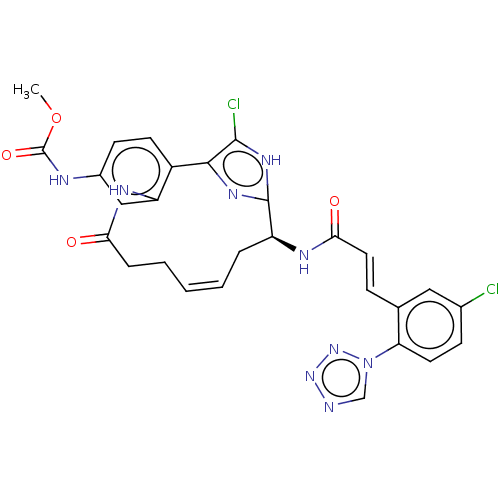 (CHEMBL4060950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230326 (CHEMBL4060950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 3833-3839 (2017) Article DOI: 10.1016/j.bmcl.2017.06.058 BindingDB Entry DOI: 10.7270/Q2GT5QPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50565826 (CHEMBL4794121) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0309 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC3R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50565826 (CHEMBL4794121) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC3R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50565828 (CHEMBL4798971) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC3R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50565828 (CHEMBL4798971) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0324 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC3R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565824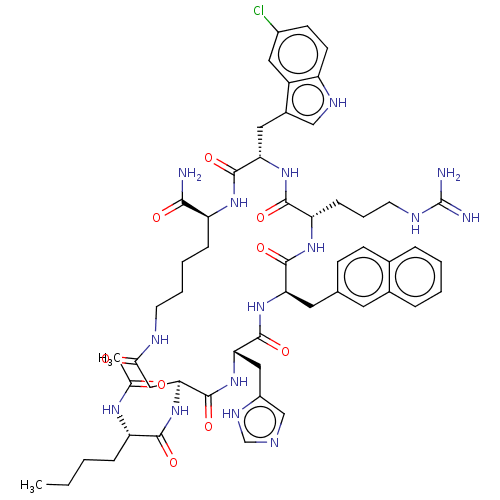 (CHEMBL4800268) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565824 (CHEMBL4800268) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565818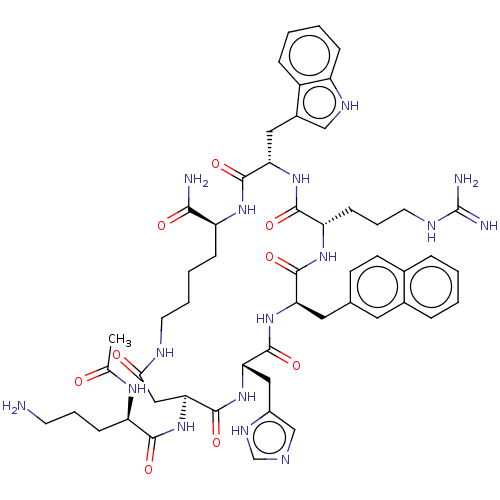 (CHEMBL4776915) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50565818 (CHEMBL4776915) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC4R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50193861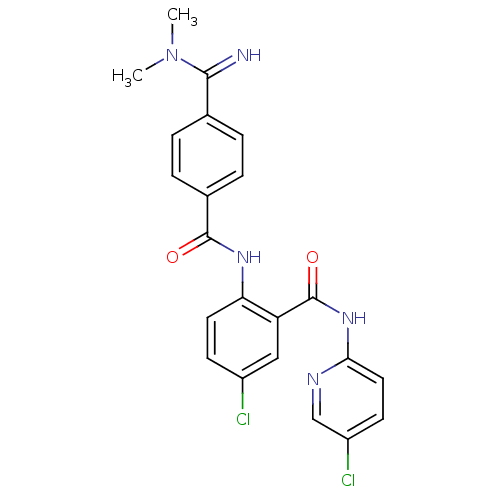 (5-chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of Factor 10a (unknown origin) | Bioorg Med Chem Lett 19: 2179-85 (2009) Article DOI: 10.1016/j.bmcl.2009.02.111 BindingDB Entry DOI: 10.7270/Q22Z15F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269207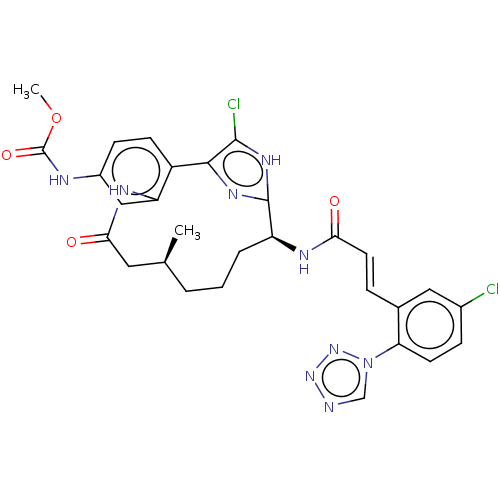 (CHEMBL4097522) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 3833-3839 (2017) Article DOI: 10.1016/j.bmcl.2017.06.058 BindingDB Entry DOI: 10.7270/Q2GT5QPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50249120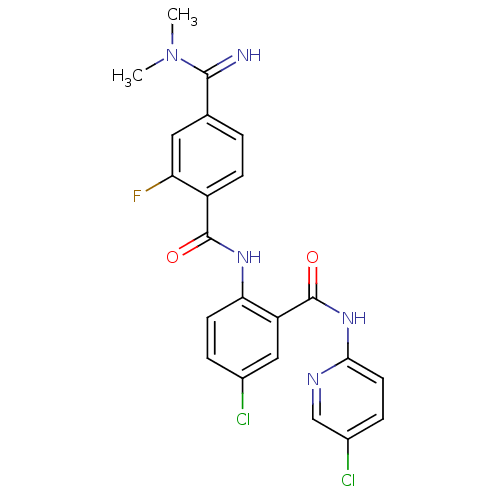 (CHEMBL472967 | N-(4-chloro-2-(5-chloropyridin-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of Factor 10a (unknown origin) | Bioorg Med Chem Lett 19: 2179-85 (2009) Article DOI: 10.1016/j.bmcl.2009.02.111 BindingDB Entry DOI: 10.7270/Q22Z15F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269199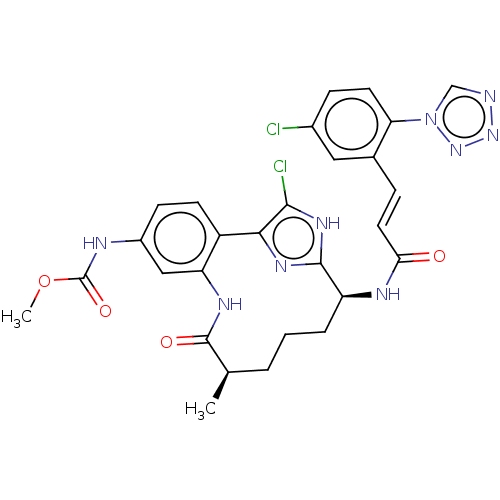 (CHEMBL4083769) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 3833-3839 (2017) Article DOI: 10.1016/j.bmcl.2017.06.058 BindingDB Entry DOI: 10.7270/Q2GT5QPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582801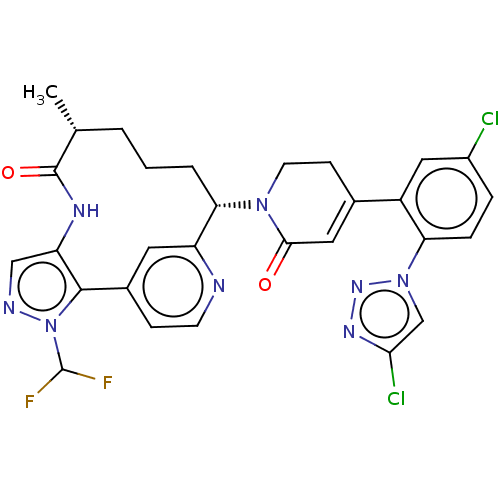 (CHEMBL5076656) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250493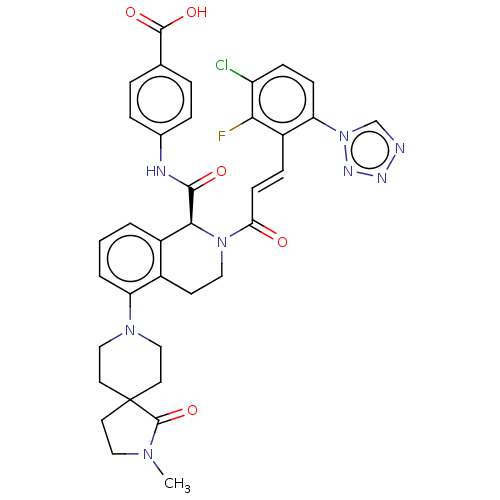 (CHEMBL4068445) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50502477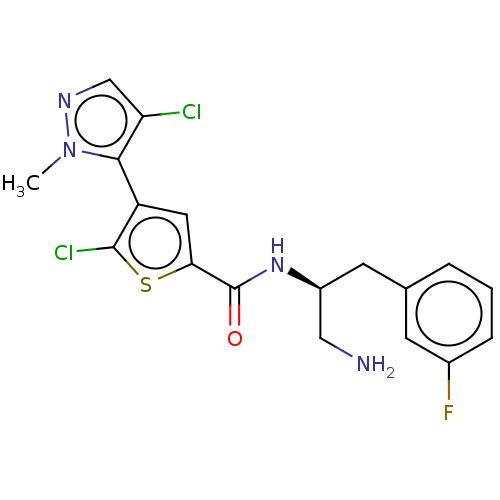 (ASB-183 | ASB183 | Afuresertib | GSK-2110183C | GS...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Institute of Innovative Medicine Curated by ChEMBL | Assay Description Inhibition of Akt1 (unknown origin) | Eur J Med Chem 180: 72-85 (2019) Article DOI: 10.1016/j.ejmech.2019.07.017 BindingDB Entry DOI: 10.7270/Q2Q243H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19023 (1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of Factor 10a (unknown origin) | Bioorg Med Chem Lett 19: 2179-85 (2009) Article DOI: 10.1016/j.bmcl.2009.02.111 BindingDB Entry DOI: 10.7270/Q22Z15F5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250492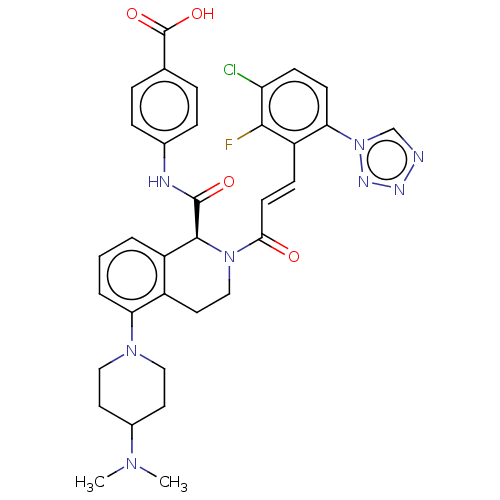 (CHEMBL4097304) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50249423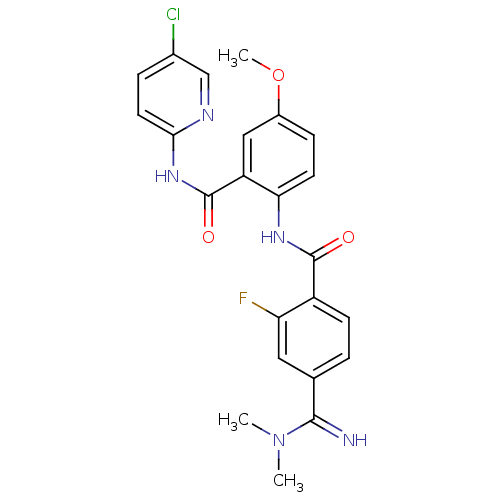 (CHEMBL515919 | N-(2-(5-chloropyridin-2-ylcarbamoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of Factor 10a (unknown origin) | Bioorg Med Chem Lett 19: 2179-85 (2009) Article DOI: 10.1016/j.bmcl.2009.02.111 BindingDB Entry DOI: 10.7270/Q22Z15F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM247411 (US10336754, Example 353 | US11053247, Example 353 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50249298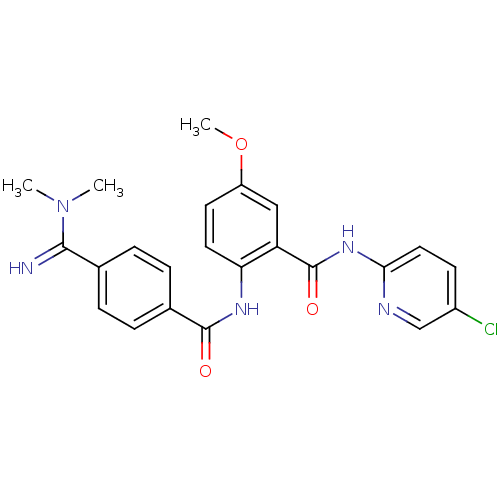 (BEVYXXA | CHEMBL512351 | N-(5-chloropyridin-2-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of Factor 10a (unknown origin) | Bioorg Med Chem Lett 19: 2179-85 (2009) Article DOI: 10.1016/j.bmcl.2009.02.111 BindingDB Entry DOI: 10.7270/Q22Z15F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582799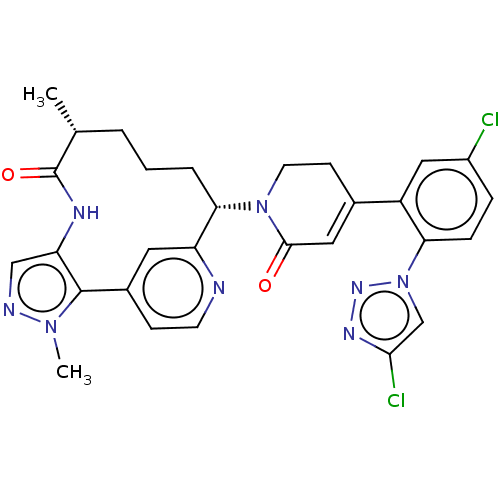 (CHEMBL5094166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50080576 (CHEMBL3422426) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC3R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50080576 (CHEMBL3422426) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[NIe,DPhe7]-alpha-MSH from human MC3R expressed in HEK293 cell membranes incubated for 120 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01620 BindingDB Entry DOI: 10.7270/Q2C53QMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 19802 total ) | Next | Last >> |