 Found 126 hits with Last Name = 'wu' and Initial = 'yq'
Found 126 hits with Last Name = 'wu' and Initial = 'yq' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50116632
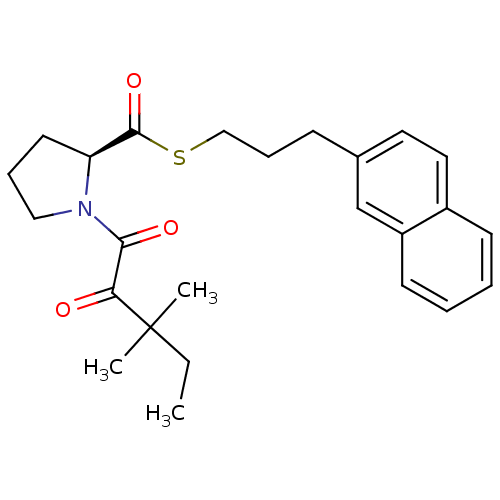
(1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)SCCCc1ccc2ccccc2c1 Show InChI InChI=1S/C25H31NO3S/c1-4-25(2,3)22(27)23(28)26-15-7-12-21(26)24(29)30-16-8-9-18-13-14-19-10-5-6-11-20(19)17-18/h5-6,10-11,13-14,17,21H,4,7-9,12,15-16H2,1-3H3/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against FK506 binding protein 12 |
J Med Chem 45: 3549-57 (2002)
BindingDB Entry DOI: 10.7270/Q2416WC5 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50116633

(1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)SCCC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C27H33NO3S/c1-4-27(2,3)24(29)25(30)28-18-11-16-23(28)26(31)32-19-17-22(20-12-7-5-8-13-20)21-14-9-6-10-15-21/h5-10,12-15,22-23H,4,11,16-19H2,1-3H3/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against FK506 binding protein 12 |
J Med Chem 45: 3549-57 (2002)
BindingDB Entry DOI: 10.7270/Q2416WC5 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM23334

(3-(pyridin-3-yl)propyl (2S)-1-(3,3-dimethyl-2-oxop...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)OCCCc1cccnc1 |r| Show InChI InChI=1S/C20H28N2O4/c1-4-20(2,3)17(23)18(24)22-12-6-10-16(22)19(25)26-13-7-9-15-8-5-11-21-14-15/h5,8,11,14,16H,4,6-7,9-10,12-13H2,1-3H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against FK506 binding protein 12 |
J Med Chem 45: 3549-57 (2002)
BindingDB Entry DOI: 10.7270/Q2416WC5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Bos taurus (bovine)) | BDBM23334

(3-(pyridin-3-yl)propyl (2S)-1-(3,3-dimethyl-2-oxop...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)OCCCc1cccnc1 |r| Show InChI InChI=1S/C20H28N2O4/c1-4-20(2,3)17(23)18(24)22-12-6-10-16(22)19(25)26-13-7-9-15-8-5-11-21-14-15/h5,8,11,14,16H,4,6-7,9-10,12-13H2,1-3H3/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 7.5 | -42.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 0 |
Guilford Pharmaceuticals, Inc.
| Assay Description
PPIase(Rotamase) activity of FKBP12 was assayed using the peptide N-succinyl Ala-Leu-Pro-Phe p-nitroanilide as substrate. It is based on the observat... |
J Med Chem 45: 3558-68 (2002)
Article DOI: 10.1021/jm0200456
BindingDB Entry DOI: 10.7270/Q2GH9G7G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50116636

(1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)SCCCc1cccnc1 Show InChI InChI=1S/C20H28N2O3S/c1-4-20(2,3)17(23)18(24)22-12-6-10-16(22)19(25)26-13-7-9-15-8-5-11-21-14-15/h5,8,11,14,16H,4,6-7,9-10,12-13H2,1-3H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against FK506 binding protein 12 |
J Med Chem 45: 3549-57 (2002)
BindingDB Entry DOI: 10.7270/Q2416WC5 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50116630

(1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)SCCCc1ccc(OC)cc1 Show InChI InChI=1S/C22H31NO4S/c1-5-22(2,3)19(24)20(25)23-14-6-9-18(23)21(26)28-15-7-8-16-10-12-17(27-4)13-11-16/h10-13,18H,5-9,14-15H2,1-4H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against FK506 binding protein 12 |
J Med Chem 45: 3549-57 (2002)
BindingDB Entry DOI: 10.7270/Q2416WC5 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50150950

(30-Ethyl-33-(1-hydroxy-2-methyl-hex-4-enyl)-6,9,18...)Show SMILES CC[C@@H]1NC(=O)[C@H](C(O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44-,45+,46-,47+,49-,50+,51+,52?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cyclophilin A rotamase |
Bioorg Med Chem Lett 14: 4549-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.028
BindingDB Entry DOI: 10.7270/Q2WH2QQQ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50116631
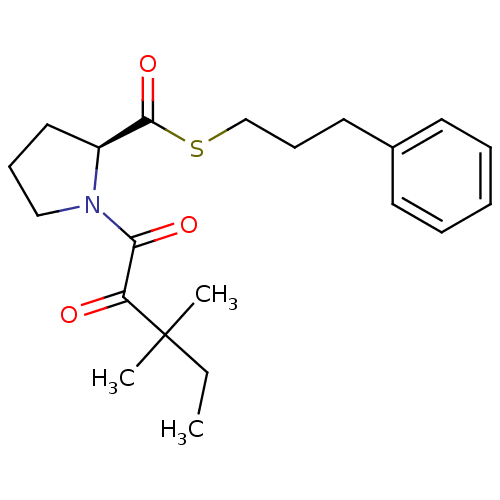
(1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)SCCCc1ccccc1 Show InChI InChI=1S/C21H29NO3S/c1-4-21(2,3)18(23)19(24)22-14-8-13-17(22)20(25)26-15-9-12-16-10-6-5-7-11-16/h5-7,10-11,17H,4,8-9,12-15H2,1-3H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against FK506 binding protein 12 |
J Med Chem 45: 3549-57 (2002)
BindingDB Entry DOI: 10.7270/Q2416WC5 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113058

(1-(Adamantan-1-ylcarbamoyl)-piperidine-2-carboxyli...)Show SMILES COc1ccc(CCCOC(=O)C2CCCCN2C(=O)NC23CC4CC(CC(C4)C2)C3)c(OC)c1 |TLB:24:25:29:23.22.28,THB:24:23:29:25.30.26,26:25:22:27.29.28,26:27:22:25.30.24| Show InChI InChI=1S/C28H40N2O5/c1-33-23-9-8-22(25(15-23)34-2)6-5-11-35-26(31)24-7-3-4-10-30(24)27(32)29-28-16-19-12-20(17-28)14-21(13-19)18-28/h8-9,15,19-21,24H,3-7,10-14,16-18H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50116635

(1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)SCCc1ccccc1 Show InChI InChI=1S/C20H27NO3S/c1-4-20(2,3)17(22)18(23)21-13-8-11-16(21)19(24)25-14-12-15-9-6-5-7-10-15/h5-7,9-10,16H,4,8,11-14H2,1-3H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against FK506 binding protein 12 |
J Med Chem 45: 3549-57 (2002)
BindingDB Entry DOI: 10.7270/Q2416WC5 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50150948

(12-Ethyl-3-(1-hydroxy-ethyl)-15-(1-hydroxy-2-methy...)Show SMILES CC[C@@H]1NC(=O)[C@H](C(O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@](C)(NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)O Show InChI InChI=1S/C62H111N11O13/c1-25-27-28-39(13)51(76)50-55(80)65-43(26-2)57(82)67(18)33-48(75)68(19)45(30-35(5)6)54(79)66-62(17,42(16)74)61(86)71(22)44(29-34(3)4)53(78)63-40(14)52(77)64-41(15)56(81)69(20)46(31-36(7)8)58(83)70(21)47(32-37(9)10)59(84)72(23)49(38(11)12)60(85)73(50)24/h25,27,34-47,49-51,74,76H,26,28-33H2,1-24H3,(H,63,78)(H,64,77)(H,65,80)(H,66,79)/b27-25+/t39-,40+,41-,42?,43+,44+,45-,46-,47+,49+,50+,51?,62-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cyclophilin A rotamase |
Bioorg Med Chem Lett 14: 4549-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.028
BindingDB Entry DOI: 10.7270/Q2WH2QQQ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50022815

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cyclophilin A rotamase |
Bioorg Med Chem Lett 14: 4549-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.028
BindingDB Entry DOI: 10.7270/Q2WH2QQQ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113095
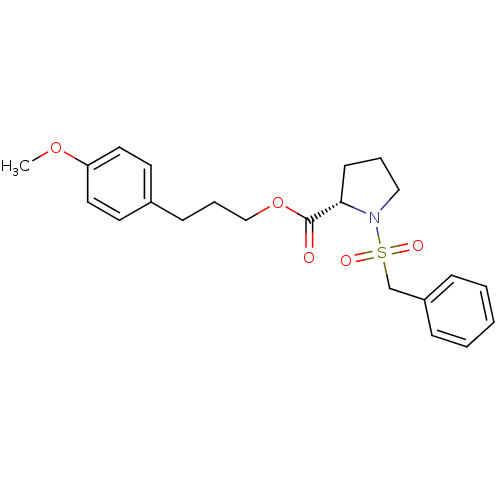
((S)-1-Phenylmethanesulfonyl-pyrrolidine-2-carboxyl...)Show SMILES COc1ccc(CCCOC(=O)[C@@H]2CCCN2S(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C22H27NO5S/c1-27-20-13-11-18(12-14-20)9-6-16-28-22(24)21-10-5-15-23(21)29(25,26)17-19-7-3-2-4-8-19/h2-4,7-8,11-14,21H,5-6,9-10,15-17H2,1H3/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50150949
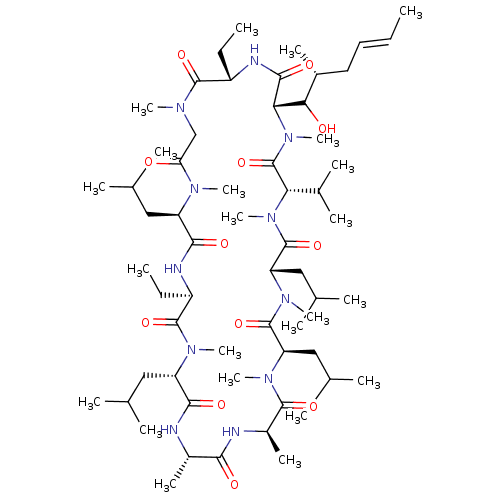
(21,30-Diethyl-33-(1-hydroxy-2-methyl-hex-4-enyl)-6...)Show SMILES CC[C@@H]1NC(=O)[C@H](C(O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](CC)NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C1=O Show InChI InChI=1S/C61H109N11O12/c1-24-27-28-39(14)51(74)50-55(78)65-42(25-2)57(80)66(17)33-48(73)67(18)44(29-34(4)5)54(77)64-43(26-3)58(81)68(19)45(30-35(6)7)53(76)62-40(15)52(75)63-41(16)56(79)69(20)46(31-36(8)9)59(82)70(21)47(32-37(10)11)60(83)71(22)49(38(12)13)61(84)72(50)23/h24,27,34-47,49-51,74H,25-26,28-33H2,1-23H3,(H,62,76)(H,63,75)(H,64,77)(H,65,78)/b27-24+/t39-,40+,41-,42+,43-,44-,45+,46-,47+,49+,50+,51?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cyclophilin A rotamase |
Bioorg Med Chem Lett 14: 4549-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.028
BindingDB Entry DOI: 10.7270/Q2WH2QQQ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Bos taurus (bovine)) | BDBM23319
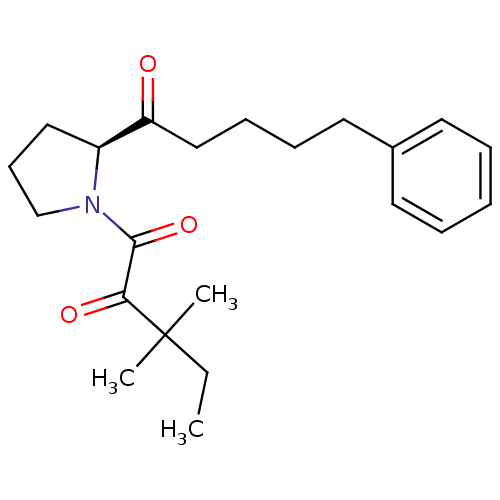
(3,3-dimethyl-1-[(2S)-2-(5-phenylpentanoyl)pyrrolid...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)CCCCc1ccccc1 |r| Show InChI InChI=1S/C22H31NO3/c1-4-22(2,3)20(25)21(26)23-16-10-14-18(23)19(24)15-9-8-13-17-11-6-5-7-12-17/h5-7,11-12,18H,4,8-10,13-16H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | -39.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 0 |
Guilford Pharmaceuticals, Inc.
| Assay Description
PPIase(Rotamase) activity of FKBP12 was assayed using the peptide N-succinyl Ala-Leu-Pro-Phe p-nitroanilide as substrate. It is based on the observat... |
J Med Chem 45: 3558-68 (2002)
Article DOI: 10.1021/jm0200456
BindingDB Entry DOI: 10.7270/Q2GH9G7G |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113063
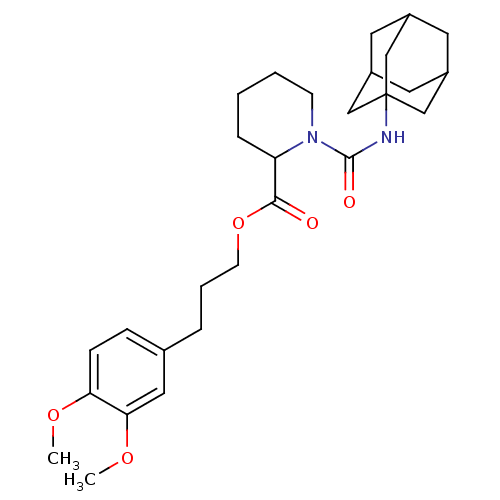
(1-(Adamantan-1-ylcarbamoyl)-piperidine-2-carboxyli...)Show SMILES COc1ccc(CCCOC(=O)C2CCCCN2C(=O)NC23CC4CC(CC(C4)C2)C3)cc1OC |TLB:24:25:29:23.22.28,THB:24:23:29:25.30.26,26:27:22:25.30.24,26:25:22:27.29.28| Show InChI InChI=1S/C28H40N2O5/c1-33-24-9-8-19(15-25(24)34-2)6-5-11-35-26(31)23-7-3-4-10-30(23)27(32)29-28-16-20-12-21(17-28)14-22(13-20)18-28/h8-9,15,20-23H,3-7,10-14,16-18H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113077
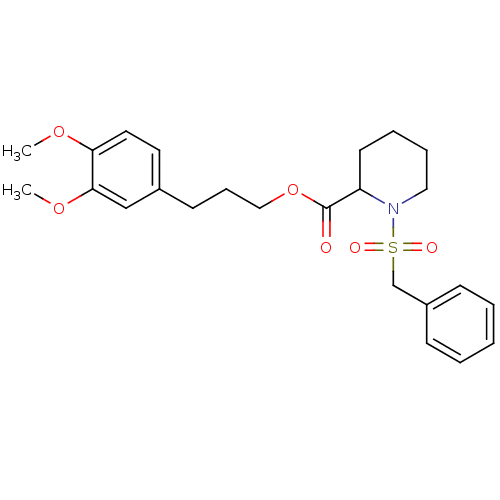
(1-Phenylmethanesulfonyl-piperidine-2-carboxylic ac...)Show SMILES COc1ccc(CCCOC(=O)C2CCCCN2S(=O)(=O)Cc2ccccc2)cc1OC Show InChI InChI=1S/C24H31NO6S/c1-29-22-14-13-19(17-23(22)30-2)11-8-16-31-24(26)21-12-6-7-15-25(21)32(27,28)18-20-9-4-3-5-10-20/h3-5,9-10,13-14,17,21H,6-8,11-12,15-16,18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50150947

(30-Ethyl-33-(1-hydroxy-2-methyl-hex-4-enyl)-6,9,18...)Show SMILES CC[C@@H]1NC(=O)[C@H](C(O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](CC(C)C)N(C)C(=O)C(=C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C Show InChI InChI=1S/C62H109N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-41,43-47,49-52,75H,17,26,28-33H2,1-16,18-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,43+,44-,45+,46-,47+,49+,50+,51+,52?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cyclophilin A rotamase |
Bioorg Med Chem Lett 14: 4549-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.028
BindingDB Entry DOI: 10.7270/Q2WH2QQQ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50116637

(1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-carb...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)SCCC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H35NO3S/c1-4-28(2,3)25(30)26(31)29-19-12-11-17-24(29)27(32)33-20-18-23(21-13-7-5-8-14-21)22-15-9-6-10-16-22/h5-10,13-16,23-24H,4,11-12,17-20H2,1-3H3/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against FK506 binding protein 12 |
J Med Chem 45: 3549-57 (2002)
BindingDB Entry DOI: 10.7270/Q2416WC5 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113070

((S)-1-Cyclohexylthiocarbamoyl-pyrrolidine-2-carbox...)Show SMILES COc1ccc(CCCOC(=O)[C@@H]2CCCN2C(=S)NC2CCCCC2)cc1OC Show InChI InChI=1S/C23H34N2O4S/c1-27-20-13-12-17(16-21(20)28-2)8-7-15-29-22(26)19-11-6-14-25(19)23(30)24-18-9-4-3-5-10-18/h12-13,16,18-19H,3-11,14-15H2,1-2H3,(H,24,30)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113056
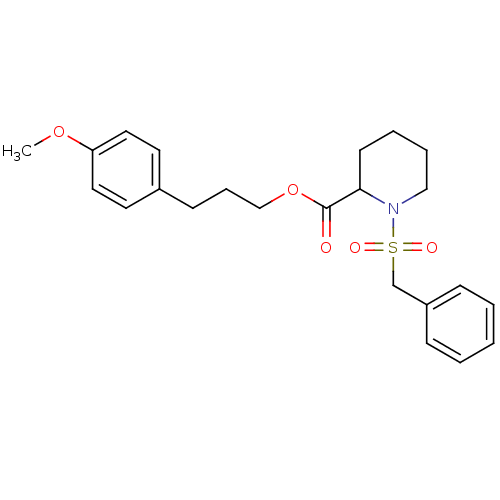
(1-Phenylmethanesulfonyl-piperidine-2-carboxylic ac...)Show SMILES COc1ccc(CCCOC(=O)C2CCCCN2S(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C23H29NO5S/c1-28-21-14-12-19(13-15-21)10-7-17-29-23(25)22-11-5-6-16-24(22)30(26,27)18-20-8-3-2-4-9-20/h2-4,8-9,12-15,22H,5-7,10-11,16-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50150952

(30-Ethyl-33-(1-hydroxy-2-methyl-hexyl)-6,9,18,24-t...)Show SMILES CCCC[C@@H](C)C(O)[C@@H]1N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C(=O)[C@H](CC)NC1=O)C(C)C Show InChI InChI=1S/C62H113N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h34-47,49-52,75H,25-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/t40-,41+,42-,43+,44-,45+,46-,47+,49+,50+,51+,52?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cyclophilin A rotamase |
Bioorg Med Chem Lett 14: 4549-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.028
BindingDB Entry DOI: 10.7270/Q2WH2QQQ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113071

(1-Phenylmethanesulfonyl-piperidine-2-carboxylic ac...)Show SMILES COc1cc(CCCOC(=O)C2CCCCN2S(=O)(=O)Cc2ccccc2)cc(OC)c1 Show InChI InChI=1S/C24H31NO6S/c1-29-21-15-20(16-22(17-21)30-2)11-8-14-31-24(26)23-12-6-7-13-25(23)32(27,28)18-19-9-4-3-5-10-19/h3-5,9-10,15-17,23H,6-8,11-14,18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Bos taurus (bovine)) | BDBM23322
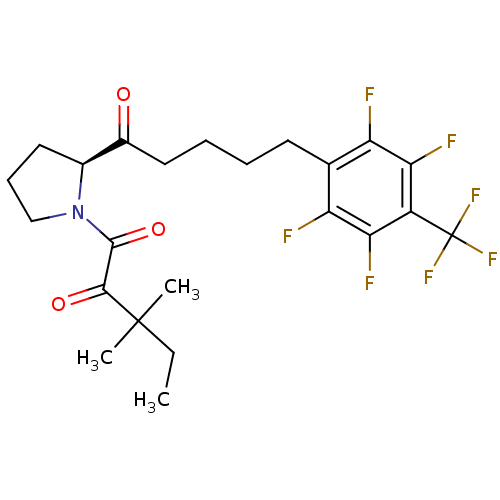
(3,3-dimethyl-1-[(2S)-2-{5-[2,3,5,6-tetrafluoro-4-(...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)CCCCc1c(F)c(F)c(c(F)c1F)C(F)(F)F |r| Show InChI InChI=1S/C23H26F7NO3/c1-4-22(2,3)20(33)21(34)31-11-7-9-13(31)14(32)10-6-5-8-12-16(24)18(26)15(23(28,29)30)19(27)17(12)25/h13H,4-11H2,1-3H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 130 | -36.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 0 |
Guilford Pharmaceuticals, Inc.
| Assay Description
PPIase(Rotamase) activity of FKBP12 was assayed using the peptide N-succinyl Ala-Leu-Pro-Phe p-nitroanilide as substrate. It is based on the observat... |
J Med Chem 45: 3558-68 (2002)
Article DOI: 10.1021/jm0200456
BindingDB Entry DOI: 10.7270/Q2GH9G7G |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113092
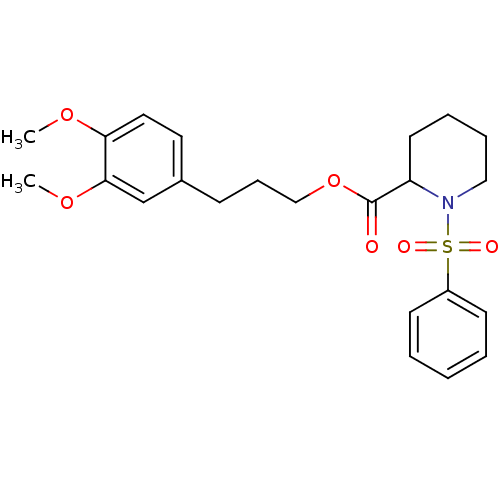
(1-Benzenesulfonyl-piperidine-2-carboxylic acid 3-(...)Show SMILES COc1ccc(CCCOC(=O)C2CCCCN2S(=O)(=O)c2ccccc2)cc1OC Show InChI InChI=1S/C23H29NO6S/c1-28-21-14-13-18(17-22(21)29-2)9-8-16-30-23(25)20-12-6-7-15-24(20)31(26,27)19-10-4-3-5-11-19/h3-5,10-11,13-14,17,20H,6-9,12,15-16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Bos taurus (bovine)) | BDBM23323
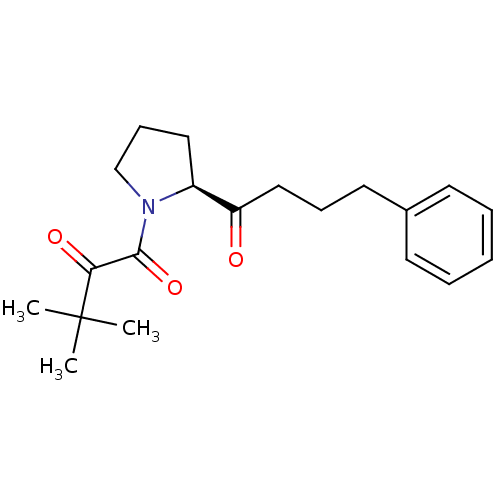
(3,3-dimethyl-1-[(2S)-2-(4-phenylbutanoyl)pyrrolidi...)Show SMILES CC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)CCCc1ccccc1 |r| Show InChI InChI=1S/C20H27NO3/c1-20(2,3)18(23)19(24)21-14-8-12-16(21)17(22)13-7-11-15-9-5-4-6-10-15/h4-6,9-10,16H,7-8,11-14H2,1-3H3/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | -35.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 0 |
Guilford Pharmaceuticals, Inc.
| Assay Description
PPIase(Rotamase) activity of FKBP12 was assayed using the peptide N-succinyl Ala-Leu-Pro-Phe p-nitroanilide as substrate. It is based on the observat... |
J Med Chem 45: 3558-68 (2002)
Article DOI: 10.1021/jm0200456
BindingDB Entry DOI: 10.7270/Q2GH9G7G |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Bos taurus (bovine)) | BDBM23325
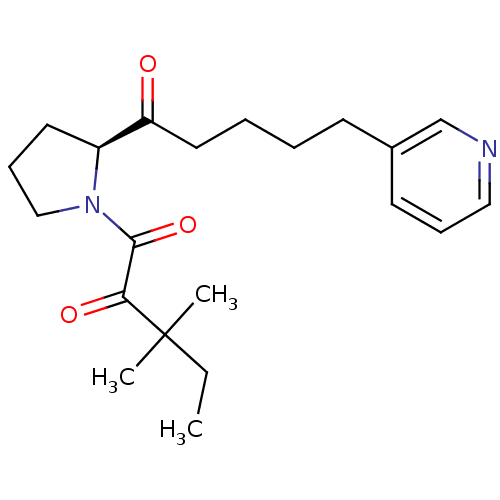
(3,3-dimethyl-1-[(2S)-2-[5-(pyridin-3-yl)pentanoyl]...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)CCCCc1cccnc1 |r| Show InChI InChI=1S/C21H30N2O3/c1-4-21(2,3)19(25)20(26)23-14-8-11-17(23)18(24)12-6-5-9-16-10-7-13-22-15-16/h7,10,13,15,17H,4-6,8-9,11-12,14H2,1-3H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 210 | -34.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 0 |
Guilford Pharmaceuticals, Inc.
| Assay Description
PPIase(Rotamase) activity of FKBP12 was assayed using the peptide N-succinyl Ala-Leu-Pro-Phe p-nitroanilide as substrate. It is based on the observat... |
J Med Chem 45: 3558-68 (2002)
Article DOI: 10.1021/jm0200456
BindingDB Entry DOI: 10.7270/Q2GH9G7G |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113091

(1-(Toluene-4-sulfonyl)-piperidine-2-carboxylic aci...)Show SMILES COc1ccc(CCCOC(=O)C2CCCCN2S(=O)(=O)c2ccc(C)cc2)cc1 Show InChI InChI=1S/C23H29NO5S/c1-18-8-14-21(15-9-18)30(26,27)24-16-4-3-7-22(24)23(25)29-17-5-6-19-10-12-20(28-2)13-11-19/h8-15,22H,3-7,16-17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Bos taurus (bovine)) | BDBM23324
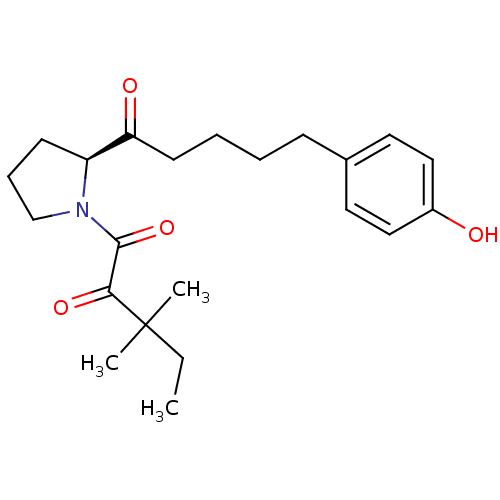
(1-[(2S)-2-[5-(4-hydroxyphenyl)pentanoyl]pyrrolidin...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)CCCCc1ccc(O)cc1 |r| Show InChI InChI=1S/C22H31NO4/c1-4-22(2,3)20(26)21(27)23-15-7-9-18(23)19(25)10-6-5-8-16-11-13-17(24)14-12-16/h11-14,18,24H,4-10,15H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | -34.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 0 |
Guilford Pharmaceuticals, Inc.
| Assay Description
PPIase(Rotamase) activity of FKBP12 was assayed using the peptide N-succinyl Ala-Leu-Pro-Phe p-nitroanilide as substrate. It is based on the observat... |
J Med Chem 45: 3558-68 (2002)
Article DOI: 10.1021/jm0200456
BindingDB Entry DOI: 10.7270/Q2GH9G7G |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113072
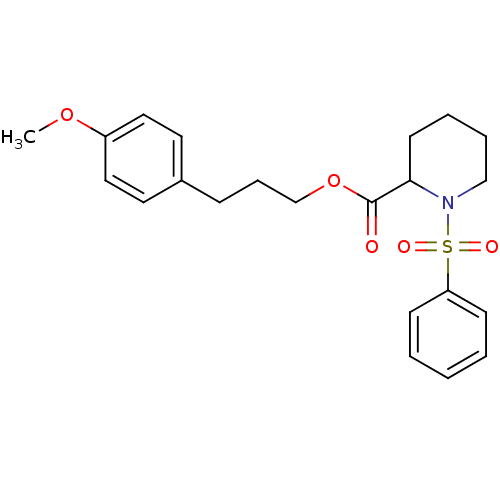
(1-Benzenesulfonyl-piperidine-2-carboxylic acid 3-(...)Show SMILES COc1ccc(CCCOC(=O)C2CCCCN2S(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C22H27NO5S/c1-27-19-14-12-18(13-15-19)8-7-17-28-22(24)21-11-5-6-16-23(21)29(25,26)20-9-3-2-4-10-20/h2-4,9-10,12-15,21H,5-8,11,16-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Bos taurus (bovine)) | BDBM23333

(3,3-dimethyl-1-[(2S)-2-(6-phenylhexanoyl)piperidin...)Show SMILES CC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)CCCCCc1ccccc1 |r| Show InChI InChI=1S/C23H33NO3/c1-23(2,3)21(26)22(27)24-17-11-10-15-19(24)20(25)16-9-5-8-14-18-12-6-4-7-13-18/h4,6-7,12-13,19H,5,8-11,14-17H2,1-3H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | -34.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 0 |
Guilford Pharmaceuticals, Inc.
| Assay Description
PPIase(Rotamase) activity of FKBP12 was assayed using the peptide N-succinyl Ala-Leu-Pro-Phe p-nitroanilide as substrate. It is based on the observat... |
J Med Chem 45: 3558-68 (2002)
Article DOI: 10.1021/jm0200456
BindingDB Entry DOI: 10.7270/Q2GH9G7G |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Bos taurus (bovine)) | BDBM23320
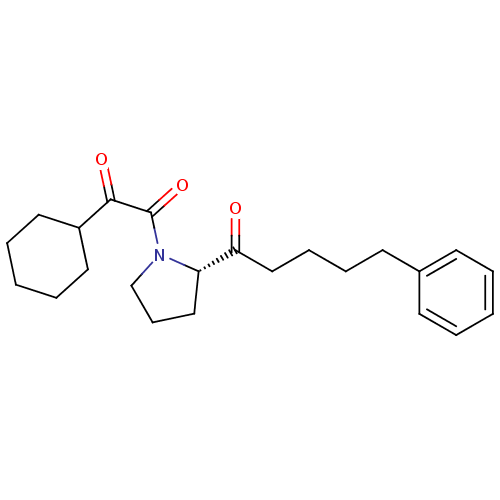
(1-cyclohexyl-2-[(2S)-2-(5-phenylpentanoyl)pyrrolid...)Show SMILES O=C(CCCCc1ccccc1)[C@@H]1CCCN1C(=O)C(=O)C1CCCCC1 |r| Show InChI InChI=1S/C23H31NO3/c25-21(16-8-7-12-18-10-3-1-4-11-18)20-15-9-17-24(20)23(27)22(26)19-13-5-2-6-14-19/h1,3-4,10-11,19-20H,2,5-9,12-17H2/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | -34.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 0 |
Guilford Pharmaceuticals, Inc.
| Assay Description
PPIase(Rotamase) activity of FKBP12 was assayed using the peptide N-succinyl Ala-Leu-Pro-Phe p-nitroanilide as substrate. It is based on the observat... |
J Med Chem 45: 3558-68 (2002)
Article DOI: 10.1021/jm0200456
BindingDB Entry DOI: 10.7270/Q2GH9G7G |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113078

(1-Phenylmethanesulfonyl-piperidine-2-carboxylic ac...)Show SMILES Fc1ccc(CCCOC(=O)C2CCCCN2S(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C22H26FNO4S/c23-20-13-11-18(12-14-20)9-6-16-28-22(25)21-10-4-5-15-24(21)29(26,27)17-19-7-2-1-3-8-19/h1-3,7-8,11-14,21H,4-6,9-10,15-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113088

(1-(Toluene-4-sulfonyl)-piperidine-2-carboxylic aci...)Show SMILES COc1cc(CCCOC(=O)C2CCCCN2S(=O)(=O)c2ccc(C)cc2)cc(OC)c1 Show InChI InChI=1S/C24H31NO6S/c1-18-9-11-22(12-10-18)32(27,28)25-13-5-4-8-23(25)24(26)31-14-6-7-19-15-20(29-2)17-21(16-19)30-3/h9-12,15-17,23H,4-8,13-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113086

(1-Phenylmethanesulfonyl-piperidine-2-carboxylic ac...)Show SMILES Clc1ccc(CCCOC(=O)C2CCCCN2S(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C22H26ClNO4S/c23-20-13-11-18(12-14-20)9-6-16-28-22(25)21-10-4-5-15-24(21)29(26,27)17-19-7-2-1-3-8-19/h1-3,7-8,11-14,21H,4-6,9-10,15-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50116629

(1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-carb...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)NCCC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H36N2O3/c1-4-28(2,3)25(31)27(33)30-20-12-11-17-24(30)26(32)29-19-18-23(21-13-7-5-8-14-21)22-15-9-6-10-16-22/h5-10,13-16,23-24H,4,11-12,17-20H2,1-3H3,(H,29,32)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against FK506 binding protein 12 |
J Med Chem 45: 3549-57 (2002)
BindingDB Entry DOI: 10.7270/Q2416WC5 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Bos taurus (bovine)) | BDBM23326
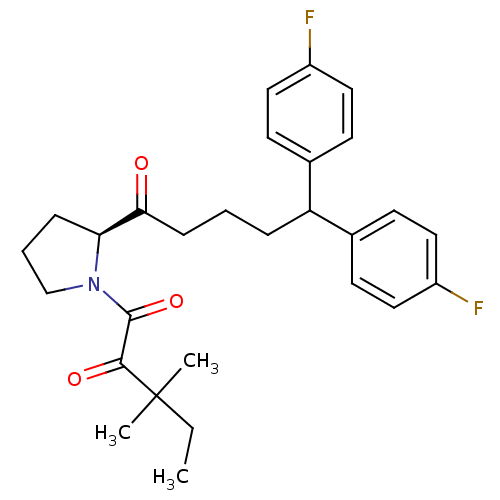
(1-[(2S)-2-[5,5-bis(4-fluorophenyl)pentanoyl]pyrrol...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)CCCC(c1ccc(F)cc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C28H33F2NO3/c1-4-28(2,3)26(33)27(34)31-18-6-8-24(31)25(32)9-5-7-23(19-10-14-21(29)15-11-19)20-12-16-22(30)17-13-20/h10-17,23-24H,4-9,18H2,1-3H3/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 460 | -33.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 0 |
Guilford Pharmaceuticals, Inc.
| Assay Description
PPIase(Rotamase) activity of FKBP12 was assayed using the peptide N-succinyl Ala-Leu-Pro-Phe p-nitroanilide as substrate. It is based on the observat... |
J Med Chem 45: 3558-68 (2002)
Article DOI: 10.1021/jm0200456
BindingDB Entry DOI: 10.7270/Q2GH9G7G |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113065

((S)-1-(Adamantan-1-ylcarbamoyl)-pyrrolidine-2-carb...)Show SMILES O=C(OCCCCc1ccccc1)[C@@H]1CCCN1C(=O)NC12C[C@H]3C[C@H](C[C@H](C3)C1)C2 |TLB:24:25:29:23.22.28,THB:24:23:29:25.30.26| Show InChI InChI=1S/C26H36N2O3/c29-24(31-12-5-4-9-19-7-2-1-3-8-19)23-10-6-11-28(23)25(30)27-26-16-20-13-21(17-26)15-22(14-20)18-26/h1-3,7-8,20-23H,4-6,9-18H2,(H,27,30)/t20-,21+,22-,23-,26?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113076

(1-Benzenesulfonyl-piperidine-2-carboxylic acid 3-(...)Show SMILES COc1cc(CCCOC(=O)C2CCCCN2S(=O)(=O)c2ccccc2)cc(OC)c1 Show InChI InChI=1S/C23H29NO6S/c1-28-19-15-18(16-20(17-19)29-2)9-8-14-30-23(25)22-12-6-7-13-24(22)31(26,27)21-10-4-3-5-11-21/h3-5,10-11,15-17,22H,6-9,12-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113059

(1-Benzenesulfonyl-piperidine-2-carboxylic acid 3-(...)Show SMILES Fc1ccc(CCCOC(=O)C2CCCCN2S(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C21H24FNO4S/c22-18-13-11-17(12-14-18)7-6-16-27-21(24)20-10-4-5-15-23(20)28(25,26)19-8-2-1-3-9-19/h1-3,8-9,11-14,20H,4-7,10,15-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Bos taurus (bovine)) | BDBM23330

(3,3-dimethyl-1-[(2S)-2-(5-phenylpentanoyl)piperidi...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)CCCCc1ccccc1 |r| Show InChI InChI=1S/C23H33NO3/c1-4-23(2,3)21(26)22(27)24-17-11-10-15-19(24)20(25)16-9-8-14-18-12-6-5-7-13-18/h5-7,12-13,19H,4,8-11,14-17H2,1-3H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 790 | -31.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 0 |
Guilford Pharmaceuticals, Inc.
| Assay Description
PPIase(Rotamase) activity of FKBP12 was assayed using the peptide N-succinyl Ala-Leu-Pro-Phe p-nitroanilide as substrate. It is based on the observat... |
J Med Chem 45: 3558-68 (2002)
Article DOI: 10.1021/jm0200456
BindingDB Entry DOI: 10.7270/Q2GH9G7G |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50116634

(1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)NCCCc1cccnc1 Show InChI InChI=1S/C20H29N3O3/c1-4-20(2,3)17(24)19(26)23-13-7-10-16(23)18(25)22-12-6-9-15-8-5-11-21-14-15/h5,8,11,14,16H,4,6-7,9-10,12-13H2,1-3H3,(H,22,25)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against FK506 binding protein 12 |
J Med Chem 45: 3549-57 (2002)
BindingDB Entry DOI: 10.7270/Q2416WC5 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113089

((S)-1-Phenylcarbamoyl-pyrrolidine-2-carboxylic aci...)Show InChI InChI=1S/C21H24N2O3/c24-20(26-16-8-11-17-9-3-1-4-10-17)19-14-7-15-23(19)21(25)22-18-12-5-2-6-13-18/h1-6,9-10,12-13,19H,7-8,11,14-16H2,(H,22,25)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113082

((S)-1-(Adamantan-1-ylthiocarbamoyl)-pyrrolidine-2-...)Show SMILES Fc1ccc(CCCOC(=O)[C@@H]2CCCN2C(=S)NC23C[C@H]4C[C@H](C[C@H](C4)C2)C3)cc1 |TLB:22:23:27:21.20.26,THB:22:21:27:23.28.24| Show InChI InChI=1S/C25H33FN2O2S/c26-21-7-5-17(6-8-21)3-2-10-30-23(29)22-4-1-9-28(22)24(31)27-25-14-18-11-19(15-25)13-20(12-18)16-25/h5-8,18-20,22H,1-4,9-16H2,(H,27,31)/t18-,19+,20-,22-,25?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Bos taurus (bovine)) | BDBM23332

(3,3-dimethyl-1-[(2S)-2-[6-(pyridin-3-yl)hexanoyl]p...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)CCCCCc1cccnc1 |r| Show InChI InChI=1S/C23H34N2O3/c1-4-23(2,3)21(27)22(28)25-16-9-8-13-19(25)20(26)14-7-5-6-11-18-12-10-15-24-17-18/h10,12,15,17,19H,4-9,11,13-14,16H2,1-3H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | -31.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 0 |
Guilford Pharmaceuticals, Inc.
| Assay Description
PPIase(Rotamase) activity of FKBP12 was assayed using the peptide N-succinyl Ala-Leu-Pro-Phe p-nitroanilide as substrate. It is based on the observat... |
J Med Chem 45: 3558-68 (2002)
Article DOI: 10.1021/jm0200456
BindingDB Entry DOI: 10.7270/Q2GH9G7G |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113094
((S)-1-(Adamantan-1-ylcarbamoyl)-pyrrolidine-2-carb...)Show SMILES O=C(OCCCc1ccccc1)[C@@H]1CCCN1C(=O)NC12C[C@H]3C[C@H](C[C@H](C3)C1)C2 |TLB:23:24:28:22.21.27,THB:23:22:28:24.29.25| Show InChI InChI=1S/C25H34N2O3/c28-23(30-11-5-8-18-6-2-1-3-7-18)22-9-4-10-27(22)24(29)26-25-15-19-12-20(16-25)14-21(13-19)17-25/h1-3,6-7,19-22H,4-5,8-17H2,(H,26,29)/t19-,20+,21-,22-,25?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113103
(1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)NCCCc1ccccc1 Show InChI InChI=1S/C21H30N2O3/c1-4-21(2,3)18(24)20(26)23-15-9-13-17(23)19(25)22-14-8-12-16-10-6-5-7-11-16/h5-7,10-11,17H,4,8-9,12-15H2,1-3H3,(H,22,25)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against FK506 binding protein 12 |
J Med Chem 45: 3549-57 (2002)
BindingDB Entry DOI: 10.7270/Q2416WC5 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113061

((S)-1-(Adamantan-1-ylcarbamoyl)-pyrrolidine-2-carb...)Show SMILES Fc1ccc(CCCOC(=O)[C@@H]2CCCN2C(=O)NC23C[C@H]4C[C@H](C[C@H](C4)C2)C3)cc1 |TLB:22:23:27:21.20.26,THB:22:21:27:23.28.24| Show InChI InChI=1S/C25H33FN2O3/c26-21-7-5-17(6-8-21)3-2-10-31-23(29)22-4-1-9-28(22)24(30)27-25-14-18-11-19(15-25)13-20(12-18)16-25/h5-8,18-20,22H,1-4,9-16H2,(H,27,30)/t18-,19+,20-,22-,25?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113073

((S)-1-(Adamantan-1-ylcarbamoyl)-pyrrolidine-2-carb...)Show SMILES O=C(OCCc1ccccc1)[C@@H]1CCCN1C(=O)NC12C[C@H]3C[C@H](C[C@H](C3)C1)C2 |TLB:22:23:27:21.20.26,THB:22:21:27:23.28.24| Show InChI InChI=1S/C24H32N2O3/c27-22(29-10-8-17-5-2-1-3-6-17)21-7-4-9-26(21)23(28)25-24-14-18-11-19(15-24)13-20(12-18)16-24/h1-3,5-6,18-21H,4,7-16H2,(H,25,28)/t18-,19+,20-,21-,24?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113062

((S)-1-Phenylcarbamoyl-pyrrolidine-2-carboxylic aci...)Show InChI InChI=1S/C20H22N2O3/c23-19(25-15-13-16-8-3-1-4-9-16)18-12-7-14-22(18)20(24)21-17-10-5-2-6-11-17/h1-6,8-11,18H,7,12-15H2,(H,21,24)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1421-8 (2002)
BindingDB Entry DOI: 10.7270/Q279441H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data