 Found 987 hits with Last Name = 'xiang' and Initial = 'h'
Found 987 hits with Last Name = 'xiang' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aurora kinase B
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora B ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora B ATP binding site by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase C
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora C ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora B ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase C
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora C ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50529647

(CHEMBL4584527 | US10806720, Compound 17 | US112305...)Show SMILES CN(C[C@](C)(O)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F)c1ccc(C#N)c(c1)-c1ccccc1 |r| Show InChI InChI=1S/C26H21F3N4O2/c1-25(35,24(34)32-20-10-8-19(15-31)23(12-20)26(27,28)29)16-33(2)21-11-9-18(14-30)22(13-21)17-6-4-3-5-7-17/h3-13,35H,16H2,1-2H3,(H,32,34)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50529667

(CHEMBL4444904 | US10806720, Compound 11 | US112305...)Show SMILES C[C@](O)(Cn1ccc2cc(F)ccc12)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H15F4N3O2/c1-19(29,11-27-7-6-12-8-14(21)3-5-17(12)27)18(28)26-15-4-2-13(10-25)16(9-15)20(22,23)24/h2-9,29H,11H2,1H3,(H,26,28)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora A ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 492 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Ubiquitin thioesterase OTUB1
(Homo sapiens) | BDBM50591324
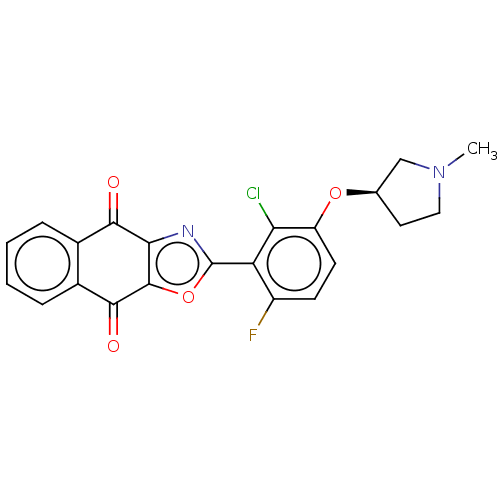
(CHEMBL5185273)Show SMILES CN1CC[C@H](C1)Oc1ccc(F)c(-c2nc3c(o2)C(=O)c2ccccc2C3=O)c1Cl |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00408
BindingDB Entry DOI: 10.7270/Q25H7M81 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341209
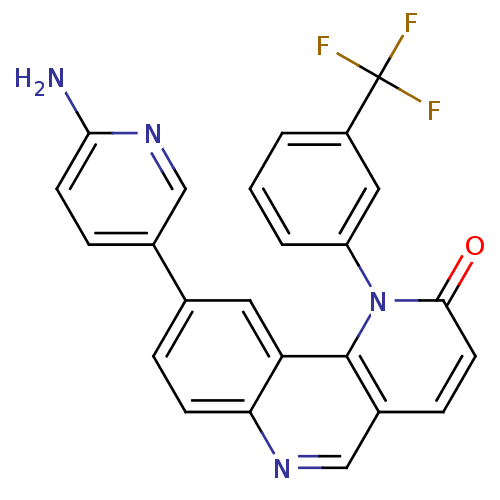
(9-(6-aminopyridin-3-yl)-1-(3-(trifluoromethyl)phen...)Show SMILES Nc1ccc(cn1)-c1ccc2ncc3ccc(=O)n(-c4cccc(c4)C(F)(F)F)c3c2c1 Show InChI InChI=1S/C24H15F3N4O/c25-24(26,27)17-2-1-3-18(11-17)31-22(32)9-6-16-13-29-20-7-4-14(10-19(20)23(16)31)15-5-8-21(28)30-12-15/h1-13H,(H2,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
Bioorg Med Chem 26: 4537-4543 (2018)
Article DOI: 10.1016/j.bmc.2018.07.047
BindingDB Entry DOI: 10.7270/Q20C4ZDW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ubiquitin carboxyl-terminal hydrolase 8
(Homo sapiens (Human)) | BDBM50591324

(CHEMBL5185273)Show SMILES CN1CC[C@H](C1)Oc1ccc(F)c(-c2nc3c(o2)C(=O)c2ccccc2C3=O)c1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00408
BindingDB Entry DOI: 10.7270/Q25H7M81 |
More data for this
Ligand-Target Pair | |
Ubiquitin thioesterase OTUB1
(Homo sapiens) | BDBM50591323
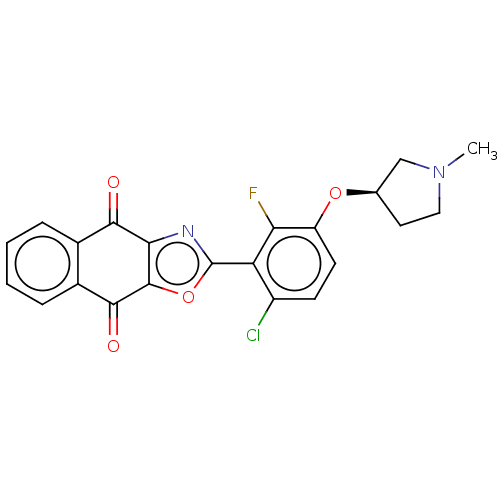
(CHEMBL5173239)Show SMILES CN1CC[C@H](C1)Oc1ccc(Cl)c(-c2nc3c(o2)C(=O)c2ccccc2C3=O)c1F |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00408
BindingDB Entry DOI: 10.7270/Q25H7M81 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) |
Bioorg Med Chem 23: 6059-68 (2015)
Article DOI: 10.1016/j.bmc.2015.05.043
BindingDB Entry DOI: 10.7270/Q2445P7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50341250
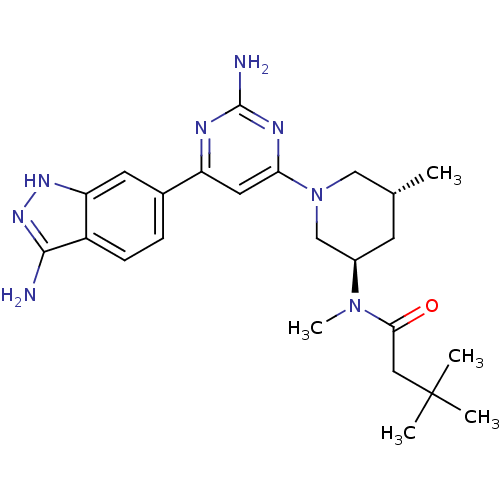
(CHEMBL1765751 | N-{(3R,5R)-1-[2-Amino-6-(3-amino-1...)Show SMILES C[C@@H]1C[C@H](CN(C1)c1cc(nc(N)n1)-c1ccc2c(N)n[nH]c2c1)N(C)C(=O)CC(C)(C)C |r| Show InChI InChI=1S/C24H34N8O/c1-14-8-16(31(5)21(33)11-24(2,3)4)13-32(12-14)20-10-18(27-23(26)28-20)15-6-7-17-19(9-15)29-30-22(17)25/h6-7,9-10,14,16H,8,11-13H2,1-5H3,(H3,25,29,30)(H2,26,27,28)/t14-,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
J Med Chem 54: 1871-95 (2011)
Article DOI: 10.1021/jm101527u
BindingDB Entry DOI: 10.7270/Q2HQ406Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by ADP-gloassay |
Eur J Med Chem 164: 304-316 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.055
BindingDB Entry DOI: 10.7270/Q23J3HF7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50341249
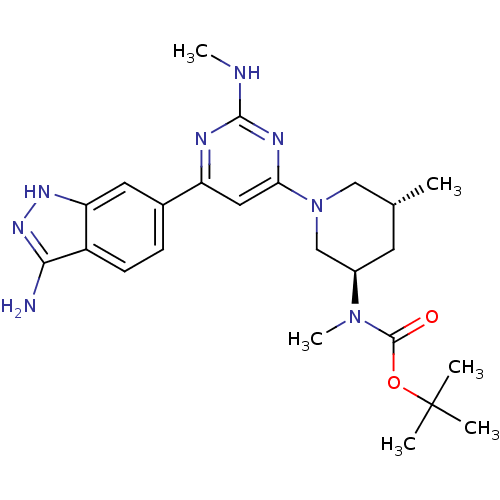
(1,1-Dimethylethyl{(3R,5R)-1-[2-Amino-6-(3-amino-1H...)Show SMILES CNc1nc(cc(n1)-c1ccc2c(N)n[nH]c2c1)N1C[C@H](C)C[C@H](C1)N(C)C(=O)OC(C)(C)C |r| Show InChI InChI=1S/C24H34N8O2/c1-14-9-16(31(6)23(33)34-24(2,3)4)13-32(12-14)20-11-18(27-22(26-5)28-20)15-7-8-17-19(10-15)29-30-21(17)25/h7-8,10-11,14,16H,9,12-13H2,1-6H3,(H3,25,29,30)(H,26,27,28)/t14-,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
J Med Chem 54: 1871-95 (2011)
Article DOI: 10.1021/jm101527u
BindingDB Entry DOI: 10.7270/Q2HQ406Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) by ADP-Glo assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112339
BindingDB Entry DOI: 10.7270/Q2DF6VZ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316497
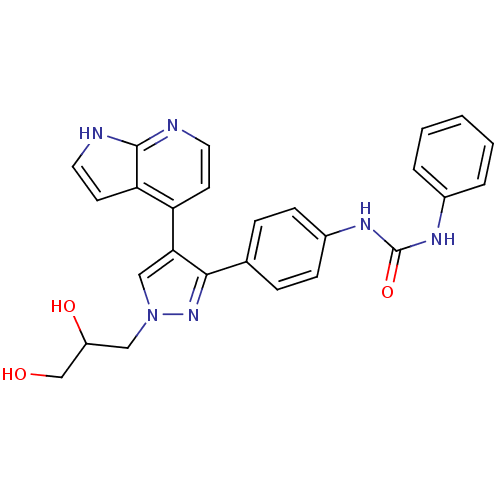
(CHEMBL1097106 | N-{4-[1-(2,3-Dihydroxypropyl)-4-(1...)Show SMILES OCC(O)Cn1cc(c(n1)-c1ccc(NC(=O)Nc2ccccc2)cc1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C26H24N6O3/c33-16-20(34)14-32-15-23(21-10-12-27-25-22(21)11-13-28-25)24(31-32)17-6-8-19(9-7-17)30-26(35)29-18-4-2-1-3-5-18/h1-13,15,20,33-34H,14,16H2,(H,27,28)(H2,29,30,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human N-terminal His tagged BTK expressed in baculovirus infected Sf9 cells using poly (4:1 Glu, Tyr) as substr... |
Bioorg Med Chem 26: 4537-4543 (2018)
Article DOI: 10.1016/j.bmc.2018.07.047
BindingDB Entry DOI: 10.7270/Q20C4ZDW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50241089
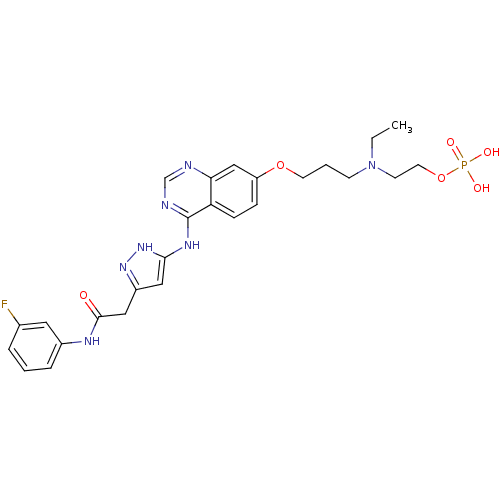
(2-(ethyl(3-(4-(5-(2-(3-fluorophenylamino)-2-oxoeth...)Show SMILES CCN(CCCOc1ccc2c(Nc3cc(CC(=O)Nc4cccc(F)c4)n[nH]3)ncnc2c1)CCOP(O)(O)=O Show InChI InChI=1S/C26H31FN7O6P/c1-2-34(10-12-40-41(36,37)38)9-4-11-39-21-7-8-22-23(16-21)28-17-29-26(22)31-24-14-20(32-33-24)15-25(35)30-19-6-3-5-18(27)13-19/h3,5-8,13-14,16-17H,2,4,9-12,15H2,1H3,(H,30,35)(H2,36,37,38)(H2,28,29,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora B ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BLK (unknown origin) by ADP-Glo assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112339
BindingDB Entry DOI: 10.7270/Q2DF6VZ0 |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 8
(Homo sapiens (Human)) | BDBM50591322

(CHEMBL5196686)Show SMILES CN1CC[C@H](C1)Oc1ccc(Cl)c(c1)-c1nc2c(o1)C(=O)c1ccccc1C2=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00408
BindingDB Entry DOI: 10.7270/Q25H7M81 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316473
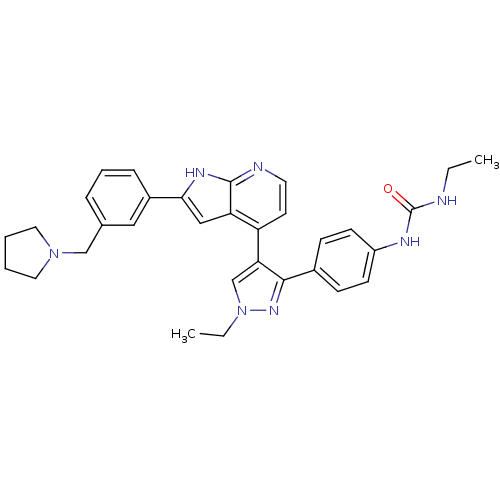
(4-[3-(4-N-Ethylcarbamylaminophenyl)-1-ethyl-1H-pyr...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nn(CC)cc1-c1ccnc2[nH]c(cc12)-c1cccc(CN2CCCC2)c1 Show InChI InChI=1S/C32H35N7O/c1-3-33-32(40)35-25-12-10-23(11-13-25)30-28(21-39(4-2)37-30)26-14-15-34-31-27(26)19-29(36-31)24-9-7-8-22(18-24)20-38-16-5-6-17-38/h7-15,18-19,21H,3-6,16-17,20H2,1-2H3,(H,34,36)(H2,33,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50239948
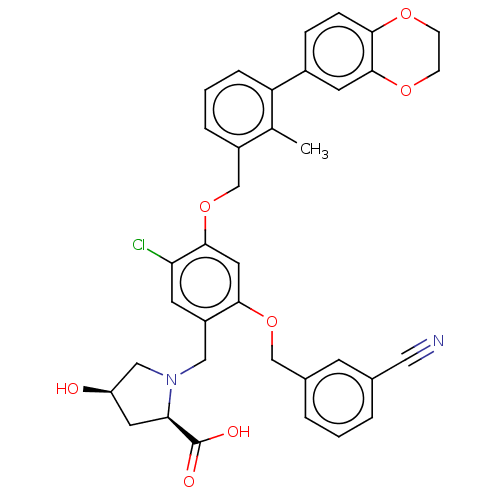
(CHEMBL4071326 | US9850225, Example 1166)Show SMILES Cc1c(COc2cc(OCc3cccc(c3)C#N)c(CN3C[C@H](O)C[C@@H]3C(O)=O)cc2Cl)cccc1-c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C36H33ClN2O7/c1-22-26(6-3-7-29(22)25-8-9-32-35(14-25)44-11-10-43-32)21-46-34-16-33(45-20-24-5-2-4-23(12-24)17-38)27(13-30(34)37)18-39-19-28(40)15-31(39)36(41)42/h2-9,12-14,16,28,31,40H,10-11,15,18-21H2,1H3,(H,41,42)/t28-,31-/m1/s1 | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316475
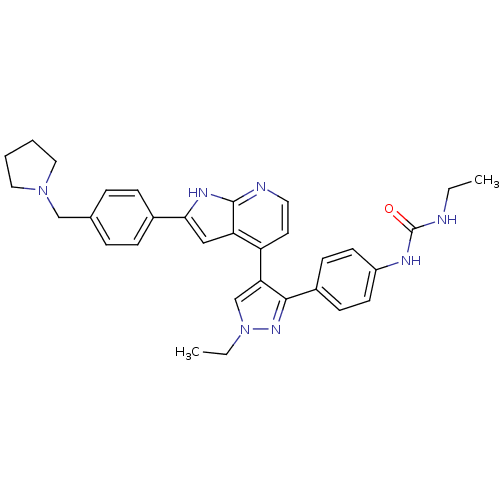
(CHEMBL1097191 | N-Ethyl-N'-[4-(1-ethyl-4-{2-[4-(1-...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nn(CC)cc1-c1ccnc2[nH]c(cc12)-c1ccc(CN2CCCC2)cc1 Show InChI InChI=1S/C32H35N7O/c1-3-33-32(40)35-25-13-11-24(12-14-25)30-28(21-39(4-2)37-30)26-15-16-34-31-27(26)19-29(36-31)23-9-7-22(8-10-23)20-38-17-5-6-18-38/h7-16,19,21H,3-6,17-18,20H2,1-2H3,(H,34,36)(H2,33,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50341241
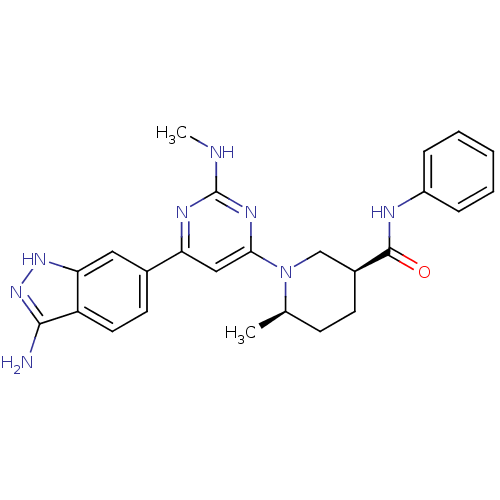
((3S,6R)-1-[6-(3-Amino-1H-indazol-6-yl)-2-(methylam...)Show SMILES CNc1nc(cc(n1)-c1ccc2c(N)n[nH]c2c1)N1C[C@H](CC[C@H]1C)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C25H28N8O/c1-15-8-9-17(24(34)28-18-6-4-3-5-7-18)14-33(15)22-13-20(29-25(27-2)30-22)16-10-11-19-21(12-16)31-32-23(19)26/h3-7,10-13,15,17H,8-9,14H2,1-2H3,(H,28,34)(H3,26,31,32)(H,27,29,30)/t15-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
J Med Chem 54: 1871-95 (2011)
Article DOI: 10.1021/jm101527u
BindingDB Entry DOI: 10.7270/Q2HQ406Q |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316471
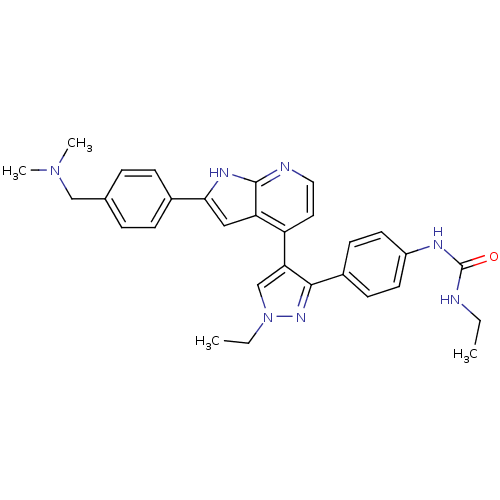
(4-[3-(4-N-Ethylcarbamylaminophenyl)-1-ethyl-1H-pyr...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nn(CC)cc1-c1ccnc2[nH]c(cc12)-c1ccc(CN(C)C)cc1 Show InChI InChI=1S/C30H33N7O/c1-5-31-30(38)33-23-13-11-22(12-14-23)28-26(19-37(6-2)35-28)24-15-16-32-29-25(24)17-27(34-29)21-9-7-20(8-10-21)18-36(3)4/h7-17,19H,5-6,18H2,1-4H3,(H,32,34)(H2,31,33,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Ubiquitin thioesterase OTUB1
(Homo sapiens) | BDBM50591321

(CHEMBL5172138) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00408
BindingDB Entry DOI: 10.7270/Q25H7M81 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PARP-2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127710
BindingDB Entry DOI: 10.7270/Q21G0QW3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ubiquitin carboxyl-terminal hydrolase 8
(Homo sapiens (Human)) | BDBM50591323

(CHEMBL5173239)Show SMILES CN1CC[C@H](C1)Oc1ccc(Cl)c(-c2nc3c(o2)C(=O)c2ccccc2C3=O)c1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00408
BindingDB Entry DOI: 10.7270/Q25H7M81 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316492
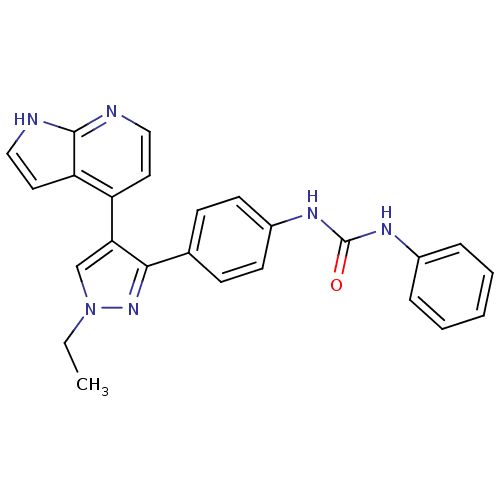
(1-(4-(1-ethyl-4-(1H-pyrrolo[2,3-b]pyridin-4-yl)-1H...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)Nc2ccccc2)cc1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C25H22N6O/c1-2-31-16-22(20-12-14-26-24-21(20)13-15-27-24)23(30-31)17-8-10-19(11-9-17)29-25(32)28-18-6-4-3-5-7-18/h3-16H,2H2,1H3,(H,26,27)(H2,28,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316498
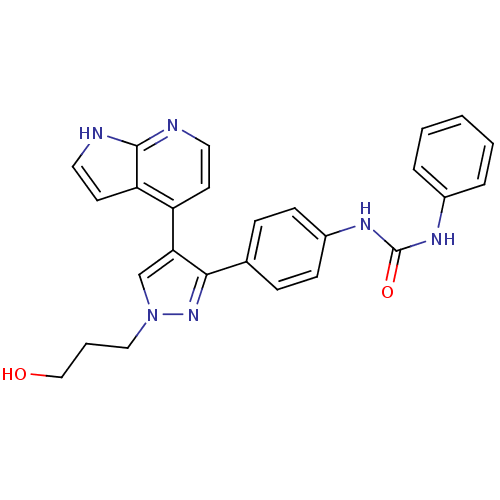
(CHEMBL1097454 | N-{4-[1-(3-Hydroxypropyl)-4-(1H-py...)Show SMILES OCCCn1cc(c(n1)-c1ccc(NC(=O)Nc2ccccc2)cc1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C26H24N6O2/c33-16-4-15-32-17-23(21-11-13-27-25-22(21)12-14-28-25)24(31-32)18-7-9-20(10-8-18)30-26(34)29-19-5-2-1-3-6-19/h1-3,5-14,17,33H,4,15-16H2,(H,27,28)(H2,29,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316474
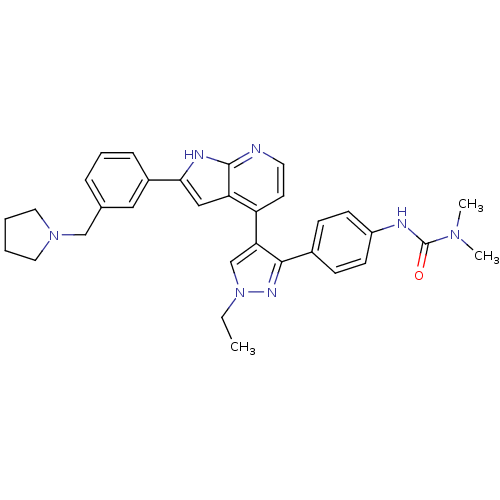
(4-[3-(4-N,N-Dimethylcarbamylaminophenyl)-1-ethyl-1...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN2CCCC2)c1 Show InChI InChI=1S/C32H35N7O/c1-4-39-21-28(30(36-39)23-10-12-25(13-11-23)34-32(40)37(2)3)26-14-15-33-31-27(26)19-29(35-31)24-9-7-8-22(18-24)20-38-16-5-6-17-38/h7-15,18-19,21H,4-6,16-17,20H2,1-3H3,(H,33,35)(H,34,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50239946
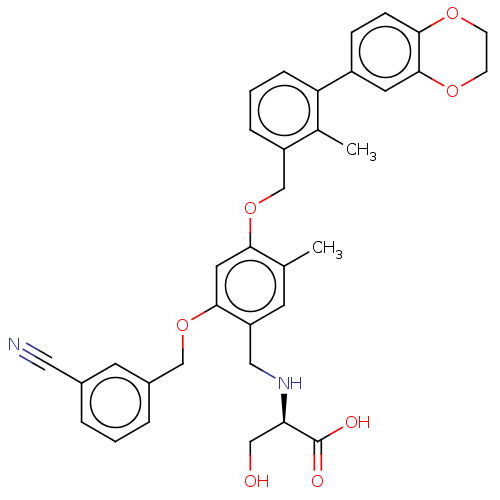
(CHEMBL4084368)Show SMILES Cc1cc(CN[C@H](CO)C(O)=O)c(OCc2cccc(c2)C#N)cc1OCc1cccc(c1C)-c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C35H34N2O7/c1-22-13-28(18-37-30(19-38)35(39)40)33(43-20-25-6-3-5-24(14-25)17-36)16-32(22)44-21-27-7-4-8-29(23(27)2)26-9-10-31-34(15-26)42-12-11-41-31/h3-10,13-16,30,37-38H,11-12,18-21H2,1-2H3,(H,39,40)/t30-/m1/s1 | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) by ADP-Glo assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112339
BindingDB Entry DOI: 10.7270/Q2DF6VZ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50341243
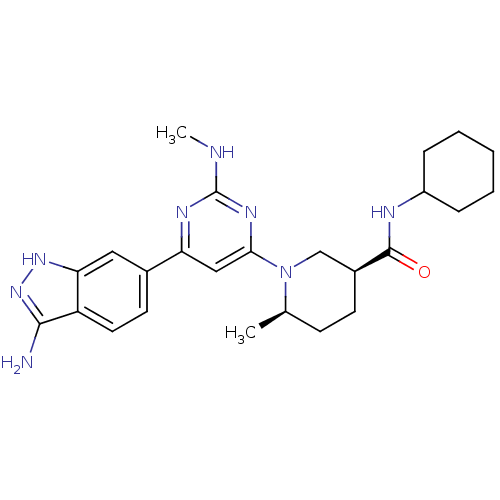
((3S,6R)-1-[6-(3-Amino-1H-indazol-6-yl)-2-(methylam...)Show SMILES CNc1nc(cc(n1)-c1ccc2c(N)n[nH]c2c1)N1C[C@H](CC[C@H]1C)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C25H34N8O/c1-15-8-9-17(24(34)28-18-6-4-3-5-7-18)14-33(15)22-13-20(29-25(27-2)30-22)16-10-11-19-21(12-16)31-32-23(19)26/h10-13,15,17-18H,3-9,14H2,1-2H3,(H,28,34)(H3,26,31,32)(H,27,29,30)/t15-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
J Med Chem 54: 1871-95 (2011)
Article DOI: 10.1021/jm101527u
BindingDB Entry DOI: 10.7270/Q2HQ406Q |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 8
(Homo sapiens (Human)) | BDBM50591321

(CHEMBL5172138) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00408
BindingDB Entry DOI: 10.7270/Q25H7M81 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316470
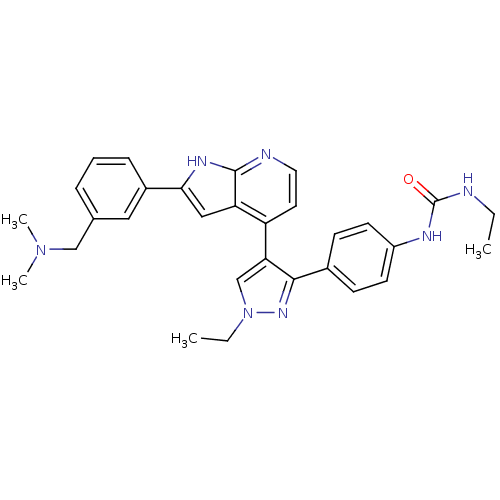
(4-[3-(4-N-Ethylcarbamylaminophenyl)-1-ethyl-1H-pyr...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nn(CC)cc1-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-5-31-30(38)33-23-12-10-21(11-13-23)28-26(19-37(6-2)35-28)24-14-15-32-29-25(24)17-27(34-29)22-9-7-8-20(16-22)18-36(3)4/h7-17,19H,5-6,18H2,1-4H3,(H,32,34)(H2,31,33,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50233644

(CHEMBL4079989)Show SMILES OCc1c(cccc1-n1ccc2cc(cc(F)c2c1=O)C1CC1)-c1ccnc2[nH]c(cc12)C1=CCN(CC1)C1COC1 |t:38| Show InChI InChI=1S/C34H31FN4O3/c35-29-15-23(20-4-5-20)14-22-9-13-39(34(41)32(22)29)31-3-1-2-25(28(31)17-40)26-6-10-36-33-27(26)16-30(37-33)21-7-11-38(12-8-21)24-18-42-19-24/h1-3,6-7,9-10,13-16,20,24,40H,4-5,8,11-12,17-19H2,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BTK in human Ramos cells assessed as inhibition of anti human IgM-induced calcium influx preincubated for 20 mins in dark followed by a... |
Bioorg Med Chem 23: 4344-53 (2015)
Article DOI: 10.1016/j.bmc.2015.06.023
BindingDB Entry DOI: 10.7270/Q25T3NR9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50233655

(CHEMBL4101864)Show SMILES OCc1c(cccc1-n1ccc2cc(cc(F)c2c1=O)C1CC1)-c1ccnc2[nH]c(cc12)C1=CCNCC1 |t:38| Show InChI InChI=1S/C31H27FN4O2/c32-26-15-21(18-4-5-18)14-20-9-13-36(31(38)29(20)26)28-3-1-2-22(25(28)17-37)23-8-12-34-30-24(23)16-27(35-30)19-6-10-33-11-7-19/h1-3,6,8-9,12-16,18,33,37H,4-5,7,10-11,17H2,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BTK in human Ramos cells assessed as inhibition of anti human IgM-induced calcium influx preincubated for 20 mins in dark followed by a... |
Bioorg Med Chem 23: 4344-53 (2015)
Article DOI: 10.1016/j.bmc.2015.06.023
BindingDB Entry DOI: 10.7270/Q25T3NR9 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316496
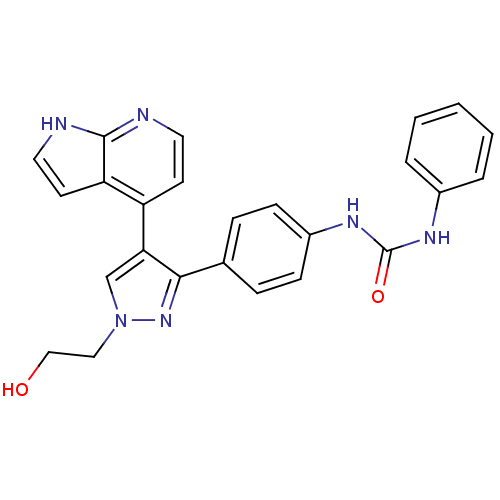
(CHEMBL1099010 | N-{4-[1-(2-Hydroxyethyl)-4-(1H-pyr...)Show SMILES OCCn1cc(c(n1)-c1ccc(NC(=O)Nc2ccccc2)cc1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C25H22N6O2/c32-15-14-31-16-22(20-10-12-26-24-21(20)11-13-27-24)23(30-31)17-6-8-19(9-7-17)29-25(33)28-18-4-2-1-3-5-18/h1-13,16,32H,14-15H2,(H,26,27)(H2,28,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50341239

((3S,6R)-1-[2-Amino-6-(3-amino-1H-indazol-6-yl)-4-p...)Show SMILES C[C@@H]1CC[C@@H](CN1c1cc(nc(N)n1)-c1ccc2c(N)[nH]nc2c1)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C24H26N8O/c1-14-7-8-16(23(33)27-17-5-3-2-4-6-17)13-32(14)21-12-19(28-24(26)29-21)15-9-10-18-20(11-15)30-31-22(18)25/h2-6,9-12,14,16H,7-8,13H2,1H3,(H,27,33)(H3,25,30,31)(H2,26,28,29)/t14-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT phosphorylation at Thr308 residue in human PC3 cells by ELISA |
J Med Chem 54: 1871-95 (2011)
Article DOI: 10.1021/jm101527u
BindingDB Entry DOI: 10.7270/Q2HQ406Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50341245

((2S,5R)-4-[6-(3-Amino-1H-indazol-6-yl)-2-(methylam...)Show SMILES CC[C@@H]1CO[C@@H](CN1c1cc(nc(NC)n1)-c1ccc2c(N)n[nH]c2c1)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C25H28N8O2/c1-3-17-14-35-21(24(34)28-16-7-5-4-6-8-16)13-33(17)22-12-19(29-25(27-2)30-22)15-9-10-18-20(11-15)31-32-23(18)26/h4-12,17,21H,3,13-14H2,1-2H3,(H,28,34)(H3,26,31,32)(H,27,29,30)/t17-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
J Med Chem 54: 1871-95 (2011)
Article DOI: 10.1021/jm101527u
BindingDB Entry DOI: 10.7270/Q2HQ406Q |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50341246
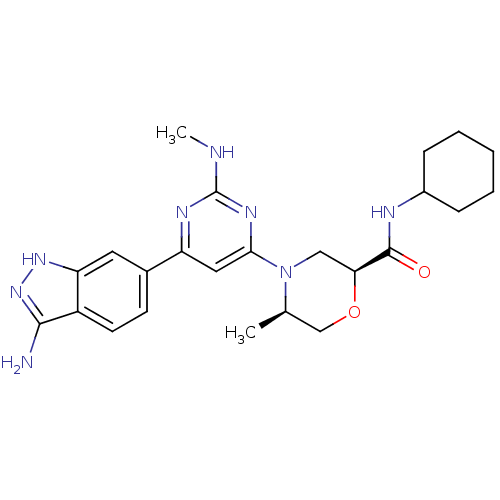
((2S,5R)-4-[6-(3-amino-1H-indazol-6-yl)-2-(methylam...)Show SMILES CNc1nc(cc(n1)-c1ccc2c(N)n[nH]c2c1)N1C[C@H](OC[C@H]1C)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C24H32N8O2/c1-14-13-34-20(23(33)27-16-6-4-3-5-7-16)12-32(14)21-11-18(28-24(26-2)29-21)15-8-9-17-19(10-15)30-31-22(17)25/h8-11,14,16,20H,3-7,12-13H2,1-2H3,(H,27,33)(H3,25,30,31)(H,26,28,29)/t14-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
J Med Chem 54: 1871-95 (2011)
Article DOI: 10.1021/jm101527u
BindingDB Entry DOI: 10.7270/Q2HQ406Q |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316472
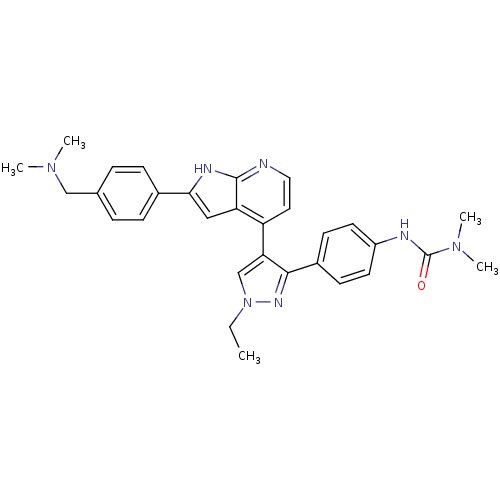
(4-[3-(4-N,N-Dimethylcarbamylaminophenyl)-1-ethyl-1...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1ccc(CN(C)C)cc1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)22-11-13-23(14-12-22)32-30(38)36(4)5)24-15-16-31-29-25(24)17-27(33-29)21-9-7-20(8-10-21)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM31093

(4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...)Show SMILES OC(=O)c1ccc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2F)cc1 |c:13| Show InChI InChI=1S/C25H15ClF2N4O2/c26-15-6-9-17-18(10-15)23(21-19(27)2-1-3-20(21)28)29-11-14-12-30-25(32-22(14)17)31-16-7-4-13(5-8-16)24(33)34/h1-10,12H,11H2,(H,33,34)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
Bioorg Med Chem 26: 4537-4543 (2018)
Article DOI: 10.1016/j.bmc.2018.07.047
BindingDB Entry DOI: 10.7270/Q20C4ZDW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM185147
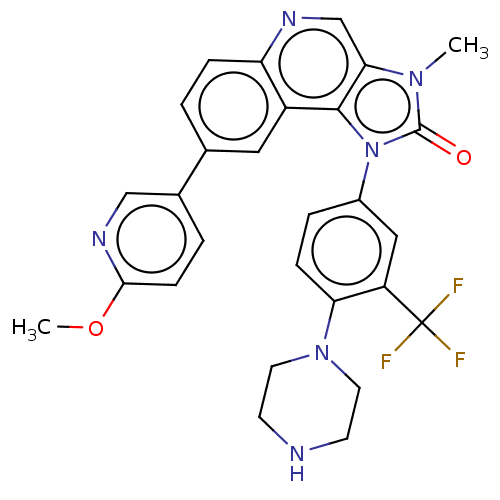
((Z)-but-2-enedioic acid;8-(6-methoxypyridin-3-yl)-...)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(-c4ccc(N5CCNCC5)c(c4)C(F)(F)F)c3c2c1 Show InChI InChI=1S/C28H25F3N6O2/c1-35-24-16-33-22-6-3-17(18-4-8-25(39-2)34-15-18)13-20(22)26(24)37(27(35)38)19-5-7-23(21(14-19)28(29,30)31)36-11-9-32-10-12-36/h3-8,13-16,32H,9-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
Bioorg Med Chem 26: 4537-4543 (2018)
Article DOI: 10.1016/j.bmc.2018.07.047
BindingDB Entry DOI: 10.7270/Q20C4ZDW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data