 Found 1046 hits with Last Name = 'xie' and Initial = 'w'
Found 1046 hits with Last Name = 'xie' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | CHEMBL5276586

| UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | CHEMBL5269400
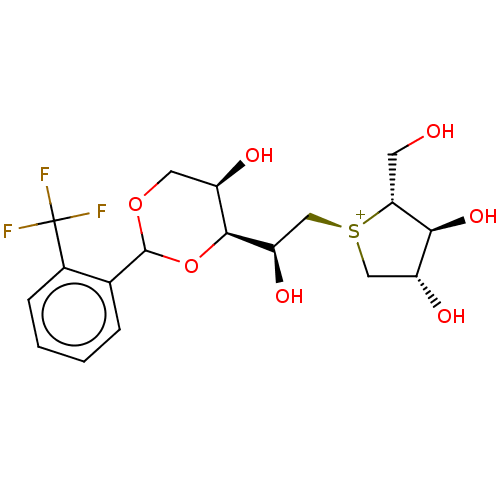
| UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | CHEMBL5268739

| UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | CHEMBL5282029

| UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | CHEMBL5283777

| UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | CHEMBL5276586

| PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | CHEMBL5269400

| PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | CHEMBL5268739

| PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | CHEMBL5282029

| PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | CHEMBL5283777

| PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50117910
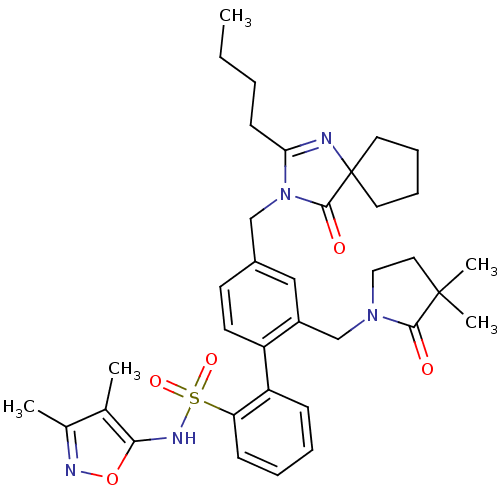
(4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(c(CN2CCC(C)(C)C2=O)c1)-c1ccccc1S(=O)(=O)Nc1onc(C)c1C |t:4| Show InChI InChI=1S/C36H45N5O5S/c1-6-7-14-31-37-36(17-10-11-18-36)34(43)41(31)22-26-15-16-28(27(21-26)23-40-20-19-35(4,5)33(40)42)29-12-8-9-13-30(29)47(44,45)39-32-24(2)25(3)38-46-32/h8-9,12-13,15-16,21,39H,6-7,10-11,14,17-20,22-23H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity at endothelin receptor subtype A |
Bioorg Med Chem 20: 4661-7 (2012)
Article DOI: 10.1016/j.bmc.2012.06.011
BindingDB Entry DOI: 10.7270/Q2G73FS6 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50117910

(4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(c(CN2CCC(C)(C)C2=O)c1)-c1ccccc1S(=O)(=O)Nc1onc(C)c1C |t:4| Show InChI InChI=1S/C36H45N5O5S/c1-6-7-14-31-37-36(17-10-11-18-36)34(43)41(31)22-26-15-16-28(27(21-26)23-40-20-19-35(4,5)33(40)42)29-12-8-9-13-30(29)47(44,45)39-32-24(2)25(3)38-46-32/h8-9,12-13,15-16,21,39H,6-7,10-11,14,17-20,22-23H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity at type 1 angiotensin 2 receptor |
Bioorg Med Chem 20: 4661-7 (2012)
Article DOI: 10.1016/j.bmc.2012.06.011
BindingDB Entry DOI: 10.7270/Q2G73FS6 |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | CHEMBL5275740
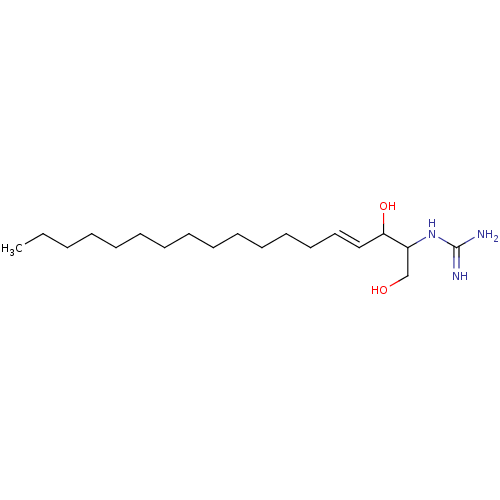
Show InChI InChI=1S/C15H11F3N4S/c16-15(17,18)8-4-6-9(7-5-8)23-11-3-1-2-10-12(11)13(19)22-14(20)21-10/h1-7H,(H4,19,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against (-)-noradrenaline-induced contraction of rat prostatic vas deferens (Alpha-1A adrenergic receptor ) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50147013
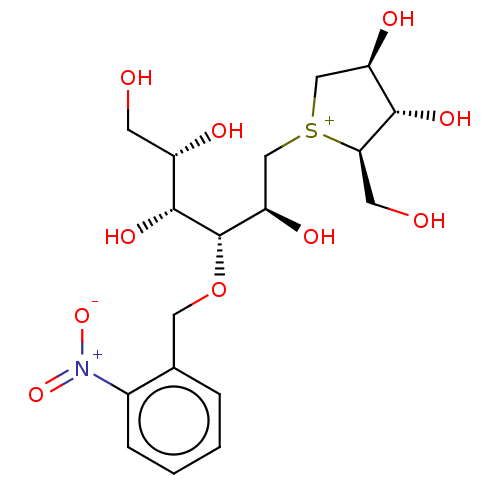
(CHEMBL3764559)Show SMILES [Cl-].OC[C@H](O)[C@@H](O)[C@@H](OCc1ccccc1[N+]([O-])=O)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C18H28NO10S.ClH/c20-5-12(22)17(26)18(29-7-10-3-1-2-4-11(10)19(27)28)14(24)9-30-8-13(23)16(25)15(30)6-21;/h1-4,12-18,20-26H,5-9H2;1H/q+1;/p-1/t12-,13+,14+,15+,16-,17+,18-,30?;/m0./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat intestinal sucrase using sucrose as substrate incubated for 30 mins by Lineweaver-Burk plot analysis |
Eur J Med Chem 110: 224-36 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.029
BindingDB Entry DOI: 10.7270/Q2NS0WSH |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50147016
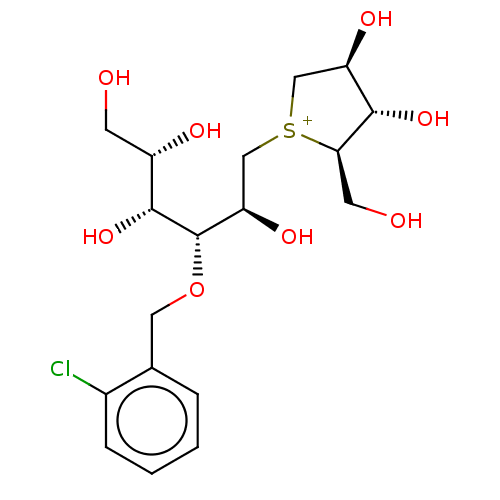
(CHEMBL3763300)Show SMILES [Cl-].OC[C@H](O)[C@@H](O)[C@@H](OCc1ccccc1Cl)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C18H28ClO8S.ClH/c19-11-4-2-1-3-10(11)7-27-18(17(26)12(22)5-20)14(24)9-28-8-13(23)16(25)15(28)6-21;/h1-4,12-18,20-26H,5-9H2;1H/q+1;/p-1/t12-,13+,14+,15+,16-,17+,18-,28?;/m0./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat intestinal sucrase using sucrose as substrate incubated for 30 mins by Lineweaver-Burk plot analysis |
Eur J Med Chem 110: 224-36 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.029
BindingDB Entry DOI: 10.7270/Q2NS0WSH |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50147107
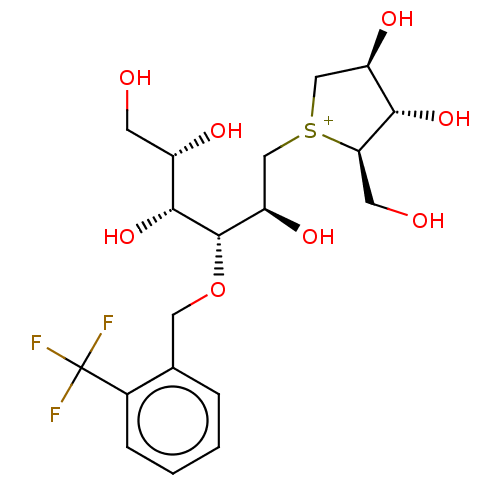
(CHEMBL3763319)Show SMILES [Cl-].OC[C@H](O)[C@@H](O)[C@@H](OCc1ccccc1C(F)(F)F)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C19H28F3O8S.ClH/c20-19(21,22)11-4-2-1-3-10(11)7-30-18(17(29)12(25)5-23)14(27)9-31-8-13(26)16(28)15(31)6-24;/h1-4,12-18,23-29H,5-9H2;1H/q+1;/p-1/t12-,13+,14+,15+,16-,17+,18-,31?;/m0./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat intestinal sucrase using sucrose as substrate incubated for 30 mins by Lineweaver-Burk plot analysis |
Eur J Med Chem 110: 224-36 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.029
BindingDB Entry DOI: 10.7270/Q2NS0WSH |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | CHEMBL5275740

Show InChI InChI=1S/C15H11F3N4S/c16-15(17,18)8-4-6-9(7-5-8)23-11-3-1-2-10-12(11)13(19)22-14(20)21-10/h1-7H,(H4,19,20,21,22) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of the cAMP-stimulated beta-galactosidase transcription in SK-N-MC cells expressing the human Histamine H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50600
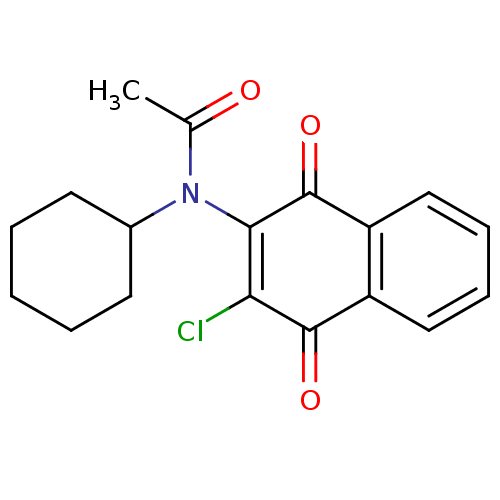
(MLS001029919 | N-(3-Chloro-1,4-dioxo-1,4-dihydro-n...)Show SMILES CC(=O)N(C1CCCCC1)C1=C(Cl)C(=O)c2ccccc2C1=O |c:11| Show InChI InChI=1S/C18H18ClNO3/c1-11(21)20(12-7-3-2-4-8-12)16-15(19)17(22)13-9-5-6-10-14(13)18(16)23/h5-6,9-10,12H,2-4,7-8H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against (-)-noradrenaline-induced contraction of rat thoracic aorta (Alpha-1D adrenergic receptor) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50147158
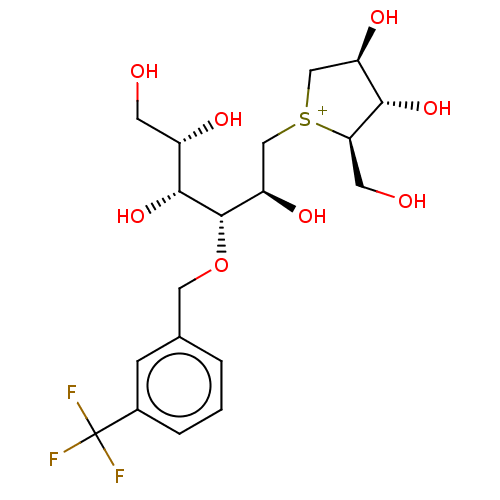
(CHEMBL3764745)Show SMILES [Cl-].OC[C@H](O)[C@@H](O)[C@@H](OCc1cccc(c1)C(F)(F)F)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C19H28F3O8S.ClH/c20-19(21,22)11-3-1-2-10(4-11)7-30-18(17(29)12(25)5-23)14(27)9-31-8-13(26)16(28)15(31)6-24;/h1-4,12-18,23-29H,5-9H2;1H/q+1;/p-1/t12-,13+,14+,15+,16-,17+,18-,31?;/m0./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 353 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat intestinal sucrase using sucrose as substrate incubated for 30 mins by Lineweaver-Burk plot analysis |
Eur J Med Chem 110: 224-36 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.029
BindingDB Entry DOI: 10.7270/Q2NS0WSH |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Mus musculus (Mouse)) | CHEMBL4595333

Show InChI InChI=1S/C18H21N5S/c1-18(2,3)10-4-6-11(7-5-10)24-15-12(19)8-9-13-14(15)16(20)23-17(21)22-13/h4-9H,19H2,1-3H3,(H4,20,21,22,23) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional Histamine H1 receptor antagonistic activity in vitro assay on guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50147159
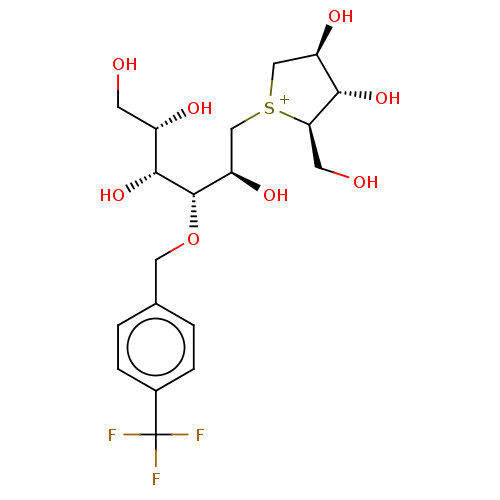
(CHEMBL3764813)Show SMILES [Cl-].OC[C@H](O)[C@@H](O)[C@@H](OCc1ccc(cc1)C(F)(F)F)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C19H28F3O8S.ClH/c20-19(21,22)11-3-1-10(2-4-11)7-30-18(17(29)12(25)5-23)14(27)9-31-8-13(26)16(28)15(31)6-24;/h1-4,12-18,23-29H,5-9H2;1H/q+1;/p-1/t12-,13+,14+,15+,16-,17+,18-,31?;/m0./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 785 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat intestinal sucrase using sucrose as substrate incubated for 30 mins by Lineweaver-Burk plot analysis |
Eur J Med Chem 110: 224-36 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.029
BindingDB Entry DOI: 10.7270/Q2NS0WSH |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Mus musculus (Mouse)) | CHEMBL5281819
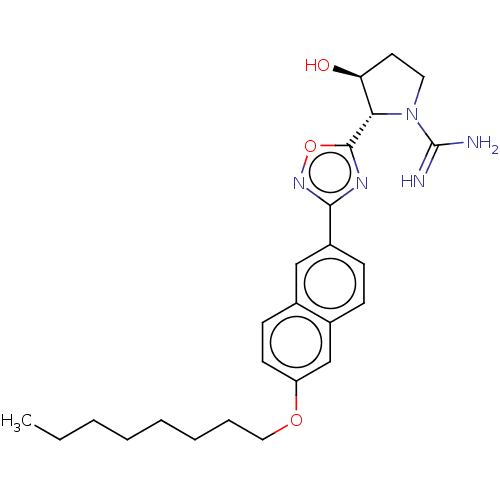
Show InChI InChI=1S/C19H22N4S/c1-4-19(2,3)12-8-10-13(11-9-12)24-15-7-5-6-14-16(15)17(20)23-18(21)22-14/h5-11H,4H2,1-3H3,(H4,20,21,22,23) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional Histamine H3 receptor antagonistic activity in vitro assay on guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50443388
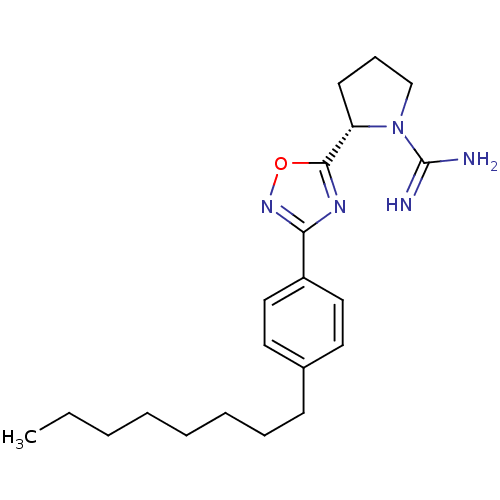
(CHEMBL3086782 | US9688668, SLR080811)Show SMILES CCCCCCCCc1ccc(cc1)-c1noc(n1)[C@@H]1CCCN1C(N)=N |r| Show InChI InChI=1S/C21H31N5O/c1-2-3-4-5-6-7-9-16-11-13-17(14-12-16)19-24-20(27-25-19)18-10-8-15-26(18)21(22)23/h11-14,18H,2-10,15H2,1H3,(H3,22,23)/t18-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50009730
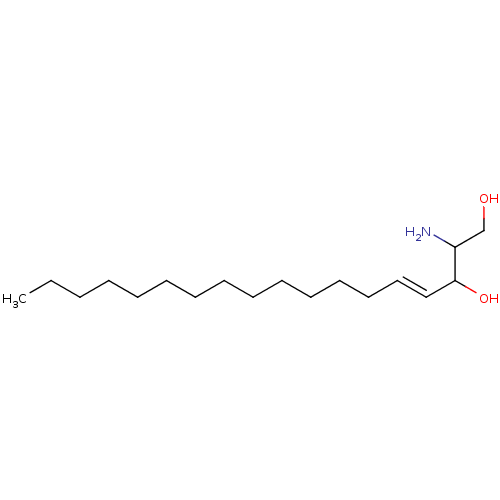
((E)-2-Amino-octadec-4-ene-1,3-diol | 2-Amino-octad...)Show InChI InChI=1S/C18H37NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(21)17(19)16-20/h14-15,17-18,20-21H,2-13,16,19H2,1H3/b15-14+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against (-)-noradrenaline-induced contraction of rat prostatic vas deferens (alpha1A receptor) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | CHEMBL447685

Show SMILES CC(C)(C)c1ccc(Sc2c(ccc3nc(N)nc(N)c23)C#N)cc1 Show InChI InChI=1S/C19H19N5S/c1-19(2,3)12-5-7-13(8-6-12)25-16-11(10-20)4-9-14-15(16)17(21)24-18(22)23-14/h4-9H,1-3H3,(H4,21,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against (-)-phenylephrine-induced contraction of rat spleen (Alpha-1B adrenergic receptor ) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50139649
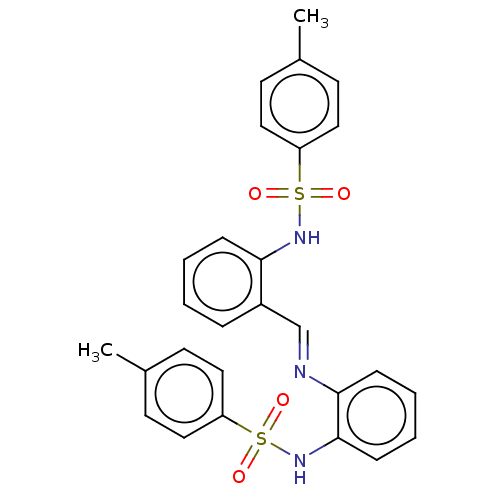
(CHEMBL3764617)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1ccccc1\C=N\c1ccccc1NS(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C27H25N3O4S2/c1-20-11-15-23(16-12-20)35(31,32)29-25-8-4-3-7-22(25)19-28-26-9-5-6-10-27(26)30-36(33,34)24-17-13-21(2)14-18-24/h3-19,29-30H,1-2H3/b28-19+ | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against (-)-noradrenaline-induced contraction of rat thoracic aorta (Alpha-1D adrenergic receptor) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50312869

(4-(4-(4-chlorophenyl)thiazol-2-ylamino)phenol | CH...)Show InChI InChI=1S/C15H11ClN2OS/c16-11-3-1-10(2-4-11)14-9-20-15(18-14)17-12-5-7-13(19)8-6-12/h1-9,19H,(H,17,18) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50393642
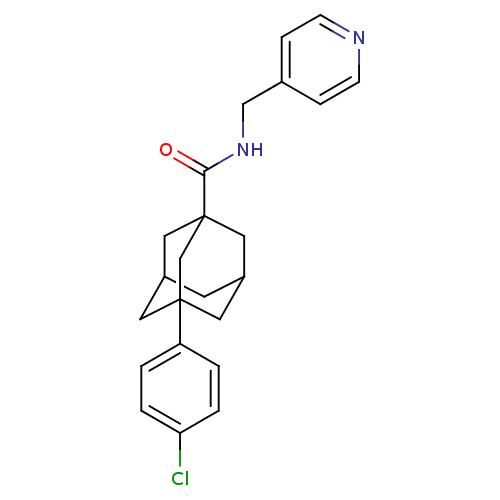
(CHEMBL2158685)Show SMILES Clc1ccc(cc1)C12CC3CC(CC(C3)(C1)C(=O)NCc1ccncc1)C2 |TLB:14:9:26:15.13.12,14:13:8.9.10:26,THB:16:13:8:10.11.26,16:13:8.9.10:26,12:13:8:10.11.26,12:11:8:15.14.13| Show InChI InChI=1S/C23H25ClN2O/c24-20-3-1-19(2-4-20)22-10-17-9-18(11-22)13-23(12-17,15-22)21(27)26-14-16-5-7-25-8-6-16/h1-8,17-18H,9-15H2,(H,26,27) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional Histamine H3 receptor antagonistic activity in vitro assay on guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50443388

(CHEMBL3086782 | US9688668, SLR080811)Show SMILES CCCCCCCCc1ccc(cc1)-c1noc(n1)[C@@H]1CCCN1C(N)=N |r| Show InChI InChI=1S/C21H31N5O/c1-2-3-4-5-6-7-9-16-11-13-17(14-12-16)19-24-20(27-25-19)18-10-8-15-26(18)21(22)23/h11-14,18H,2-10,15H2,1H3,(H3,22,23)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional Histamine H1 receptor antagonistic activity in vitro assay on guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | CHEMBL447685

Show SMILES CC(C)(C)c1ccc(Sc2c(ccc3nc(N)nc(N)c23)C#N)cc1 Show InChI InChI=1S/C19H19N5S/c1-19(2,3)12-5-7-13(8-6-12)25-16-11(10-20)4-9-14-15(16)17(21)24-18(22)23-14/h4-9H,1-3H3,(H4,21,22,23,24) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50312869

(4-(4-(4-chlorophenyl)thiazol-2-ylamino)phenol | CH...)Show InChI InChI=1S/C15H11ClN2OS/c16-11-3-1-10(2-4-11)14-9-20-15(18-14)17-12-5-7-13(19)8-6-12/h1-9,19H,(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against (-)-noradrenaline-induced contraction of rat thoracic aorta (Alpha-1D adrenergic receptor) |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine kinase 1
(Mus musculus) | CHEMBL4595333

Show InChI InChI=1S/C18H21N5S/c1-18(2,3)10-4-6-11(7-5-10)24-15-12(19)8-9-13-14(15)16(20)23-17(21)22-13/h4-9H,19H2,1-3H3,(H4,20,21,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Mus musculus) | CHEMBL5281819

Show InChI InChI=1S/C19H22N4S/c1-4-19(2,3)12-8-10-13(11-9-12)24-15-7-5-6-14-16(15)17(20)23-18(21)22-14/h5-11H,4H2,1-3H3,(H4,20,21,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional Histamine H3 receptor antagonistic activity in vitro assay on guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50139649

(CHEMBL3764617)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1ccccc1\C=N\c1ccccc1NS(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C27H25N3O4S2/c1-20-11-15-23(16-12-20)35(31,32)29-25-8-4-3-7-22(25)19-28-26-9-5-6-10-27(26)30-36(33,34)24-17-13-21(2)14-18-24/h3-19,29-30H,1-2H3/b28-19+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against (-)-noradrenaline-induced contraction of rat thoracic aorta (Alpha-1D adrenergic receptor) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50189781
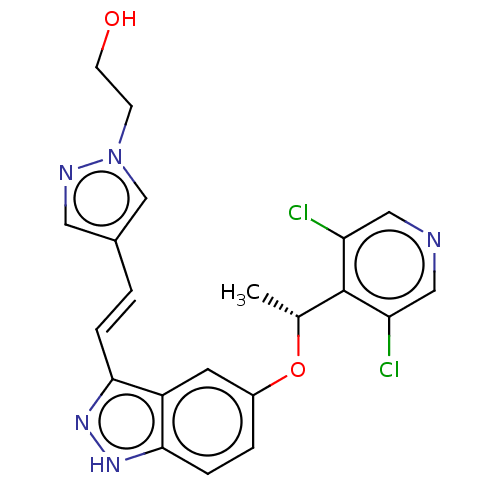
(CHEMBL3828009)Show SMILES C[C@@H](Oc1ccc2[nH]nc(\C=C\c3cnn(CCO)c3)c2c1)c1c(Cl)cncc1Cl |r| Show InChI InChI=1S/C21H19Cl2N5O2/c1-13(21-17(22)10-24-11-18(21)23)30-15-3-5-20-16(8-15)19(26-27-20)4-2-14-9-25-28(12-14)6-7-29/h2-5,8-13,29H,6-7H2,1H3,(H,26,27)/b4-2+/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435764

(CHEMBL2392692)Show SMILES CNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccc(Br)cc1OC(F)(F)F Show InChI InChI=1S/C19H22BrF3N6O2/c1-11-26-17(24-2)28-18(27-11)29-7-5-12(6-8-29)16(30)25-10-13-3-4-14(20)9-15(13)31-19(21,22)23/h3-4,9,12H,5-8,10H2,1-2H3,(H,25,30)(H,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | CHEMBL5279959
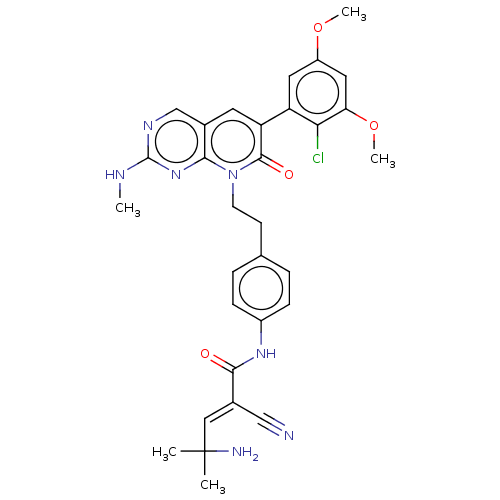
| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50189781

(CHEMBL3828009)Show SMILES C[C@@H](Oc1ccc2[nH]nc(\C=C\c3cnn(CCO)c3)c2c1)c1c(Cl)cncc1Cl |r| Show InChI InChI=1S/C21H19Cl2N5O2/c1-13(21-17(22)10-24-11-18(21)23)30-15-3-5-20-16(8-15)19(26-27-20)4-2-14-9-25-28(12-14)6-7-29/h2-5,8-13,29H,6-7H2,1H3,(H,26,27)/b4-2+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50431385
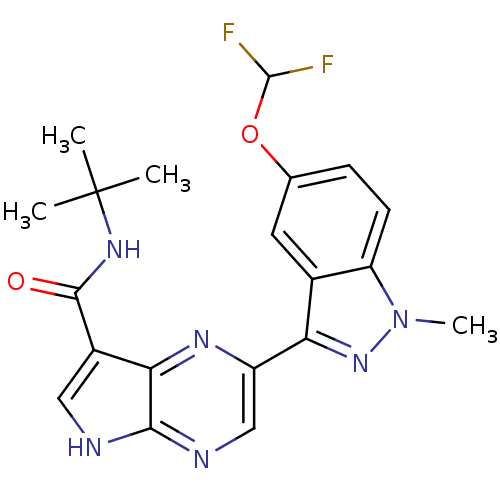
(CHEMBL2346686)Show SMILES Cn1nc(-c2cnc3[nH]cc(C(=O)NC(C)(C)C)c3n2)c2cc(OC(F)F)ccc12 Show InChI InChI=1S/C20H20F2N6O2/c1-20(2,3)26-18(29)12-8-23-17-16(12)25-13(9-24-17)15-11-7-10(30-19(21)22)5-6-14(11)28(4)27-15/h5-9,19H,1-4H3,(H,23,24)(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... |
J Med Chem 56: 1677-92 (2013)
Article DOI: 10.1021/jm301720p
BindingDB Entry DOI: 10.7270/Q22N53M0 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435765
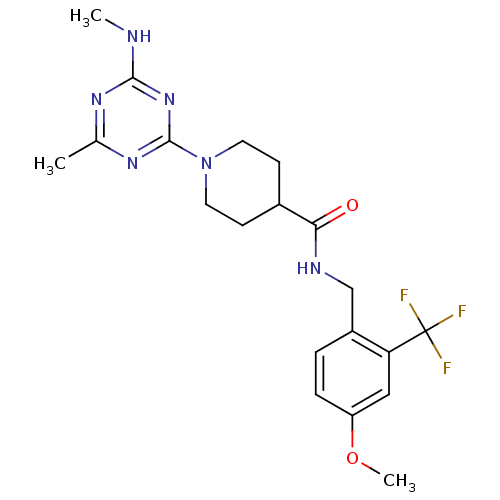
(CHEMBL2392691)Show SMILES CNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccc(OC)cc1C(F)(F)F Show InChI InChI=1S/C20H25F3N6O2/c1-12-26-18(24-2)28-19(27-12)29-8-6-13(7-9-29)17(30)25-11-14-4-5-15(31-3)10-16(14)20(21,22)23/h4-5,10,13H,6-9,11H2,1-3H3,(H,25,30)(H,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50400047

(BIIB-057 | CHEMBL2177736 | US9579320, Example 87)Show SMILES N[C@H]1CCCC[C@H]1Nc1ncc(C(N)=O)c(Nc2cccc(c2)-n2nccn2)n1 |r| Show InChI InChI=1S/C19H23N9O/c20-15-6-1-2-7-16(15)26-19-22-11-14(17(21)29)18(27-19)25-12-4-3-5-13(10-12)28-23-8-9-24-28/h3-5,8-11,15-16H,1-2,6-7,20H2,(H2,21,29)(H2,22,25,26,27)/t15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant spleen tyrosine kinase (360 to 635 amino acid residues) after 10 mins by scintillation counting analysis |
J Med Chem 55: 10414-23 (2012)
Article DOI: 10.1021/jm301367c
BindingDB Entry DOI: 10.7270/Q2057H23 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50400044
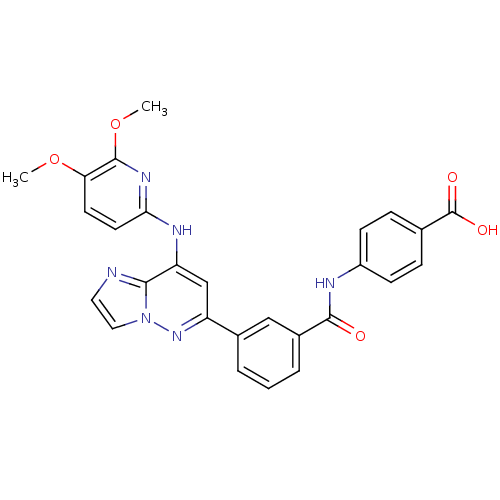
(CHEMBL2177726 | US9169259, I-54)Show SMILES COc1ccc(Nc2cc(nn3ccnc23)-c2cccc(c2)C(=O)Nc2ccc(cc2)C(O)=O)nc1OC Show InChI InChI=1S/C27H22N6O5/c1-37-22-10-11-23(31-26(22)38-2)30-21-15-20(32-33-13-12-28-24(21)33)17-4-3-5-18(14-17)25(34)29-19-8-6-16(7-9-19)27(35)36/h3-15H,1-2H3,(H,29,34)(H,30,31)(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant spleen tyrosine kinase (360 to 635 amino acid residues) after 10 mins by scintillation counting analysis |
J Med Chem 55: 10414-23 (2012)
Article DOI: 10.1021/jm301367c
BindingDB Entry DOI: 10.7270/Q2057H23 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435745
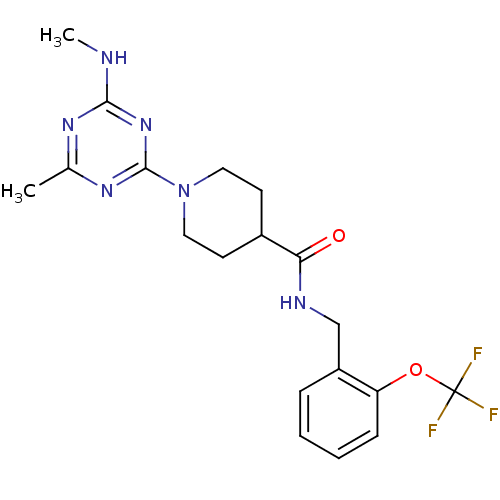
(CHEMBL2392706)Show SMILES CNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1OC(F)(F)F Show InChI InChI=1S/C19H23F3N6O2/c1-12-25-17(23-2)27-18(26-12)28-9-7-13(8-10-28)16(29)24-11-14-5-3-4-6-15(14)30-19(20,21)22/h3-6,13H,7-11H2,1-2H3,(H,24,29)(H,23,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435753

(CHEMBL2392698)Show SMILES COCCNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C21H27F3N6O2/c1-14-27-19(25-9-12-32-2)29-20(28-14)30-10-7-15(8-11-30)18(31)26-13-16-5-3-4-6-17(16)21(22,23)24/h3-6,15H,7-13H2,1-2H3,(H,26,31)(H,25,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50400047

(BIIB-057 | CHEMBL2177736 | US9579320, Example 87)Show SMILES N[C@H]1CCCC[C@H]1Nc1ncc(C(N)=O)c(Nc2cccc(c2)-n2nccn2)n1 |r| Show InChI InChI=1S/C19H23N9O/c20-15-6-1-2-7-16(15)26-19-22-11-14(17(21)29)18(27-19)25-12-4-3-5-13(10-12)28-23-8-9-24-28/h3-5,8-11,15-16H,1-2,6-7,20H2,(H2,21,29)(H2,22,25,26,27)/t15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... |
J Med Chem 56: 1677-92 (2013)
Article DOI: 10.1021/jm301720p
BindingDB Entry DOI: 10.7270/Q22N53M0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50185178

(1-tert-butyl-3-(3-(5-(4-(piperidin-1-yl)piperidin-...)Show SMILES CC(C)(C)NC(=O)Nc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1 Show InChI InChI=1S/C29H38N8O/c1-29(2,3)33-28(38)30-19-7-9-23-22(17-19)26(35-34-23)27-31-24-10-8-21(18-25(24)32-27)37-15-11-20(12-16-37)36-13-5-4-6-14-36/h7-10,17-18,20H,4-6,11-16H2,1-3H3,(H,31,32)(H,34,35)(H2,30,33,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435754
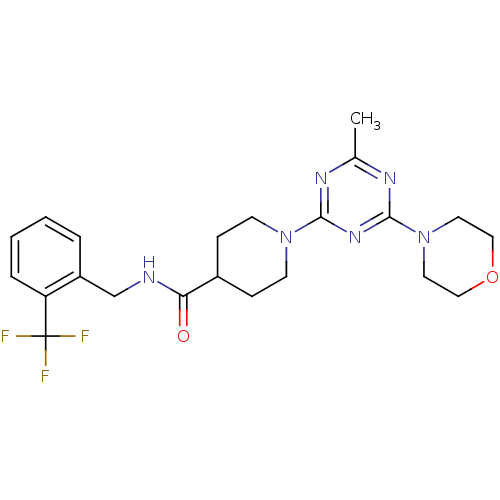
(CHEMBL2392697)Show SMILES Cc1nc(nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C22H27F3N6O2/c1-15-27-20(29-21(28-15)31-10-12-33-13-11-31)30-8-6-16(7-9-30)19(32)26-14-17-4-2-3-5-18(17)22(23,24)25/h2-5,16H,6-14H2,1H3,(H,26,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435755

(CHEMBL2392696)Show SMILES CC(C)Nc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C21H27F3N6O/c1-13(2)26-19-27-14(3)28-20(29-19)30-10-8-15(9-11-30)18(31)25-12-16-6-4-5-7-17(16)21(22,23)24/h4-7,13,15H,8-12H2,1-3H3,(H,25,31)(H,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435756

(CHEMBL2392695)Show SMILES CN(C)c1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C20H25F3N6O/c1-13-25-18(28(2)3)27-19(26-13)29-10-8-14(9-11-29)17(30)24-12-15-6-4-5-7-16(15)20(21,22)23/h4-7,14H,8-12H2,1-3H3,(H,24,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435757
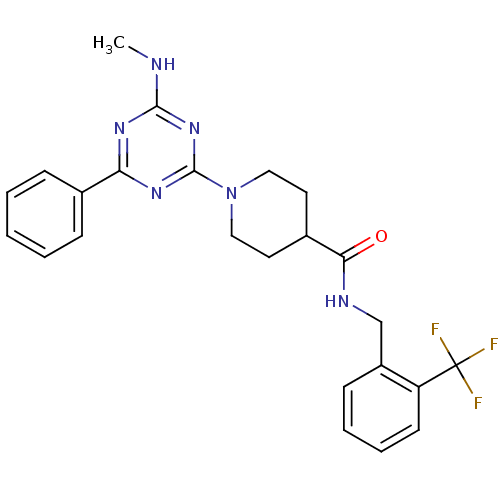
(CHEMBL2392694)Show SMILES CNc1nc(nc(n1)-c1ccccc1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C24H25F3N6O/c1-28-22-30-20(16-7-3-2-4-8-16)31-23(32-22)33-13-11-17(12-14-33)21(34)29-15-18-9-5-6-10-19(18)24(25,26)27/h2-10,17H,11-15H2,1H3,(H,29,34)(H,28,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data