

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ketohexokinase (Homo sapiens (Human)) | BDBM319585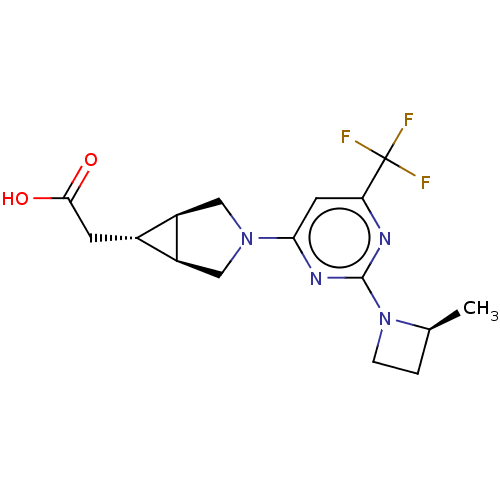 (US10174007, Example 4 | US10787438, Example 4 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed noncompetitive inhibition of recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50569864 (CHEMBL4869517) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to beta2 adrenoceptor (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01195 BindingDB Entry DOI: 10.7270/Q24F1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50569866 (CHEMBL4860571) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to beta2 adrenoceptor (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01195 BindingDB Entry DOI: 10.7270/Q24F1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50000504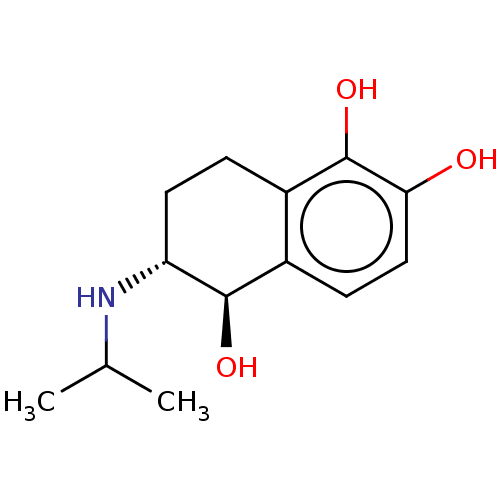 (6-Isopropylamino-5,6,7,8-tetrahydro-naphthalene-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to beta2 adrenoceptor (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01195 BindingDB Entry DOI: 10.7270/Q24F1VJ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50569865 (CHEMBL4871979) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to beta2 adrenoceptor (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01195 BindingDB Entry DOI: 10.7270/Q24F1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50133601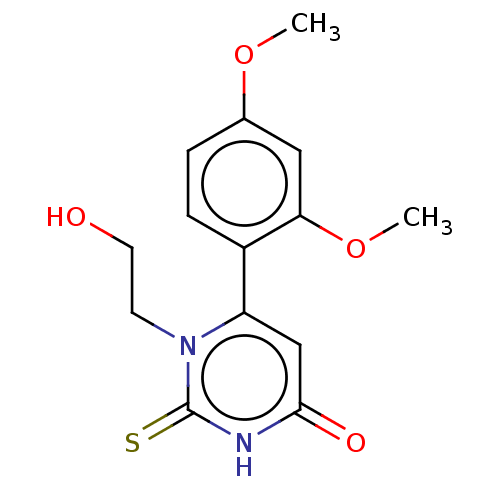 (CHEMBL3633251) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... | J Med Chem 58: 8513-28 (2015) Article DOI: 10.1021/acs.jmedchem.5b00963 BindingDB Entry DOI: 10.7270/Q2SQ926X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50133602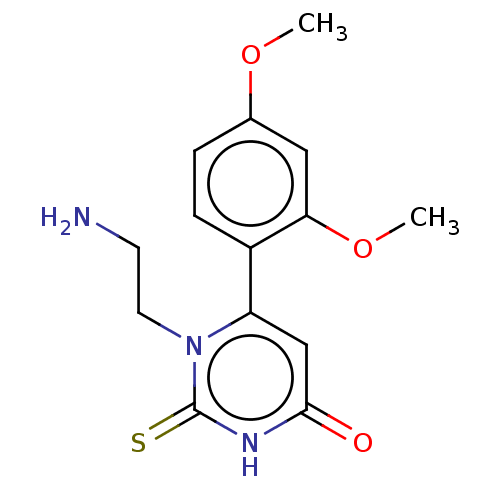 (CHEMBL3633250) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... | J Med Chem 58: 8513-28 (2015) Article DOI: 10.1021/acs.jmedchem.5b00963 BindingDB Entry DOI: 10.7270/Q2SQ926X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50133595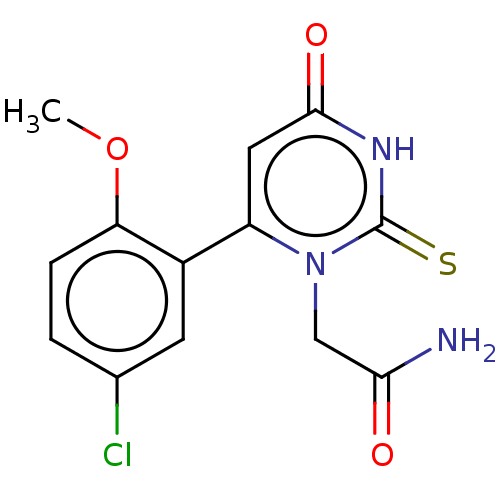 (CHEMBL3633460) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... | J Med Chem 58: 8513-28 (2015) Article DOI: 10.1021/acs.jmedchem.5b00963 BindingDB Entry DOI: 10.7270/Q2SQ926X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50133596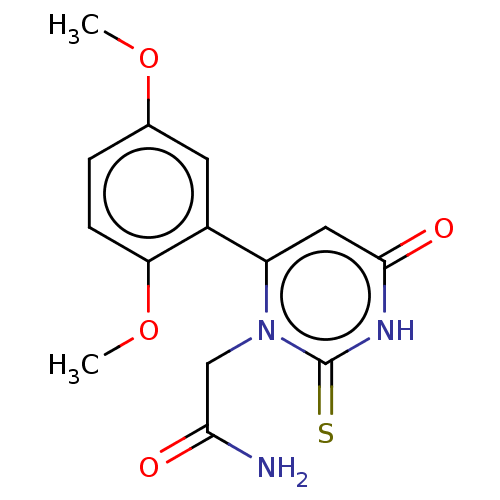 (CHEMBL3633459) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... | J Med Chem 58: 8513-28 (2015) Article DOI: 10.1021/acs.jmedchem.5b00963 BindingDB Entry DOI: 10.7270/Q2SQ926X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50133603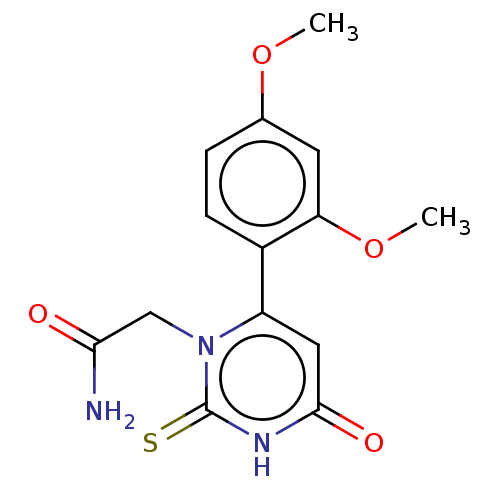 (CHEMBL3633248) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... | J Med Chem 58: 8513-28 (2015) Article DOI: 10.1021/acs.jmedchem.5b00963 BindingDB Entry DOI: 10.7270/Q2SQ926X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM119872 (3‐(2‐ethoxypropyl)‐2‐sulfa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 413 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Worldwide Research and Development | Assay Description Assay mixtures (100 µL) contained 50 mM NaPi (pH 7.4), 150 mM NaCl, 1 mM DTPA, 2% DMSO, the indicated concentrations of H2O2, and Amplex Red. Th... | Biochemistry 52: 9187-201 (2013) Article DOI: 10.1021/bi401354d BindingDB Entry DOI: 10.7270/Q2K35SBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM119874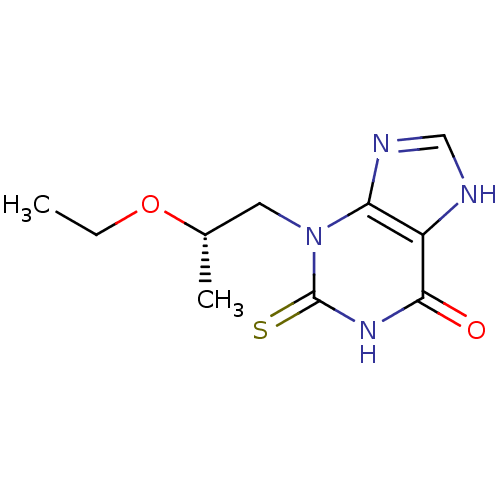 (3‐[(2S)‐2‐ethoxypropyl]‐2&...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 429 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Worldwide Research and Development | Assay Description Assay mixtures (100 µL) contained 50 mM NaPi (pH 7.4), 150 mM NaCl, 1 mM DTPA, 2% DMSO, the indicated concentrations of H2O2, and Amplex Red. Th... | Biochemistry 52: 9187-201 (2013) Article DOI: 10.1021/bi401354d BindingDB Entry DOI: 10.7270/Q2K35SBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50133600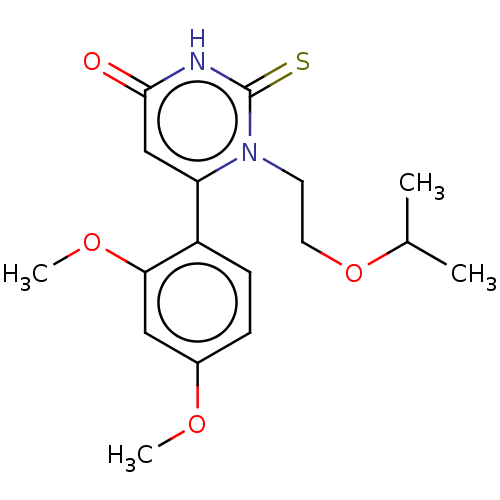 (CHEMBL3633457) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... | J Med Chem 58: 8513-28 (2015) Article DOI: 10.1021/acs.jmedchem.5b00963 BindingDB Entry DOI: 10.7270/Q2SQ926X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM119873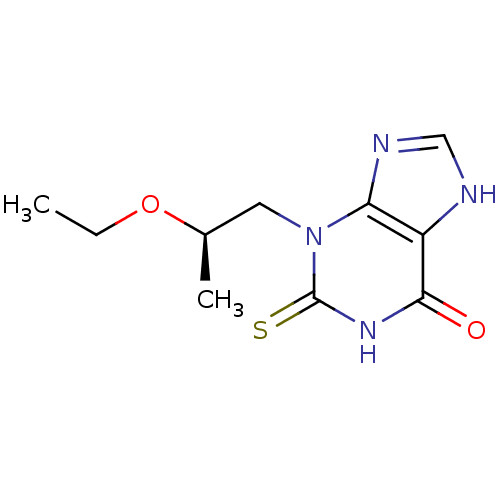 (3‐[(2R)‐2‐ethoxypropyl]‐2&...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 546 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Worldwide Research and Development | Assay Description Assay mixtures (100 µL) contained 50 mM NaPi (pH 7.4), 150 mM NaCl, 1 mM DTPA, 2% DMSO, the indicated concentrations of H2O2, and Amplex Red. Th... | Biochemistry 52: 9187-201 (2013) Article DOI: 10.1021/bi401354d BindingDB Entry DOI: 10.7270/Q2K35SBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319582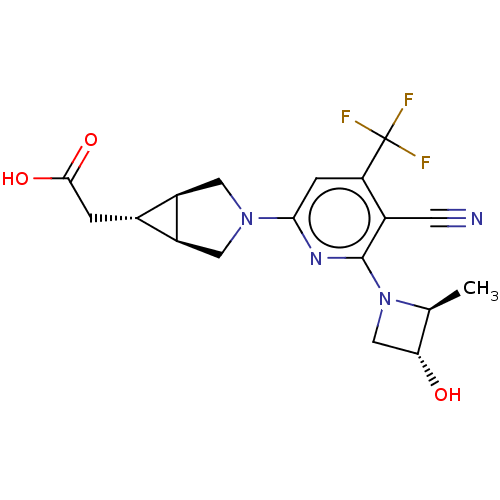 (US10174007, Example 1 | US10787438, Example 1 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 1 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319582 (US10174007, Example 1 | US10787438, Example 1 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 10 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319585 (US10174007, Example 4 | US10787438, Example 4 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 1 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM50380246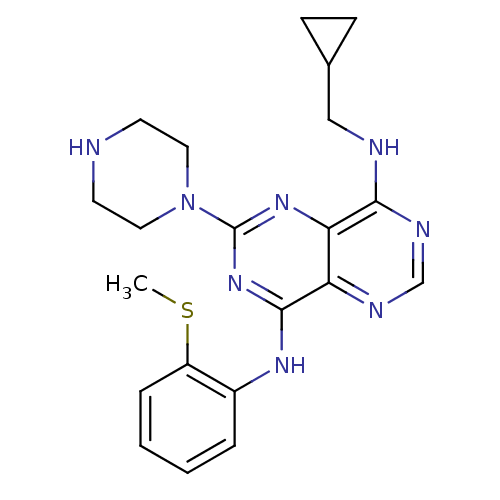 (CHEMBL2017214) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of KHK (unknown origin) using D-fructose as substrate after 60 mins in presence of ATP by LC-MS analysis | J Med Chem 60: 7835-7849 (2017) Article DOI: 10.1021/acs.jmedchem.7b00947 BindingDB Entry DOI: 10.7270/Q2H997CM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319586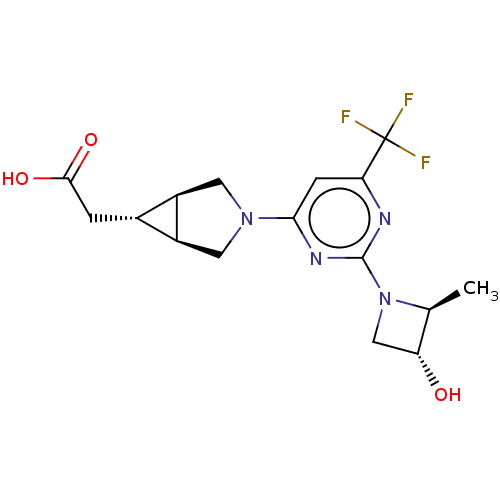 (US10174007, Example 5 | US10787438, Example 5 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 1 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319585 (US10174007, Example 4 | US10787438, Example 4 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using 8 mM fructose as substrate preincubated for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM50548426 (CHEMBL4760155) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 10 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319585 (US10174007, Example 4 | US10787438, Example 4 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His-tagged KHKA expressed in Escherichia coli BL21 (DE3) using 8 mM fructose as substrate preincubated for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Rattus norvegicus) | BDBM319585 (US10174007, Example 4 | US10787438, Example 4 | US...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant rat N-terminal His-tagged KHK expressed in Escherichia coli BL21 (DE3) using 8 mM fructose as substrate preincubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Rattus norvegicus) | BDBM50241178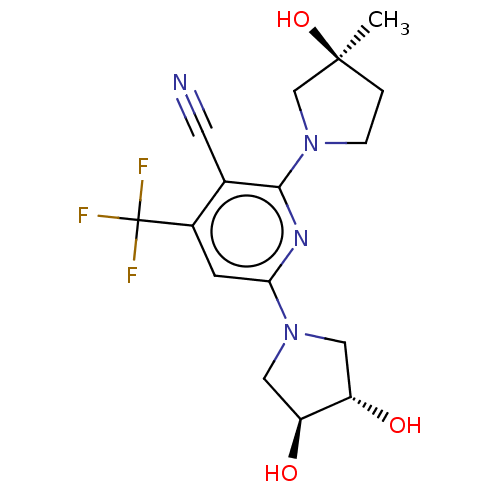 (CHEMBL4070442) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant rat N-terminal His-tagged KHK expressed in Escherichia coli BL21(DE3) using fructose as substrate incubated for 30 mins fol... | J Med Chem 60: 7835-7849 (2017) Article DOI: 10.1021/acs.jmedchem.7b00947 BindingDB Entry DOI: 10.7270/Q2H997CM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ketohexokinase (Rattus norvegicus) | BDBM50241178 (CHEMBL4070442) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant rat N-terminal His-tagged KHK expressed in Escherichia coli BL21(DE3) using fructose as substrate incubated for 30 mins fol... | J Med Chem 60: 7835-7849 (2017) Article DOI: 10.1021/acs.jmedchem.7b00947 BindingDB Entry DOI: 10.7270/Q2H997CM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM50241178 (CHEMBL4070442) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 389 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged KHK-A expressed in Escherichia coli BL21(DE3) using fructose as substrate incubated for 30 mins... | J Med Chem 60: 7835-7849 (2017) Article DOI: 10.1021/acs.jmedchem.7b00947 BindingDB Entry DOI: 10.7270/Q2H997CM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM50548423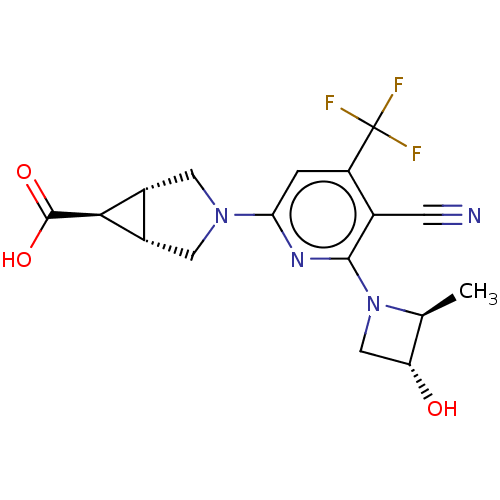 (CHEMBL4793621) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 10 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM50241178 (CHEMBL4070442) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged KHK-A expressed in Escherichia coli BL21(DE3) using fructose as substrate incubated for 30 mins... | J Med Chem 60: 7835-7849 (2017) Article DOI: 10.1021/acs.jmedchem.7b00947 BindingDB Entry DOI: 10.7270/Q2H997CM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM50548424 (CHEMBL4762437) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 10 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM50241178 (CHEMBL4070442) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 10 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM50241178 (CHEMBL4070442) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged KHK-C expressed in Escherichia coli BL21(DE3) using fructose as substrate incubated for 30 mins... | J Med Chem 60: 7835-7849 (2017) Article DOI: 10.1021/acs.jmedchem.7b00947 BindingDB Entry DOI: 10.7270/Q2H997CM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM50241178 (CHEMBL4070442) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged KHK-C expressed in Escherichia coli BL21(DE3) using fructose as substrate incubated for 30 mins... | J Med Chem 60: 7835-7849 (2017) Article DOI: 10.1021/acs.jmedchem.7b00947 BindingDB Entry DOI: 10.7270/Q2H997CM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM50548425 (CHEMBL4798024) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 10 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50133602 (CHEMBL3633250) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of MPO in LPS-stimulated human whole blood after 4 hrs by Amplex Red/H2O2-based fluorescence plate reader analysis | J Med Chem 58: 8513-28 (2015) Article DOI: 10.1021/acs.jmedchem.5b00963 BindingDB Entry DOI: 10.7270/Q2SQ926X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM50349755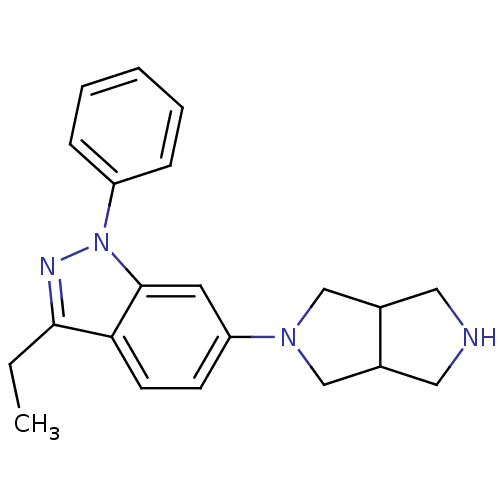 (CHEMBL1809182 | US8822447, 50 | US9771375, Example...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of KHK (unknown origin) using D-fructose as substrate after 60 mins in presence of ATP by LC-MS analysis | J Med Chem 60: 7835-7849 (2017) Article DOI: 10.1021/acs.jmedchem.7b00947 BindingDB Entry DOI: 10.7270/Q2H997CM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50133604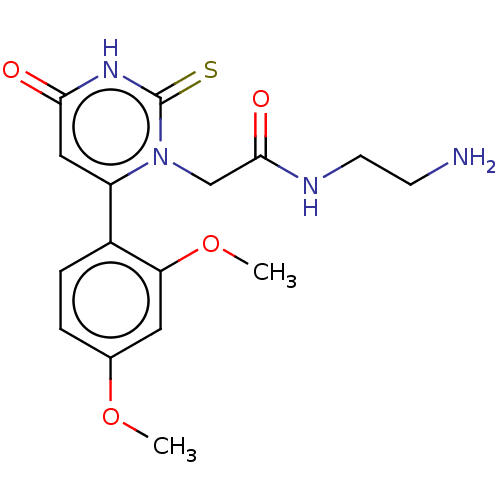 (CHEMBL3633249) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of MPO in LPS-stimulated human whole blood after 4 hrs by Amplex Red/H2O2-based fluorescence plate reader analysis | J Med Chem 58: 8513-28 (2015) Article DOI: 10.1021/acs.jmedchem.5b00963 BindingDB Entry DOI: 10.7270/Q2SQ926X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Rattus norvegicus) | BDBM50241195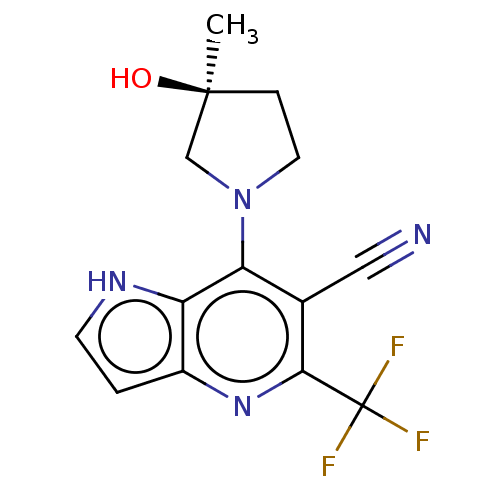 (CHEMBL4063606) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant rat N-terminal His-tagged KHK expressed in Escherichia coli BL21(DE3) using fructose as substrate incubated for 30 mins fol... | J Med Chem 60: 7835-7849 (2017) Article DOI: 10.1021/acs.jmedchem.7b00947 BindingDB Entry DOI: 10.7270/Q2H997CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Rattus norvegicus) | BDBM50241195 (CHEMBL4063606) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 646 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant rat N-terminal His-tagged KHK expressed in Escherichia coli BL21(DE3) using fructose as substrate incubated for 30 mins fol... | J Med Chem 60: 7835-7849 (2017) Article DOI: 10.1021/acs.jmedchem.7b00947 BindingDB Entry DOI: 10.7270/Q2H997CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM50241195 (CHEMBL4063606) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged KHK expressed in Escherichia coli BL21(DE3) using fructose as substrate incubated for 30 mins f... | J Med Chem 60: 7835-7849 (2017) Article DOI: 10.1021/acs.jmedchem.7b00947 BindingDB Entry DOI: 10.7270/Q2H997CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM50241195 (CHEMBL4063606) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 676 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged KHK expressed in Escherichia coli BL21(DE3) using fructose as substrate incubated for 30 mins f... | J Med Chem 60: 7835-7849 (2017) Article DOI: 10.1021/acs.jmedchem.7b00947 BindingDB Entry DOI: 10.7270/Q2H997CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM50241197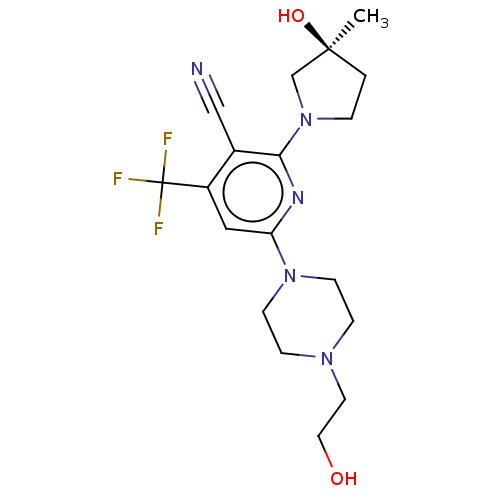 (CHEMBL4072209) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged KHK expressed in Escherichia coli BL21(DE3) using fructose as substrate incubated for 30 mins f... | J Med Chem 60: 7835-7849 (2017) Article DOI: 10.1021/acs.jmedchem.7b00947 BindingDB Entry DOI: 10.7270/Q2H997CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM50241209 (CHEMBL4101660) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged KHK expressed in Escherichia coli BL21(DE3) using fructose as substrate incubated for 30 mins f... | J Med Chem 60: 7835-7849 (2017) Article DOI: 10.1021/acs.jmedchem.7b00947 BindingDB Entry DOI: 10.7270/Q2H997CM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50133596 (CHEMBL3633459) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of MPO in LPS-stimulated human whole blood after 4 hrs by Amplex Red/H2O2-based fluorescence plate reader analysis | J Med Chem 58: 8513-28 (2015) Article DOI: 10.1021/acs.jmedchem.5b00963 BindingDB Entry DOI: 10.7270/Q2SQ926X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM50241209 (CHEMBL4101660) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged KHK expressed in Escherichia coli BL21(DE3) using fructose as substrate incubated for 30 mins f... | J Med Chem 60: 7835-7849 (2017) Article DOI: 10.1021/acs.jmedchem.7b00947 BindingDB Entry DOI: 10.7270/Q2H997CM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50133595 (CHEMBL3633460) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of MPO in LPS-stimulated human whole blood after 4 hrs by Amplex Red/H2O2-based fluorescence plate reader analysis | J Med Chem 58: 8513-28 (2015) Article DOI: 10.1021/acs.jmedchem.5b00963 BindingDB Entry DOI: 10.7270/Q2SQ926X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50193079 (CHEBI:5834 | CHEMBL1237210) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of dopamine transporter (unknown origin) assessed as suppression of synaptosomal uptake of dopamine | J Nat Prod 79: 1538-47 (2016) Article DOI: 10.1021/acs.jnatprod.5b01063 BindingDB Entry DOI: 10.7270/Q2F76FG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50193394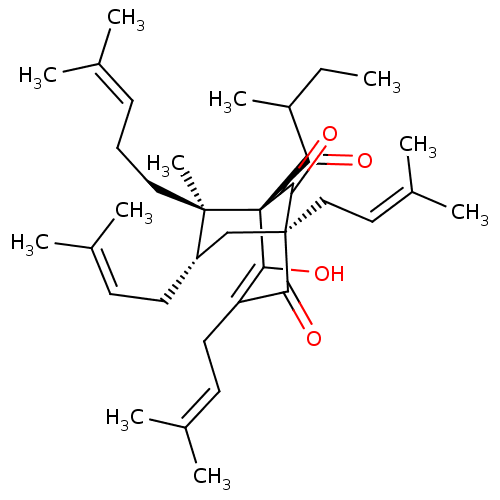 (CHEMBL536352) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of norepinephrine transporter (unknown origin) assessed as suppression of synaptosomal uptake of norepinephrine | J Nat Prod 79: 1538-47 (2016) Article DOI: 10.1021/acs.jnatprod.5b01063 BindingDB Entry DOI: 10.7270/Q2F76FG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50193079 (CHEBI:5834 | CHEMBL1237210) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of norepinephrine transporter (unknown origin) assessed as suppression of synaptosomal uptake of norepinephrine | J Nat Prod 79: 1538-47 (2016) Article DOI: 10.1021/acs.jnatprod.5b01063 BindingDB Entry DOI: 10.7270/Q2F76FG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50193394 (CHEMBL536352) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of serotonin transporter (unknown origin) assessed as suppression of synaptosomal uptake of serotonin | J Nat Prod 79: 1538-47 (2016) Article DOI: 10.1021/acs.jnatprod.5b01063 BindingDB Entry DOI: 10.7270/Q2F76FG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50193079 (CHEBI:5834 | CHEMBL1237210) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of serotonin transporter (unknown origin) assessed as suppression of synaptosomal uptake of serotonin | J Nat Prod 79: 1538-47 (2016) Article DOI: 10.1021/acs.jnatprod.5b01063 BindingDB Entry DOI: 10.7270/Q2F76FG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 402 total ) | Next | Last >> |