

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50033532 (CHEMBL435380 | N-[(3S,4R)-1-((R)-2-Hydroxy-2-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 using [3H]DAMGO as radioligand. | J Med Chem 38: 1547-57 (1995) BindingDB Entry DOI: 10.7270/Q2GQ6ZDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50033536 (CHEMBL121494 | N-[(3R,4R)-1-((R)-2-Hydroxy-2-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 using [3H]DAMGO as radioligand. | J Med Chem 38: 1547-57 (1995) BindingDB Entry DOI: 10.7270/Q2GQ6ZDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50102711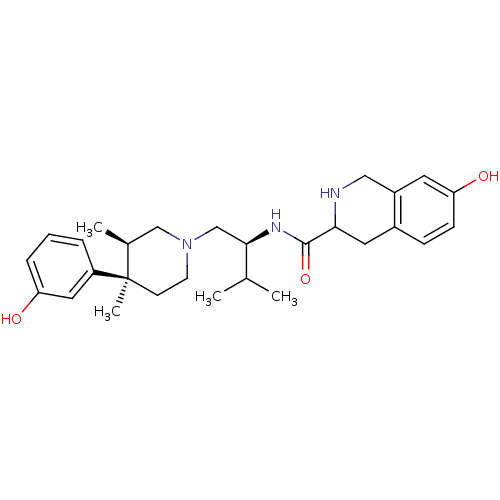 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Antagonist activity on agonist (U50,488) stimulated [35S]GTP-gamma-S, binding in cloned opioid receptor kappa 1 | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50064515 (3-[(3R,4R)-3,4-Dimethyl-1-((E)-3-o-tolyl-allyl)-pi...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding to Opioid receptor mu 1 in Guinea pig caudate st... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50033535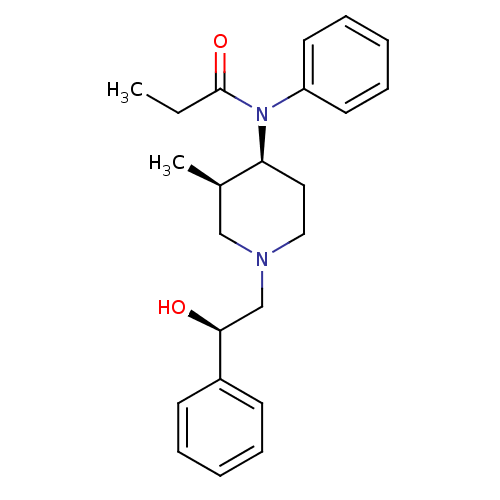 (CHEMBL331883 | N-[(3R,4S)-1-((R)-2-Hydroxy-2-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 using [3H]DAMGO as radioligand. | J Med Chem 38: 1547-57 (1995) BindingDB Entry DOI: 10.7270/Q2GQ6ZDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50034623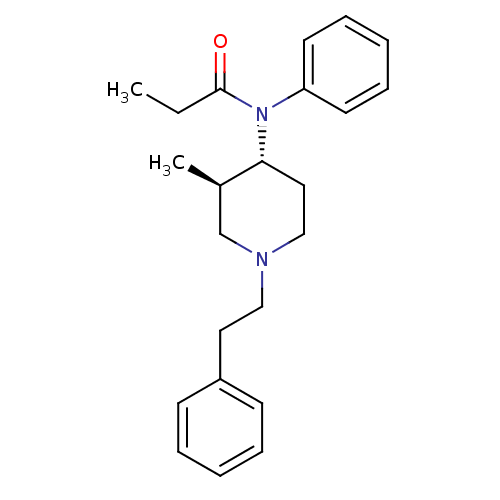 (CHEMBL38685 | N-((3R,4R)-3-Methyl-1-phenethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 using [3H]DAMGO as radioligand. | J Med Chem 38: 1547-57 (1995) BindingDB Entry DOI: 10.7270/Q2GQ6ZDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50102711 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of stimulation of [35S]GTP-gamma-S, binding produced by the selective agonist (U69593, kappa-receptor), in guinea pig caudate membranes. | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding to Opioid receptor mu 1 in Guinea pig caudate st... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50064518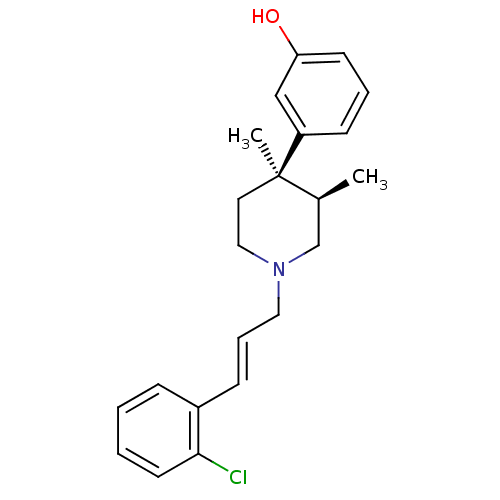 (3-{(3R,4R)-1-[(E)-3-(2-Chloro-phenyl)-allyl]-3,4-d...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding to Opioid receptor mu 1 in Guinea pig caudate st... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50370067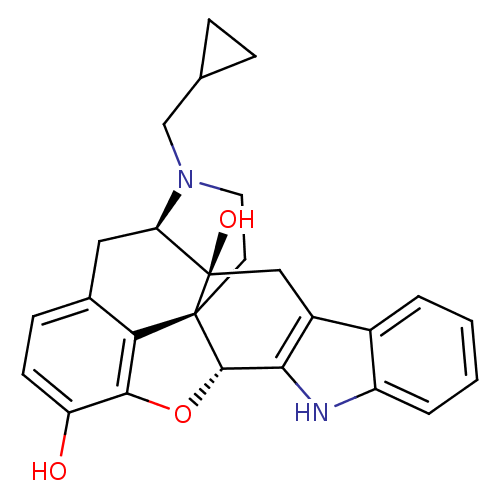 (CHEMBL1237164) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity of compound on agonist stimulated [35S]GTP-gamma-S binding on delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of stimulation of [35S]GTP-gamma-S, binding produced by the selective agonist (U69593, kappa-receptor), in guinea pig caudate membranes | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor kappa 1 in Guinea pig Caudate stimulated by U69,593 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50064521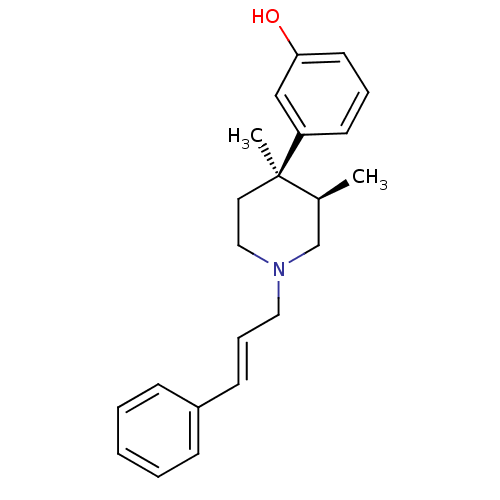 (3-[(3R,4R)-3,4-Dimethyl-1-((E)-3-phenyl-allyl)-pip...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding to Opioid receptor mu 1 in Guinea pig caudate st... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50291970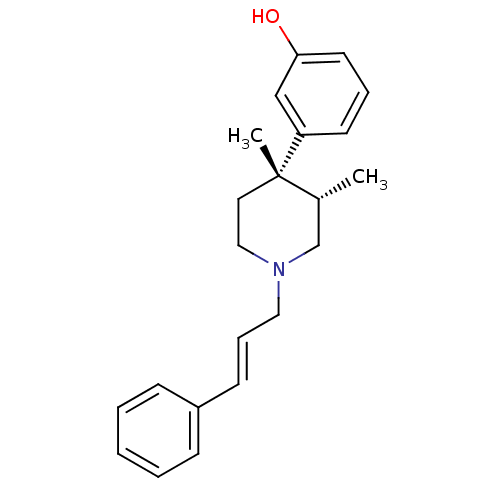 (3-[(3S,4S)-3,4-Dimethyl-1-((E)-3-phenyl-allyl)-pip...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor mu 1 in Guinea pig Caudate stimulated by DAMGO | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50026614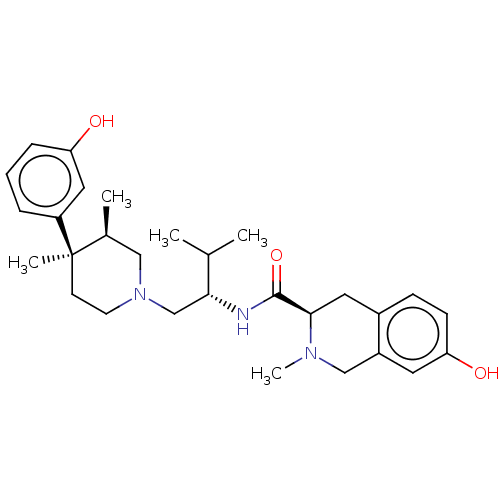 (CHEMBL575508) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]U-69593 binding to Opioid receptor kappa 1 of guinea pig brain | J Med Chem 46: 3127-37 (2003) Article DOI: 10.1021/jm030094y BindingDB Entry DOI: 10.7270/Q2319WM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4779 (CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis | J Med Chem 59: 2005-24 (2016) Article DOI: 10.1021/acs.jmedchem.5b01633 BindingDB Entry DOI: 10.7270/Q2KS6TDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50064515 (3-[(3R,4R)-3,4-Dimethyl-1-((E)-3-o-tolyl-allyl)-pi...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding in Guinea pig caudate stimulated by U69,593 to O... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50027039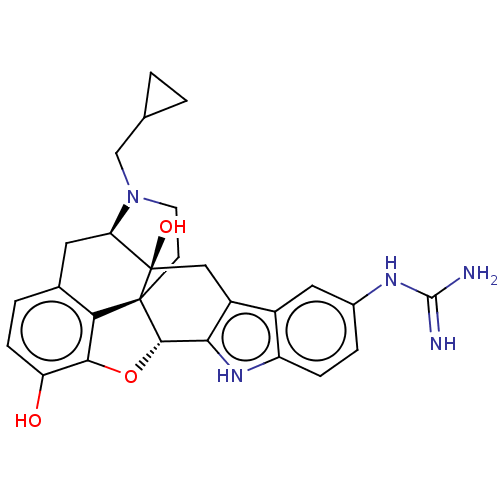 (5''-Guanidinonaltrindole | 5''-Guanidinylnaltrindo...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]U-69593 binding to Opioid receptor kappa 1 of guinea pig brain | J Med Chem 46: 3127-37 (2003) Article DOI: 10.1021/jm030094y BindingDB Entry DOI: 10.7270/Q2319WM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50596723 (CHEMBL5205903 | US20230348421, Compound 59) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114246 BindingDB Entry DOI: 10.7270/Q20P142F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50370067 (CHEMBL1237164) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DADLE at delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from morphine-insensitive Opioid receptor delta 1 (presence of 50 nM morphine) of rat brain membranes | J Med Chem 41: 2872-81 (1998) Article DOI: 10.1021/jm980083i BindingDB Entry DOI: 10.7270/Q24F1RFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor kappa 1 in Guinea pig brain membranes using radioligand [3H]U-69593 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from Opioid receptor delta 1 of rat brain membranes (DAMGO quenching mu receptor) | J Med Chem 41: 2872-81 (1998) Article DOI: 10.1021/jm980083i BindingDB Entry DOI: 10.7270/Q24F1RFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.312 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding in Guinea pig caudate stimulated by SMC-80 (Opio... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards human cloned Opioid receptor kappa 1 using [3H]U-69593 | J Med Chem 46: 3127-37 (2003) Article DOI: 10.1021/jm030094y BindingDB Entry DOI: 10.7270/Q2319WM4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to Opioid receptor mu 1 of rat brain membranes | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]U-69593 binding to Opioid receptor kappa 1 of guinea pig brain | J Med Chem 46: 3127-37 (2003) Article DOI: 10.1021/jm030094y BindingDB Entry DOI: 10.7270/Q2319WM4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50102711 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity to opioid receptor kappa 1 of guinea pig brain, using [3H]U-69593 as radioligand | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding in Guinea pig caudate stimulated by U69,593 to O... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50064515 (3-[(3R,4R)-3,4-Dimethyl-1-((E)-3-o-tolyl-allyl)-pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding in Guinea pig caudate stimulated by SMC-80 (Opio... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM50562182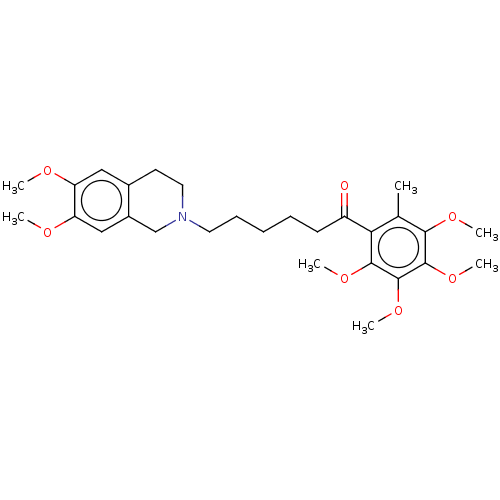 (CHEMBL4744466) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-RHM-4 from sigma 2 receptor in rat liver membranes after 120 mins by microbeta scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112906 BindingDB Entry DOI: 10.7270/Q23T9MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398759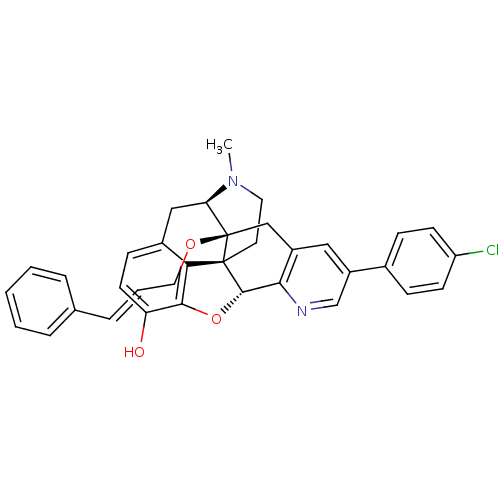 (CHEMBL2179655) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor delta 1 was determined by inhibition of binding of [3H]DADLE (1.3-2.0 nM) to rat brain membranes | J Med Chem 42: 3527-38 (1999) Article DOI: 10.1021/jm990039i BindingDB Entry DOI: 10.7270/Q2QR4XSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.430 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee Health Science Center | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Bioorg Med Chem 14: 6525-38 (2006) Article DOI: 10.1016/j.bmc.2006.06.019 BindingDB Entry DOI: 10.7270/Q2WQ022N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50065862 (22-cyclopropylmethyl-9-phenyl-14-oxa-11,22-diazahe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from morphine-insensitive Opioid receptor delta 1 (presence of 50 nM morphine) of rat brain membranes | J Med Chem 41: 2872-81 (1998) Article DOI: 10.1021/jm980083i BindingDB Entry DOI: 10.7270/Q24F1RFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50033537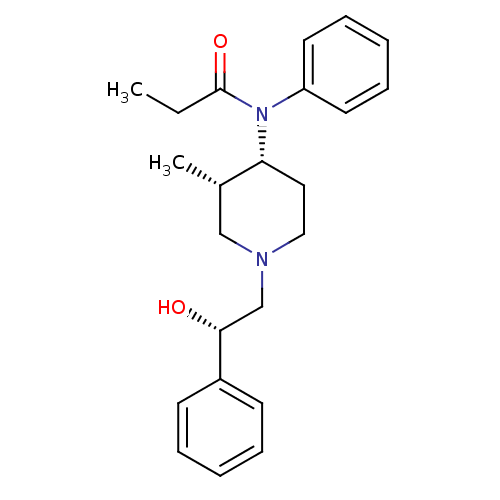 (CHEMBL121060 | N-[(3S,4R)-1-((S)-2-Hydroxy-2-pheny...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 using [3H]U-69593 as radioligand. | J Med Chem 38: 1547-57 (1995) BindingDB Entry DOI: 10.7270/Q2GQ6ZDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50454092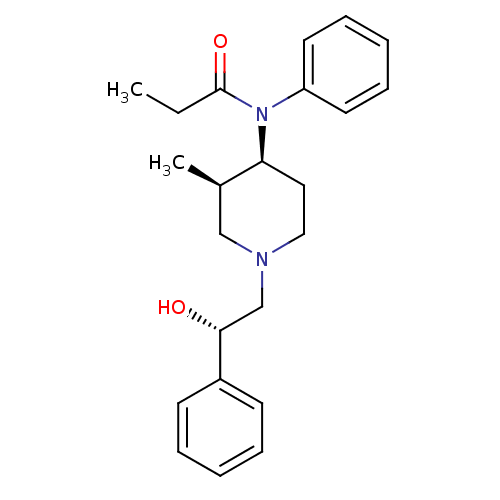 (CHEMBL2373185) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 using [3H]U-69593 as radioligand. | J Med Chem 38: 1547-57 (1995) BindingDB Entry DOI: 10.7270/Q2GQ6ZDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398758 (CHEMBL2179656) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity was determined as ability to displace [3H]-U-69, radioligand from Opioid receptor kappa 1 | Bioorg Med Chem Lett 8: 3149-52 (1999) BindingDB Entry DOI: 10.7270/Q2S75FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50064518 (3-{(3R,4R)-1-[(E)-3-(2-Chloro-phenyl)-allyl]-3,4-d...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.567 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding in Guinea pig caudate stimulated by U69,593 to O... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50366779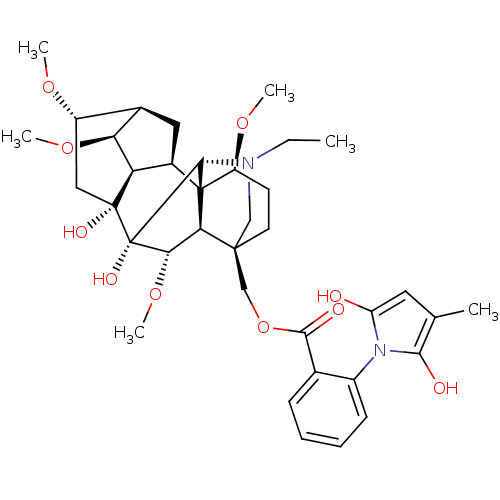 (METHYLLYCACONITINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity against alpha7 nAChR (nicotinic acetylcholine receptor) using [3H]-MLA as a radioligand relative to alpha4-beta2 | J Med Chem 43: 142-5 (2000) Article DOI: 10.1021/jm990544f BindingDB Entry DOI: 10.7270/Q2G163KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18681 ((2R)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee Health Science Center | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Bioorg Med Chem 14: 6525-38 (2006) Article DOI: 10.1016/j.bmc.2006.06.019 BindingDB Entry DOI: 10.7270/Q2WQ022N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50065854 (22-cyclopropylmethyl-9-phenoxy-14-oxa-11,22-diazah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from morphine-insensitive Opioid receptor delta 1 (presence of 50 nM morphine) of rat brain membranes | J Med Chem 41: 2872-81 (1998) Article DOI: 10.1021/jm980083i BindingDB Entry DOI: 10.7270/Q24F1RFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50027052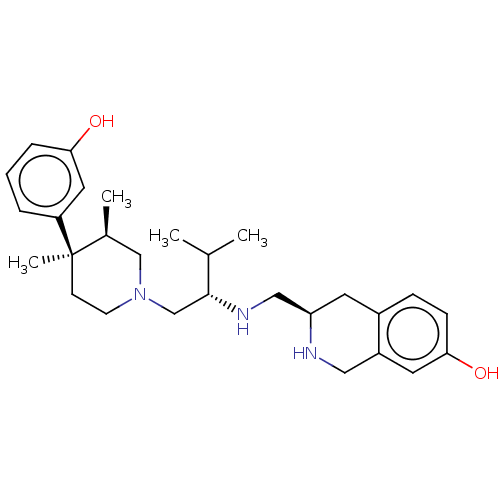 (CHEMBL2112473) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]U-69593 binding to Opioid receptor kappa 1 of guinea pig brain | J Med Chem 46: 3127-37 (2003) Article DOI: 10.1021/jm030094y BindingDB Entry DOI: 10.7270/Q2319WM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398760 (CHEMBL2179652) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50098674 ((E)-17-(2-butenyl)-6,7-dehydro-4,5alpha-epoxy-3,14...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity of compound on agonist stimulated [35S]GTP-gamma-S binding on delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50065854 (22-cyclopropylmethyl-9-phenoxy-14-oxa-11,22-diazah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from Opioid receptor delta 1 of rat brain membranes (DAMGO quenching mu receptor) | J Med Chem 41: 2872-81 (1998) Article DOI: 10.1021/jm980083i BindingDB Entry DOI: 10.7270/Q24F1RFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50064521 (3-[(3R,4R)-3,4-Dimethyl-1-((E)-3-phenyl-allyl)-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to Opioid receptor mu 1 of rat brain membranes | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50291970 (3-[(3S,4S)-3,4-Dimethyl-1-((E)-3-phenyl-allyl)-pip...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor mu 1 in Guinea pig brain membranes using radioligand [3H]DAMGO | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123273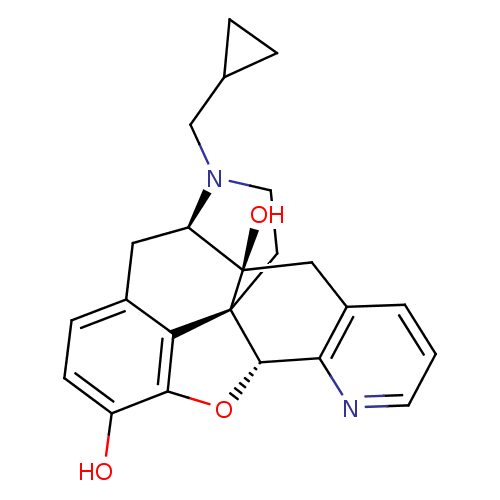 (19-cyclopropylmethyl-(2S,10R)-11-oxa-8,19-diazahex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor delta 1 was determined by inhibition of binding of [3H]DADLE (1.3-2.0 nM) to rat brain membranes | J Med Chem 42: 3527-38 (1999) Article DOI: 10.1021/jm990039i BindingDB Entry DOI: 10.7270/Q2QR4XSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 9590 total ) | Next | Last >> |