 Found 386 hits with Last Name = 'xu' and Initial = 'yg'
Found 386 hits with Last Name = 'xu' and Initial = 'yg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50112086

(3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...)Show SMILES Cn1c(CNc2ccc(cc2)C(N)=N)nc2cc(ccc12)C(=O)N(CCC(O)=O)c1ccccn1 Show InChI InChI=1S/C25H25N7O3/c1-31-20-10-7-17(25(35)32(13-11-23(33)34)21-4-2-3-12-28-21)14-19(20)30-22(31)15-29-18-8-5-16(6-9-18)24(26)27/h2-10,12,14,29H,11,13,15H2,1H3,(H3,26,27)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Eur J Med Chem 57: 21-8 (2012)
Article DOI: 10.1016/j.ejmech.2012.09.016
BindingDB Entry DOI: 10.7270/Q2D50P1S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50432209
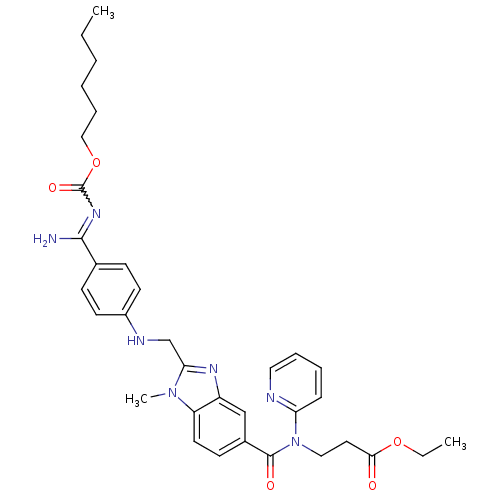
(BIBR-1048 | BIBR-1048-BS-RS1 | DABIGATRAN ETEXILAT...)Show SMILES CCCCCCOC(=O)N=C(N)c1ccc(NCc2nc3cc(ccc3n2C)C(=O)N(CCC(=O)OCC)c2ccccn2)cc1 |w:9.8| Show InChI InChI=1S/C34H41N7O5/c1-4-6-7-10-21-46-34(44)39-32(35)24-12-15-26(16-13-24)37-23-30-38-27-22-25(14-17-28(27)40(30)3)33(43)41(20-18-31(42)45-5-2)29-11-8-9-19-36-29/h8-9,11-17,19,22,37H,4-7,10,18,20-21,23H2,1-3H3,(H2,35,39,44) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 23: 2089-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.126
BindingDB Entry DOI: 10.7270/Q2HH6MF0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50353115
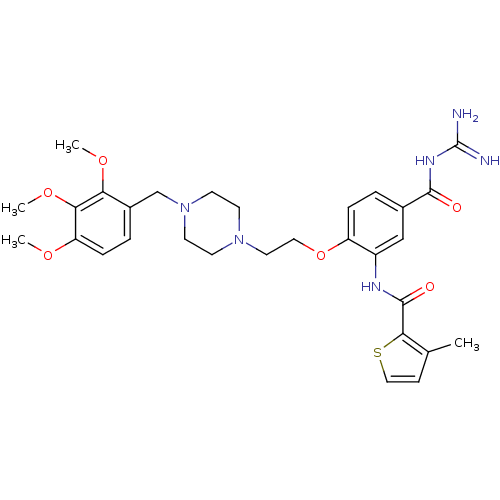
(CHEMBL1829127)Show SMILES COc1ccc(CN2CCN(CCOc3ccc(cc3NC(=O)c3sccc3C)C(=O)NC(N)=N)CC2)c(OC)c1OC Show InChI InChI=1S/C30H38N6O6S/c1-19-9-16-43-27(19)29(38)33-22-17-20(28(37)34-30(31)32)5-7-23(22)42-15-14-35-10-12-36(13-11-35)18-21-6-8-24(39-2)26(41-4)25(21)40-3/h5-9,16-17H,10-15,18H2,1-4H3,(H,33,38)(H4,31,32,34,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat assessed as inhibition of acid-induced platelet swelling by spectrophotometric analysis |
Eur J Med Chem 46: 4107-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.06.011
BindingDB Entry DOI: 10.7270/Q27081T0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50353110
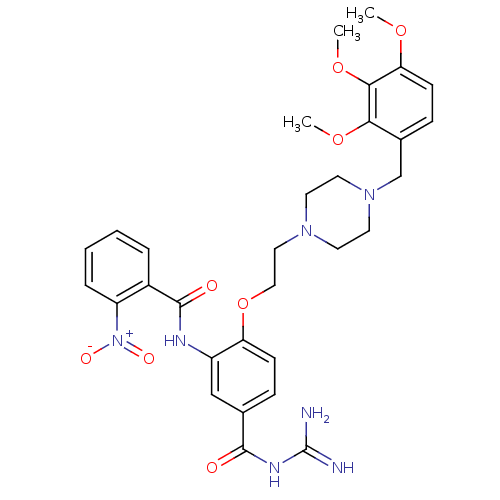
(CHEMBL1829122)Show SMILES COc1ccc(CN2CCN(CCOc3ccc(cc3NC(=O)c3ccccc3[N+]([O-])=O)C(=O)NC(N)=N)CC2)c(OC)c1OC Show InChI InChI=1S/C31H37N7O8/c1-43-26-11-9-21(27(44-2)28(26)45-3)19-37-14-12-36(13-15-37)16-17-46-25-10-8-20(29(39)35-31(32)33)18-23(25)34-30(40)22-6-4-5-7-24(22)38(41)42/h4-11,18H,12-17,19H2,1-3H3,(H,34,40)(H4,32,33,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat assessed as inhibition of acid-induced platelet swelling by spectrophotometric analysis |
Eur J Med Chem 46: 4107-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.06.011
BindingDB Entry DOI: 10.7270/Q27081T0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50353113
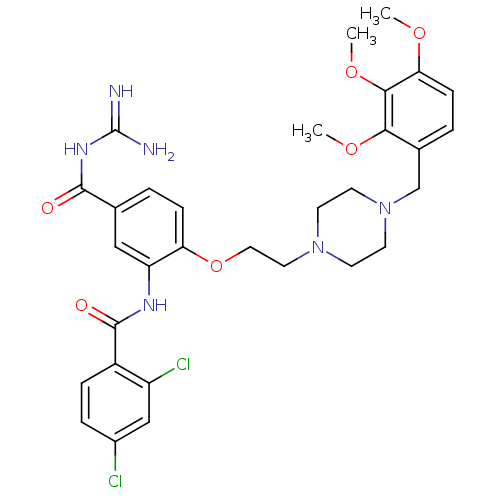
(CHEMBL1829125)Show SMILES COc1ccc(CN2CCN(CCOc3ccc(cc3NC(=O)c3ccc(Cl)cc3Cl)C(=O)NC(N)=N)CC2)c(OC)c1OC Show InChI InChI=1S/C31H36Cl2N6O6/c1-42-26-9-5-20(27(43-2)28(26)44-3)18-39-12-10-38(11-13-39)14-15-45-25-8-4-19(29(40)37-31(34)35)16-24(25)36-30(41)22-7-6-21(32)17-23(22)33/h4-9,16-17H,10-15,18H2,1-3H3,(H,36,41)(H4,34,35,37,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat assessed as inhibition of acid-induced platelet swelling by spectrophotometric analysis |
Eur J Med Chem 46: 4107-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.06.011
BindingDB Entry DOI: 10.7270/Q27081T0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50353114
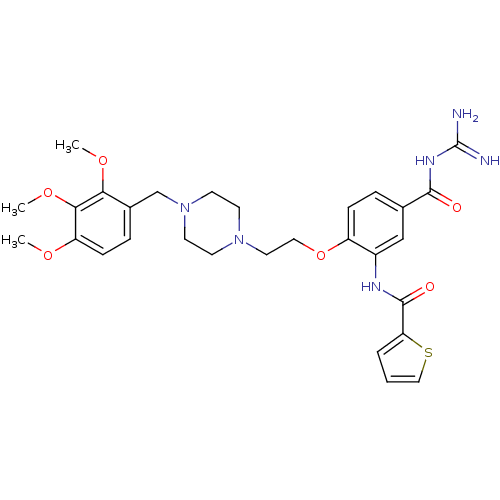
(CHEMBL1829126)Show SMILES COc1ccc(CN2CCN(CCOc3ccc(cc3NC(=O)c3cccs3)C(=O)NC(N)=N)CC2)c(OC)c1OC Show InChI InChI=1S/C29H36N6O6S/c1-38-23-9-7-20(25(39-2)26(23)40-3)18-35-12-10-34(11-13-35)14-15-41-22-8-6-19(27(36)33-29(30)31)17-21(22)32-28(37)24-5-4-16-42-24/h4-9,16-17H,10-15,18H2,1-3H3,(H,32,37)(H4,30,31,33,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat assessed as inhibition of acid-induced platelet swelling by spectrophotometric analysis |
Eur J Med Chem 46: 4107-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.06.011
BindingDB Entry DOI: 10.7270/Q27081T0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50353111
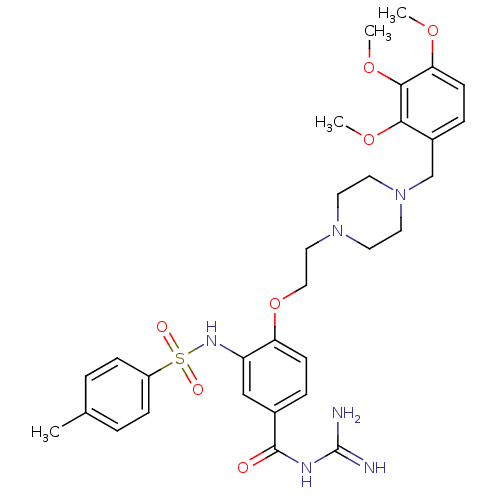
(CHEMBL1829123)Show SMILES COc1ccc(CN2CCN(CCOc3ccc(cc3NS(=O)(=O)c3ccc(C)cc3)C(=O)NC(N)=N)CC2)c(OC)c1OC Show InChI InChI=1S/C31H40N6O7S/c1-21-5-9-24(10-6-21)45(39,40)35-25-19-22(30(38)34-31(32)33)7-11-26(25)44-18-17-36-13-15-37(16-14-36)20-23-8-12-27(41-2)29(43-4)28(23)42-3/h5-12,19,35H,13-18,20H2,1-4H3,(H4,32,33,34,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat assessed as inhibition of acid-induced platelet swelling by spectrophotometric analysis |
Eur J Med Chem 46: 4107-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.06.011
BindingDB Entry DOI: 10.7270/Q27081T0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50353097
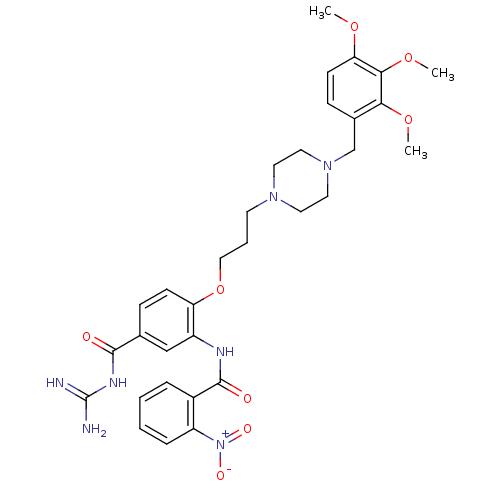
(CHEMBL1829132)Show SMILES COc1ccc(CN2CCN(CCCOc3ccc(cc3NC(=O)c3ccccc3[N+]([O-])=O)C(=O)NC(N)=N)CC2)c(OC)c1OC Show InChI InChI=1S/C32H39N7O8/c1-44-27-12-10-22(28(45-2)29(27)46-3)20-38-16-14-37(15-17-38)13-6-18-47-26-11-9-21(30(40)36-32(33)34)19-24(26)35-31(41)23-7-4-5-8-25(23)39(42)43/h4-5,7-12,19H,6,13-18,20H2,1-3H3,(H,35,41)(H4,33,34,36,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat assessed as inhibition of acid-induced platelet swelling by spectrophotometric analysis |
Eur J Med Chem 46: 4107-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.06.011
BindingDB Entry DOI: 10.7270/Q27081T0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50353116
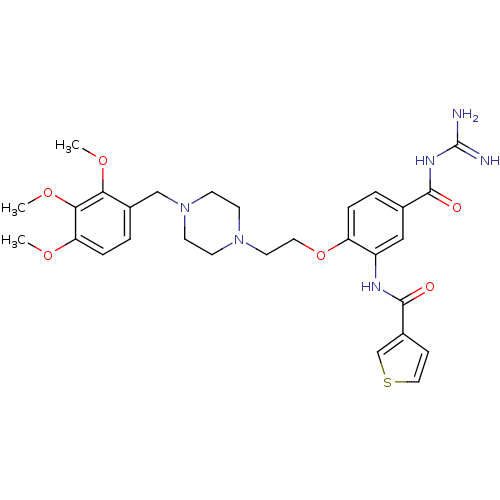
(CHEMBL1829128)Show SMILES COc1ccc(CN2CCN(CCOc3ccc(cc3NC(=O)c3ccsc3)C(=O)NC(N)=N)CC2)c(OC)c1OC Show InChI InChI=1S/C29H36N6O6S/c1-38-24-7-5-20(25(39-2)26(24)40-3)17-35-11-9-34(10-12-35)13-14-41-23-6-4-19(27(36)33-29(30)31)16-22(23)32-28(37)21-8-15-42-18-21/h4-8,15-16,18H,9-14,17H2,1-3H3,(H,32,37)(H4,30,31,33,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat assessed as inhibition of acid-induced platelet swelling by spectrophotometric analysis |
Eur J Med Chem 46: 4107-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.06.011
BindingDB Entry DOI: 10.7270/Q27081T0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50353098
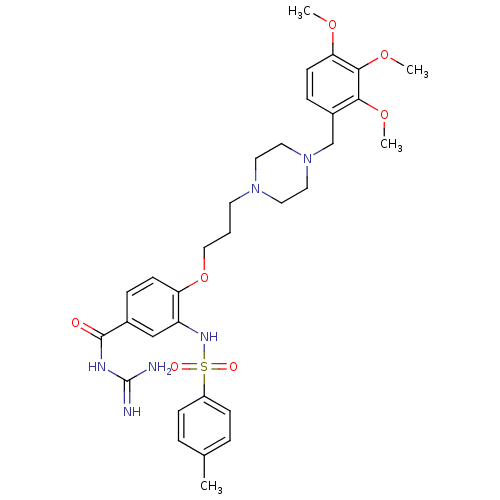
(CHEMBL1829133)Show SMILES COc1ccc(CN2CCN(CCCOc3ccc(cc3NS(=O)(=O)c3ccc(C)cc3)C(=O)NC(N)=N)CC2)c(OC)c1OC Show InChI InChI=1S/C32H42N6O7S/c1-22-6-10-25(11-7-22)46(40,41)36-26-20-23(31(39)35-32(33)34)8-12-27(26)45-19-5-14-37-15-17-38(18-16-37)21-24-9-13-28(42-2)30(44-4)29(24)43-3/h6-13,20,36H,5,14-19,21H2,1-4H3,(H4,33,34,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat assessed as inhibition of acid-induced platelet swelling by spectrophotometric analysis |
Eur J Med Chem 46: 4107-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.06.011
BindingDB Entry DOI: 10.7270/Q27081T0 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PARP1 (unknown origin) incubated for 45 mins in presence of biotinylated-NAD+ by microplate reader analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01535
BindingDB Entry DOI: 10.7270/Q22N566V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114116
BindingDB Entry DOI: 10.7270/Q2930Z6Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50353103
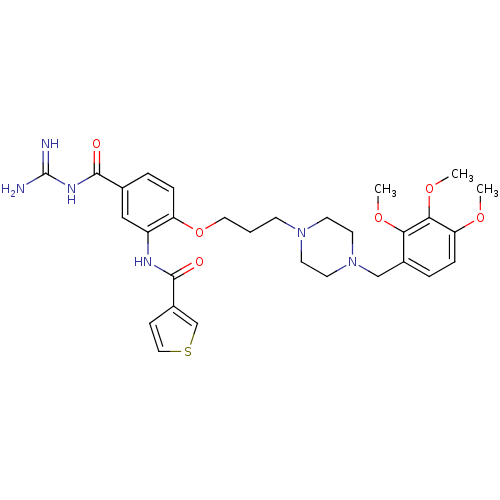
(CHEMBL1829139)Show SMILES COc1ccc(CN2CCN(CCCOc3ccc(cc3NC(=O)c3ccsc3)C(=O)NC(N)=N)CC2)c(OC)c1OC Show InChI InChI=1S/C30H38N6O6S/c1-39-25-8-6-21(26(40-2)27(25)41-3)18-36-13-11-35(12-14-36)10-4-15-42-24-7-5-20(28(37)34-30(31)32)17-23(24)33-29(38)22-9-16-43-19-22/h5-9,16-17,19H,4,10-15,18H2,1-3H3,(H,33,38)(H4,31,32,34,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat assessed as inhibition of acid-induced platelet swelling by spectrophotometric analysis |
Eur J Med Chem 46: 4107-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.06.011
BindingDB Entry DOI: 10.7270/Q27081T0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50353102
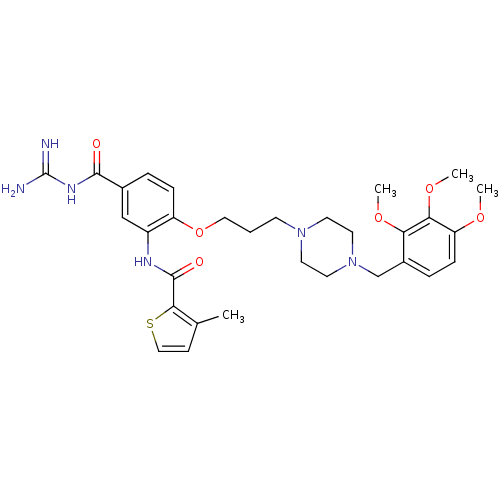
(CHEMBL1829138)Show SMILES COc1ccc(CN2CCN(CCCOc3ccc(cc3NC(=O)c3sccc3C)C(=O)NC(N)=N)CC2)c(OC)c1OC Show InChI InChI=1S/C31H40N6O6S/c1-20-10-17-44-28(20)30(39)34-23-18-21(29(38)35-31(32)33)6-8-24(23)43-16-5-11-36-12-14-37(15-13-36)19-22-7-9-25(40-2)27(42-4)26(22)41-3/h6-10,17-18H,5,11-16,19H2,1-4H3,(H,34,39)(H4,32,33,35,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat assessed as inhibition of acid-induced platelet swelling by spectrophotometric analysis |
Eur J Med Chem 46: 4107-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.06.011
BindingDB Entry DOI: 10.7270/Q27081T0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50378116
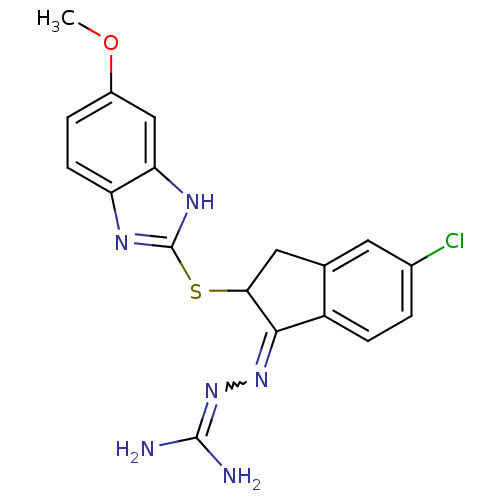
(CHEMBL1202327)Show SMILES COc1ccc2nc(SC3Cc4cc(Cl)ccc4C3=NN=C(N)N)[nH]c2c1 |w:19.21,(-3.73,2.77,;-3.72,1.54,;-2.38,.77,;-2.38,-.77,;-1.03,-1.56,;.3,-.77,;1.76,-1.24,;2.66,.02,;4.21,.04,;4.96,1.39,;4.31,2.74,;5.42,3.8,;5.37,5.35,;6.71,6.16,;6.68,7.39,;8.06,5.42,;8.1,3.86,;6.77,3.07,;6.48,1.56,;7.53,.42,;9.03,.76,;10.08,-.38,;11.29,-.11,;9.71,-1.55,;1.76,1.24,;.3,.77,;-1.03,1.56,)| Show InChI InChI=1S/C18H17ClN6OS/c1-26-11-3-5-13-14(8-11)23-18(22-13)27-15-7-9-6-10(19)2-4-12(9)16(15)24-25-17(20)21/h2-6,8,15H,7H2,1H3,(H,22,23)(H4,20,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat platelet-rich plasma by optical swelling assay |
Eur J Med Chem 44: 3771-6 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.036
BindingDB Entry DOI: 10.7270/Q2474BS7 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50210747
(CHEMBL3968039)Show SMILES COc1cc2c(Nc3ccc(NC(=O)Nc4ccc(C)c(C)c4)c(Cl)c3)ncnc2cc1OCCCN1CCN(C)CC1 Show InChI InChI=1S/C32H38ClN7O3/c1-21-6-7-23(16-22(21)2)37-32(41)38-27-9-8-24(17-26(27)33)36-31-25-18-29(42-4)30(19-28(25)34-20-35-31)43-15-5-10-40-13-11-39(3)12-14-40/h6-9,16-20H,5,10-15H2,1-4H3,(H,34,35,36)(H2,37,38,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human EGFR cytoplasmic domain (669 to 1210 residues) expressed in baculovirus expression system preincubated for ... |
Eur J Med Chem 125: 245-254 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.039
BindingDB Entry DOI: 10.7270/Q2QJ7K9C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50210697
(CHEMBL3962233)Show SMILES COc1cc2c(Nc3ccc(NC(=O)Nc4ccc(Cl)cc4)c(Cl)c3)ncnc2cc1OCCCN1CCN(C)CC1 Show InChI InChI=1S/C30H33Cl2N7O3/c1-38-11-13-39(14-12-38)10-3-15-42-28-18-26-23(17-27(28)41-2)29(34-19-33-26)35-22-8-9-25(24(32)16-22)37-30(40)36-21-6-4-20(31)5-7-21/h4-9,16-19H,3,10-15H2,1-2H3,(H,33,34,35)(H2,36,37,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human EGFR cytoplasmic domain (669 to 1210 residues) expressed in baculovirus expression system preincubated for ... |
Eur J Med Chem 125: 245-254 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.039
BindingDB Entry DOI: 10.7270/Q2QJ7K9C |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50353105
(CHEMBL1829117)Show SMILES COc1ccc(CN2CCN(CCOc3ccc(cc3[N+]([O-])=O)C(=O)[NH+]=C(N)[NH-])CC2)c(OC)c1OC |w:25.25| Show InChI InChI=1S/C24H32N6O7/c1-34-20-7-5-17(21(35-2)22(20)36-3)15-29-10-8-28(9-11-29)12-13-37-19-6-4-16(14-18(19)30(32)33)23(31)27-24(25)26/h4-7,14H,8-13,15H2,1-3H3,(H4,25,26,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.06 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat assessed as inhibition of acid-induced platelet swelling by spectrophotometric analysis |
Eur J Med Chem 46: 4107-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.06.011
BindingDB Entry DOI: 10.7270/Q27081T0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50353099
(CHEMBL1829134)Show SMILES COc1ccc(CN2CCN(CCCOc3ccc(cc3NC(=O)c3ccc(cc3)C(F)(F)F)C(=O)NC(N)=N)CC2)c(OC)c1OC Show InChI InChI=1S/C33H39F3N6O6/c1-45-27-12-8-23(28(46-2)29(27)47-3)20-42-16-14-41(15-17-42)13-4-18-48-26-11-7-22(31(44)40-32(37)38)19-25(26)39-30(43)21-5-9-24(10-6-21)33(34,35)36/h5-12,19H,4,13-18,20H2,1-3H3,(H,39,43)(H4,37,38,40,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat assessed as inhibition of acid-induced platelet swelling by spectrophotometric analysis |
Eur J Med Chem 46: 4107-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.06.011
BindingDB Entry DOI: 10.7270/Q27081T0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50297583
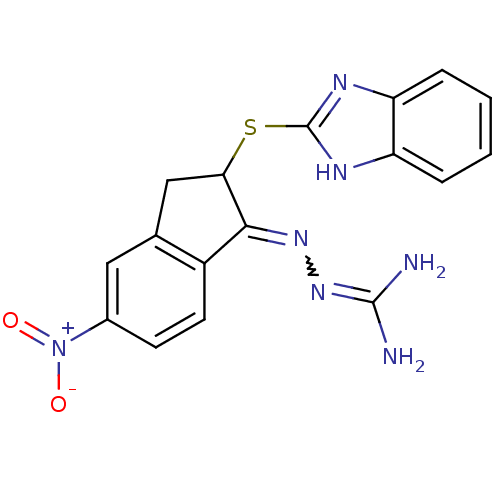
(CHEMBL560806 | N''-[2-(1H-benzimidazol-2-ylthio)-5...)Show SMILES NC(N)=NN=C1C(Cc2cc(ccc12)[N+]([O-])=O)Sc1nc2ccccc2[nH]1 |w:4.3,(23.89,-33.56,;22.38,-33.25,;21.9,-31.79,;21.36,-34.4,;19.85,-34.09,;18.83,-35.25,;17.29,-35.09,;16.68,-36.51,;17.83,-37.52,;17.84,-39.05,;19.17,-39.81,;20.5,-39.03,;20.48,-37.5,;19.16,-36.75,;19.19,-41.36,;17.86,-42.14,;20.53,-42.12,;16.51,-33.77,;14.97,-33.78,;14.05,-32.53,;12.59,-33.02,;11.25,-32.25,;9.92,-33.03,;9.92,-34.57,;11.25,-35.34,;12.6,-34.56,;14.07,-35.03,)| Show InChI InChI=1S/C17H15N7O2S/c18-16(19)23-22-15-11-6-5-10(24(25)26)7-9(11)8-14(15)27-17-20-12-3-1-2-4-13(12)21-17/h1-7,14H,8H2,(H,20,21)(H4,18,19,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat platelet-rich plasma by optical swelling assay |
Eur J Med Chem 44: 3771-6 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.036
BindingDB Entry DOI: 10.7270/Q2474BS7 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50207580
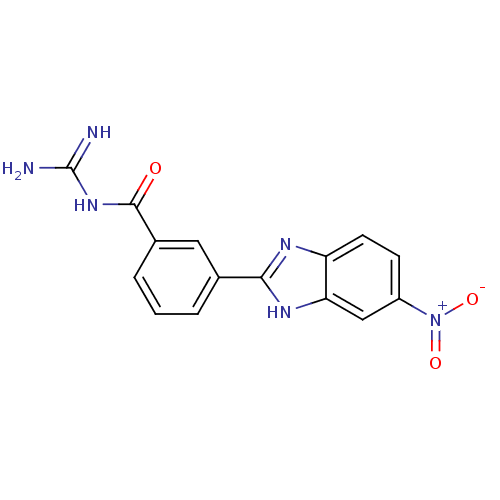
(CHEMBL234074 | N-[3-(5-nitro-1H-benzoimidazol-2-yl...)Show SMILES NC(=N)NC(=O)c1cccc(c1)-c1nc2ccc(cc2[nH]1)[N+]([O-])=O Show InChI InChI=1S/C15H12N6O3/c16-15(17)20-14(22)9-3-1-2-8(6-9)13-18-11-5-4-10(21(23)24)7-12(11)19-13/h1-7H,(H,18,19)(H4,16,17,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.42 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in rat platelets by Platelet swelling assay |
Bioorg Med Chem Lett 17: 2430-3 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.035
BindingDB Entry DOI: 10.7270/Q21C1WJ1 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50353101
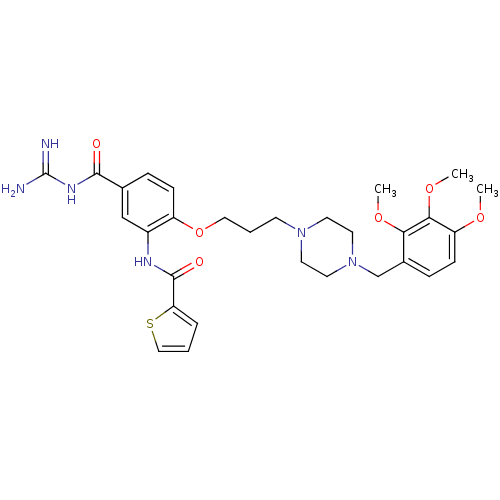
(CHEMBL1829137)Show SMILES COc1ccc(CN2CCN(CCCOc3ccc(cc3NC(=O)c3cccs3)C(=O)NC(N)=N)CC2)c(OC)c1OC Show InChI InChI=1S/C30H38N6O6S/c1-39-24-10-8-21(26(40-2)27(24)41-3)19-36-14-12-35(13-15-36)11-5-16-42-23-9-7-20(28(37)34-30(31)32)18-22(23)33-29(38)25-6-4-17-43-25/h4,6-10,17-18H,5,11-16,19H2,1-3H3,(H,33,38)(H4,31,32,34,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat assessed as inhibition of acid-induced platelet swelling by spectrophotometric analysis |
Eur J Med Chem 46: 4107-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.06.011
BindingDB Entry DOI: 10.7270/Q27081T0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50297577
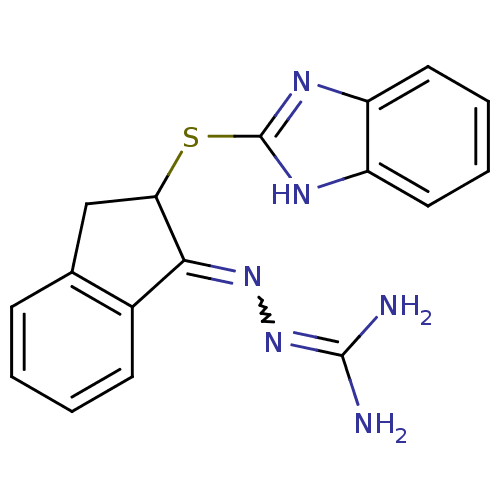
(CHEMBL549392 | N''-[2-(1H-benzimidazol-2-ylthio)-2...)Show SMILES NC(N)=NN=C1C(Cc2ccccc12)Sc1nc2ccccc2[nH]1 |w:4.3,(18.41,3.83,;16.9,4.15,;16.42,5.61,;15.87,3,;14.36,3.31,;13.33,2.17,;11.8,2.32,;11.17,.92,;12.32,-.11,;12.32,-1.64,;13.65,-2.41,;14.98,-1.63,;14.98,-.11,;13.65,.66,;11.03,3.65,;9.49,3.65,;8.57,4.91,;7.1,4.43,;5.75,5.19,;4.43,4.42,;4.42,2.87,;5.76,2.1,;7.1,2.87,;8.58,2.39,)| Show InChI InChI=1S/C17H16N6S/c18-16(19)23-22-15-11-6-2-1-5-10(11)9-14(15)24-17-20-12-7-3-4-8-13(12)21-17/h1-8,14H,9H2,(H,20,21)(H4,18,19,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.73 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat platelet-rich plasma by optical swelling assay |
Eur J Med Chem 44: 3771-6 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.036
BindingDB Entry DOI: 10.7270/Q2474BS7 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50297571
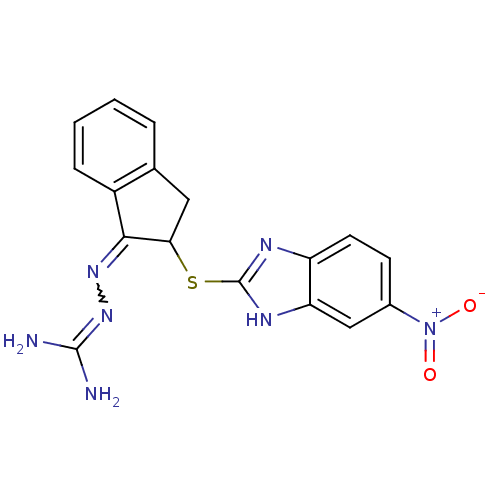
(CHEMBL561343 | N''-{2-[(5-nitro-1H-benzimidazol-2-...)Show SMILES NC(N)=NN=C1C(Cc2ccccc12)Sc1nc2ccc(cc2[nH]1)[N+]([O-])=O |w:4.3,(19.8,-22.61,;18.29,-22.3,;17.81,-20.83,;17.27,-23.45,;15.76,-23.13,;14.73,-24.28,;13.2,-24.13,;12.57,-25.53,;13.72,-26.55,;13.72,-28.08,;15.04,-28.85,;16.38,-28.08,;16.37,-26.55,;15.05,-25.79,;12.43,-22.79,;10.88,-22.79,;9.97,-24.05,;8.49,-23.57,;7.15,-24.34,;5.82,-23.57,;5.82,-22.02,;7.15,-21.25,;8.49,-22.02,;9.97,-21.54,;4.48,-21.26,;4.48,-19.71,;3.15,-22.03,)| Show InChI InChI=1S/C17H15N7O2S/c18-16(19)23-22-15-11-4-2-1-3-9(11)7-14(15)27-17-20-12-6-5-10(24(25)26)8-13(12)21-17/h1-6,8,14H,7H2,(H,20,21)(H4,18,19,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.74 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat platelet-rich plasma by optical swelling assay |
Eur J Med Chem 44: 3771-6 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.036
BindingDB Entry DOI: 10.7270/Q2474BS7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50142796
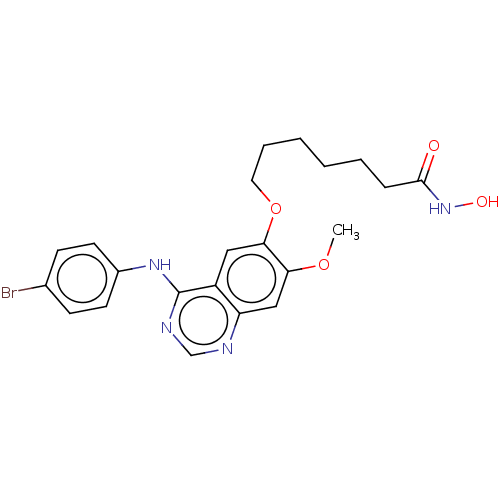
(CHEMBL3759186)Show SMILES COc1cc2ncnc(Nc3ccc(Br)cc3)c2cc1OCCCCCCC(=O)NO Show InChI InChI=1S/C22H25BrN4O4/c1-30-19-13-18-17(12-20(19)31-11-5-3-2-4-6-21(28)27-29)22(25-14-24-18)26-16-9-7-15(23)8-10-16/h7-10,12-14,29H,2-6,11H2,1H3,(H,27,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorometric assay |
Eur J Med Chem 109: 1-12 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.033
BindingDB Entry DOI: 10.7270/Q2377BJX |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50345911
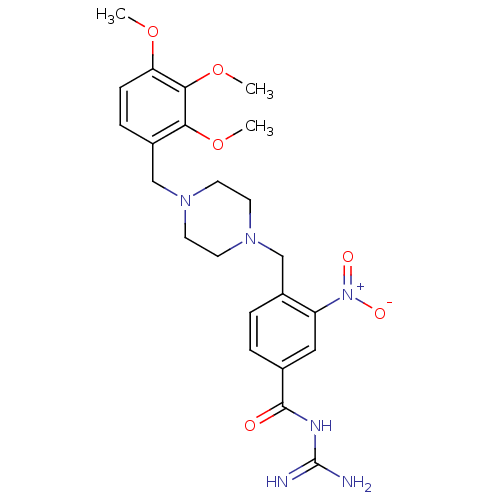
(CHEMBL1783852 | CHEMBL1789669 | N-(diaminomethylen...)Show SMILES COc1ccc(CN2CCN(Cc3ccc(cc3[N+]([O-])=O)C(=O)NC(N)=N)CC2)c(OC)c1OC Show InChI InChI=1S/C23H30N6O6/c1-33-19-7-6-17(20(34-2)21(19)35-3)14-28-10-8-27(9-11-28)13-16-5-4-15(12-18(16)29(31)32)22(30)26-23(24)25/h4-7,12H,8-11,13-14H2,1-3H3,(H4,24,25,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.82 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat assessed as inhibition of acid-induced platelet swelling by spectrophotometric analysis |
Eur J Med Chem 46: 4107-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.06.011
BindingDB Entry DOI: 10.7270/Q27081T0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50297563
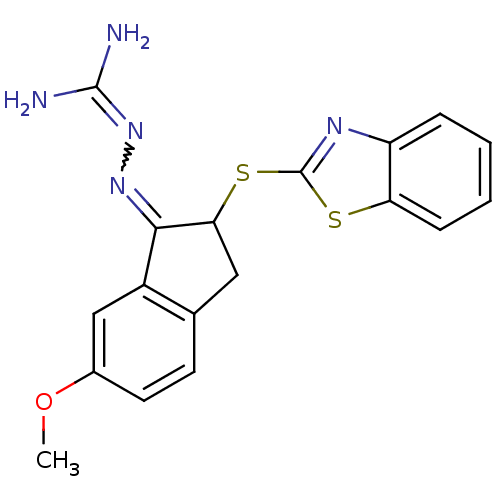
(CHEMBL550813 | N''-[2-(1,3-benzothiazol-2-ylthio)-...)Show SMILES [#6]-[#8]-c1ccc2-[#6]-[#6](-[#16]-c3nc4ccccc4s3)-[#6](=[#7]\[#7]=[#6](\[#7])-[#7])-c2c1 |w:19.21| Show InChI InChI=1S/C18H17N5OS2/c1-24-11-7-6-10-8-15(16(12(10)9-11)22-23-17(19)20)26-18-21-13-4-2-3-5-14(13)25-18/h2-7,9,15H,8H2,1H3,(H4,19,20,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat platelet-rich plasma by optical swelling assay |
Eur J Med Chem 44: 3771-6 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.036
BindingDB Entry DOI: 10.7270/Q2474BS7 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50297578
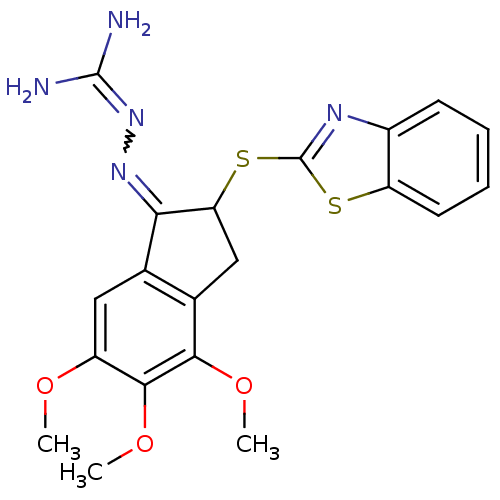
(2-{[(1E)-2-(1,3-benzothiazol-2-ylsulfanyl)-4,5,6-t...)Show SMILES [#6]-[#8]-c1cc2-[#6](=[#7]\[#7]=[#6](\[#7])-[#7])-[#6](-[#6]-c2c(-[#8]-[#6])c1-[#8]-[#6])-[#16]-c1nc2ccccc2s1 |w:6.6| Show InChI InChI=1S/C20H21N5O3S2/c1-26-13-8-10-11(17(27-2)18(13)28-3)9-15(16(10)24-25-19(21)22)30-20-23-12-6-4-5-7-14(12)29-20/h4-8,15H,9H2,1-3H3,(H4,21,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.98 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat platelet-rich plasma by optical swelling assay |
Eur J Med Chem 44: 3771-6 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.036
BindingDB Entry DOI: 10.7270/Q2474BS7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50142796

(CHEMBL3759186)Show SMILES COc1cc2ncnc(Nc3ccc(Br)cc3)c2cc1OCCCCCCC(=O)NO Show InChI InChI=1S/C22H25BrN4O4/c1-30-19-13-18-17(12-20(19)31-11-5-3-2-4-6-21(28)27-29)22(25-14-24-18)26-16-9-7-15(23)8-10-16/h7-10,12-14,29H,2-6,11H2,1H3,(H,27,28)(H,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorom... |
Eur J Med Chem 109: 1-12 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.033
BindingDB Entry DOI: 10.7270/Q2377BJX |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50297580
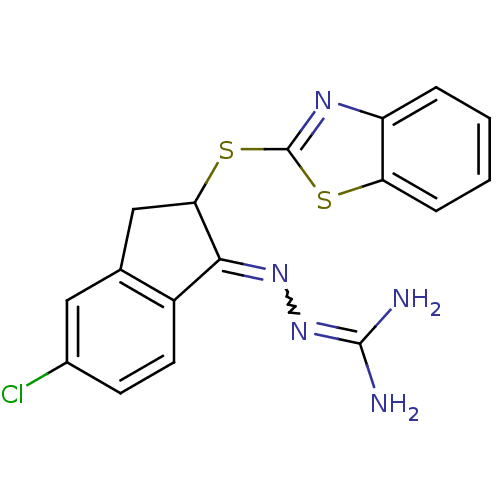
(CHEMBL560884 | N''-[2-(1,3-benzothiazol-2-ylthio)-...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]=[#6]-1-[#6](-[#6]-c2cc(Cl)ccc-12)-[#16]-c1nc2ccccc2s1 |w:4.3| Show InChI InChI=1S/C17H14ClN5S2/c18-10-5-6-11-9(7-10)8-14(15(11)22-23-16(19)20)25-17-21-12-3-1-2-4-13(12)24-17/h1-7,14H,8H2,(H4,19,20,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.34 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat platelet-rich plasma by optical swelling assay |
Eur J Med Chem 44: 3771-6 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.036
BindingDB Entry DOI: 10.7270/Q2474BS7 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50297562

(CHEMBL563178 | N''-[2-(1,3-benzothiazol-2-ylthio)-...)Show SMILES [#6]-[#8]-c1cc2-[#6]-[#6](-[#16]-c3nc4ccccc4s3)-[#6](=[#7]\[#7]=[#6](\[#7])-[#7])-c2cc1-[#8]-[#6] |w:18.20| Show InChI InChI=1S/C19H19N5O2S2/c1-25-13-7-10-8-16(28-19-22-12-5-3-4-6-15(12)27-19)17(23-24-18(20)21)11(10)9-14(13)26-2/h3-7,9,16H,8H2,1-2H3,(H4,20,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.44 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat platelet-rich plasma by optical swelling assay |
Eur J Med Chem 44: 3771-6 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.036
BindingDB Entry DOI: 10.7270/Q2474BS7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50142857
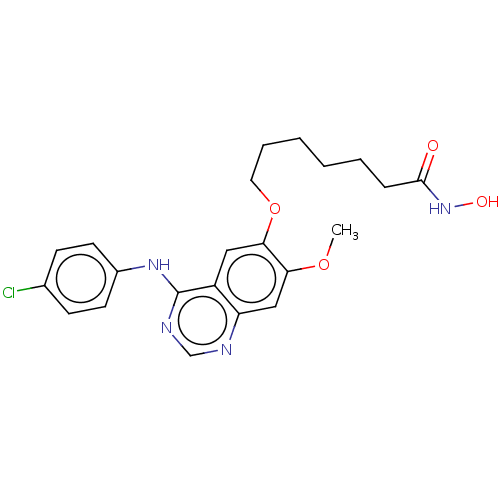
(CHEMBL3758839)Show SMILES COc1cc2ncnc(Nc3ccc(Cl)cc3)c2cc1OCCCCCCC(=O)NO Show InChI InChI=1S/C22H25ClN4O4/c1-30-19-13-18-17(12-20(19)31-11-5-3-2-4-6-21(28)27-29)22(25-14-24-18)26-16-9-7-15(23)8-10-16/h7-10,12-14,29H,2-6,11H2,1H3,(H,27,28)(H,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorom... |
Eur J Med Chem 109: 1-12 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.033
BindingDB Entry DOI: 10.7270/Q2377BJX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50142796

(CHEMBL3759186)Show SMILES COc1cc2ncnc(Nc3ccc(Br)cc3)c2cc1OCCCCCCC(=O)NO Show InChI InChI=1S/C22H25BrN4O4/c1-30-19-13-18-17(12-20(19)31-11-5-3-2-4-6-21(28)27-29)22(25-14-24-18)26-16-9-7-15(23)8-10-16/h7-10,12-14,29H,2-6,11H2,1H3,(H,27,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorometric assay |
Eur J Med Chem 109: 1-12 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.033
BindingDB Entry DOI: 10.7270/Q2377BJX |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50345911

(CHEMBL1783852 | CHEMBL1789669 | N-(diaminomethylen...)Show SMILES COc1ccc(CN2CCN(Cc3ccc(cc3[N+]([O-])=O)C(=O)NC(N)=N)CC2)c(OC)c1OC Show InChI InChI=1S/C23H30N6O6/c1-33-19-7-6-17(20(34-2)21(19)35-3)14-28-10-8-27(9-11-28)13-16-5-4-15(12-18(16)29(31)32)22(30)26-23(24)25/h4-7,12H,8-11,13-14H2,1-3H3,(H4,24,25,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1-mediated rat platelet swelling |
Bioorg Med Chem Lett 19: 3283-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.079
BindingDB Entry DOI: 10.7270/Q2BR8SJ1 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50207583
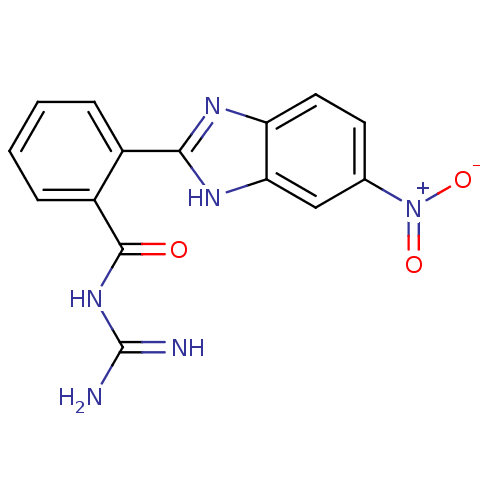
(CHEMBL234279 | N-[2-(5-nitro-1H-benzoimidazol-2-yl...)Show SMILES NC(=N)NC(=O)c1ccccc1-c1nc2ccc(cc2[nH]1)[N+]([O-])=O Show InChI InChI=1S/C15H12N6O3/c16-15(17)20-14(22)10-4-2-1-3-9(10)13-18-11-6-5-8(21(23)24)7-12(11)19-13/h1-7H,(H,18,19)(H4,16,17,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.05 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in rat platelets by Platelet swelling assay |
Bioorg Med Chem Lett 17: 2430-3 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.035
BindingDB Entry DOI: 10.7270/Q21C1WJ1 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50345917
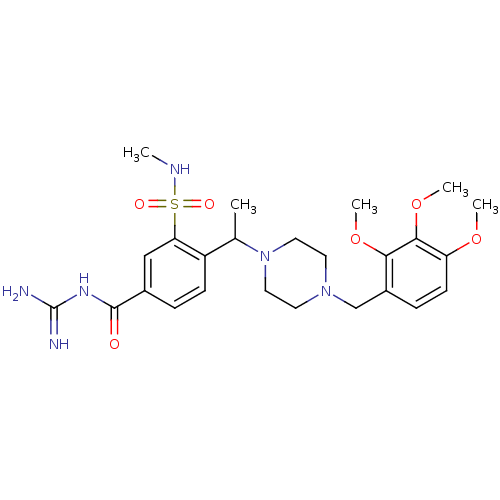
(CHEMBL1783857 | N-(diaminomethylene)-3-(N-methylsu...)Show SMILES CNS(=O)(=O)c1cc(ccc1C(C)N1CCN(Cc2ccc(OC)c(OC)c2OC)CC1)C(=O)NC(N)=N Show InChI InChI=1S/C25H36N6O6S/c1-16(19-8-6-17(24(32)29-25(26)27)14-21(19)38(33,34)28-2)31-12-10-30(11-13-31)15-18-7-9-20(35-3)23(37-5)22(18)36-4/h6-9,14,16,28H,10-13,15H2,1-5H3,(H4,26,27,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.48 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1-mediated rat platelet swelling |
Bioorg Med Chem Lett 19: 3283-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.079
BindingDB Entry DOI: 10.7270/Q2BR8SJ1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50142796

(CHEMBL3759186)Show SMILES COc1cc2ncnc(Nc3ccc(Br)cc3)c2cc1OCCCCCCC(=O)NO Show InChI InChI=1S/C22H25BrN4O4/c1-30-19-13-18-17(12-20(19)31-11-5-3-2-4-6-21(28)27-29)22(25-14-24-18)26-16-9-7-15(23)8-10-16/h7-10,12-14,29H,2-6,11H2,1H3,(H,27,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorometric assay |
Eur J Med Chem 109: 1-12 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.033
BindingDB Entry DOI: 10.7270/Q2377BJX |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50586865
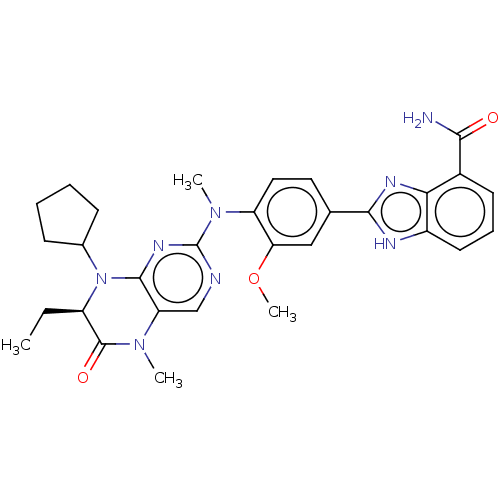
(CHEMBL5084388)Show SMILES CC[C@H]1N(C2CCCC2)c2nc(ncc2N(C)C1=O)N(C)c1ccc(cc1OC)-c1nc2c(cccc2[nH]1)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant PARP2 (unknown origin) incubated for 45 mins in presence of biotinylated-NAD+ by ELISA |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01535
BindingDB Entry DOI: 10.7270/Q22N566V |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50142898
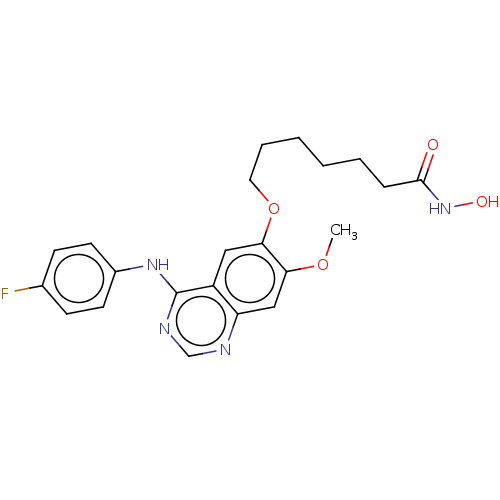
(CHEMBL3758380)Show SMILES COc1cc2ncnc(Nc3ccc(F)cc3)c2cc1OCCCCCCC(=O)NO Show InChI InChI=1S/C22H25FN4O4/c1-30-19-13-18-17(12-20(19)31-11-5-3-2-4-6-21(28)27-29)22(25-14-24-18)26-16-9-7-15(23)8-10-16/h7-10,12-14,29H,2-6,11H2,1H3,(H,27,28)(H,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorom... |
Eur J Med Chem 109: 1-12 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.033
BindingDB Entry DOI: 10.7270/Q2377BJX |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50142845
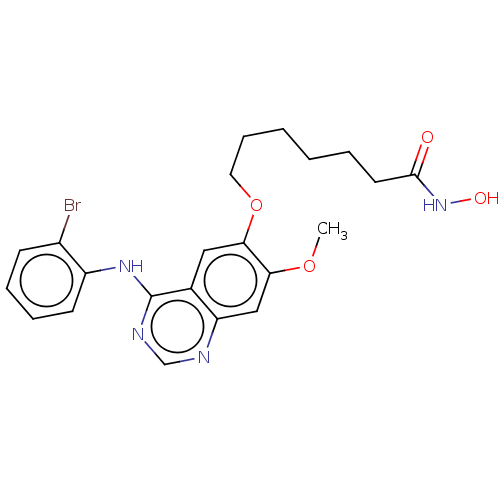
(CHEMBL3759591)Show SMILES COc1cc2ncnc(Nc3ccccc3Br)c2cc1OCCCCCCC(=O)NO Show InChI InChI=1S/C22H25BrN4O4/c1-30-19-13-18-15(12-20(19)31-11-7-3-2-4-10-21(28)27-29)22(25-14-24-18)26-17-9-6-5-8-16(17)23/h5-6,8-9,12-14,29H,2-4,7,10-11H2,1H3,(H,27,28)(H,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorom... |
Eur J Med Chem 109: 1-12 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.033
BindingDB Entry DOI: 10.7270/Q2377BJX |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human VEGFR-2 preincubated for 10 mins followed by addition of FAM-labeled peptide and incubated for 10 mins by Caliper motility shift ... |
Eur J Med Chem 109: 371-9 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.032
BindingDB Entry DOI: 10.7270/Q2M32XKS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50297575
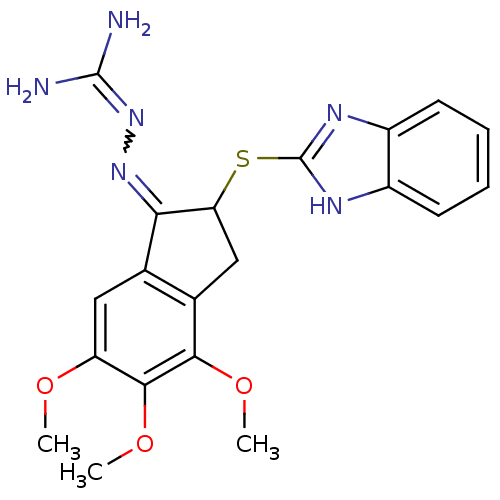
(2-{[(1E)-2-(1H-1,3-benzodiazol-2-ylsulfanyl)-4,5,6...)Show SMILES COc1cc2C(=NN=C(N)N)C(Cc2c(OC)c1OC)Sc1nc2ccccc2[nH]1 |w:6.6,(.51,-14.95,;.5,-13.41,;-.83,-12.64,;-.84,-11.11,;-2.16,-10.35,;-2.48,-8.84,;-1.45,-7.7,;.06,-8.01,;1.08,-6.86,;2.59,-7.18,;.6,-5.4,;-4.01,-8.69,;-4.64,-10.09,;-3.49,-11.11,;-3.49,-12.64,;-4.83,-13.41,;-6.16,-12.64,;-2.16,-13.41,;-2.16,-14.95,;-3.5,-15.73,;-4.78,-7.36,;-6.32,-7.36,;-7.23,-6.1,;-8.71,-6.58,;-10.05,-5.82,;-11.38,-6.59,;-11.38,-8.14,;-10.05,-8.91,;-8.71,-8.14,;-7.23,-8.61,)| Show InChI InChI=1S/C20H22N6O3S/c1-27-14-8-10-11(17(28-2)18(14)29-3)9-15(16(10)25-26-19(21)22)30-20-23-12-6-4-5-7-13(12)24-20/h4-8,15H,9H2,1-3H3,(H,23,24)(H4,21,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.8 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat platelet-rich plasma by optical swelling assay |
Eur J Med Chem 44: 3771-6 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.036
BindingDB Entry DOI: 10.7270/Q2474BS7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50142859
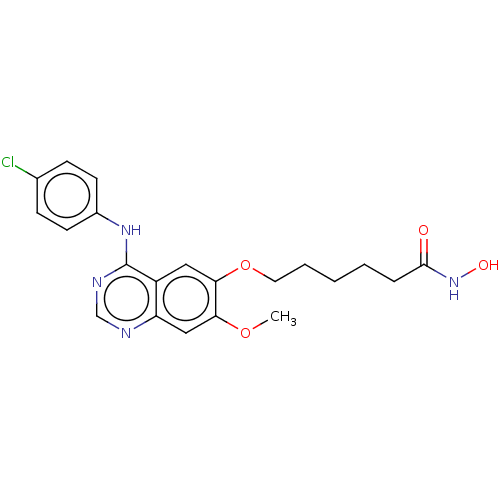
(CHEMBL3759261)Show SMILES COc1cc2ncnc(Nc3ccc(Cl)cc3)c2cc1OCCCCCC(=O)NO Show InChI InChI=1S/C21H23ClN4O4/c1-29-18-12-17-16(11-19(18)30-10-4-2-3-5-20(27)26-28)21(24-13-23-17)25-15-8-6-14(22)7-9-15/h6-9,11-13,28H,2-5,10H2,1H3,(H,26,27)(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorom... |
Eur J Med Chem 109: 1-12 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.033
BindingDB Entry DOI: 10.7270/Q2377BJX |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50207572
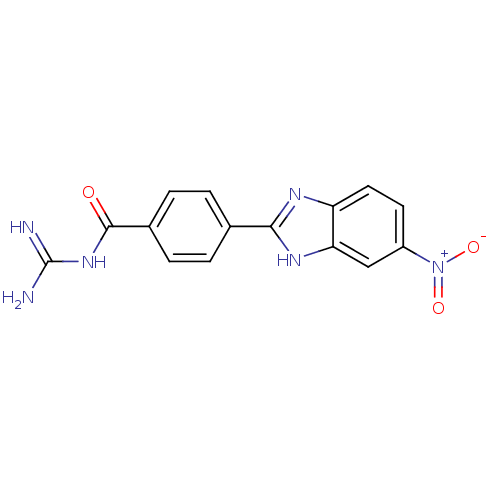
(CHEMBL234072 | N-[4-(5-nitro-1H-benzoimidazol-2-yl...)Show SMILES NC(=N)NC(=O)c1ccc(cc1)-c1nc2ccc(cc2[nH]1)[N+]([O-])=O Show InChI InChI=1S/C15H12N6O3/c16-15(17)20-14(22)9-3-1-8(2-4-9)13-18-11-6-5-10(21(23)24)7-12(11)19-13/h1-7H,(H,18,19)(H4,16,17,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in rat platelets by Platelet swelling assay |
Bioorg Med Chem Lett 17: 2430-3 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.035
BindingDB Entry DOI: 10.7270/Q21C1WJ1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM21
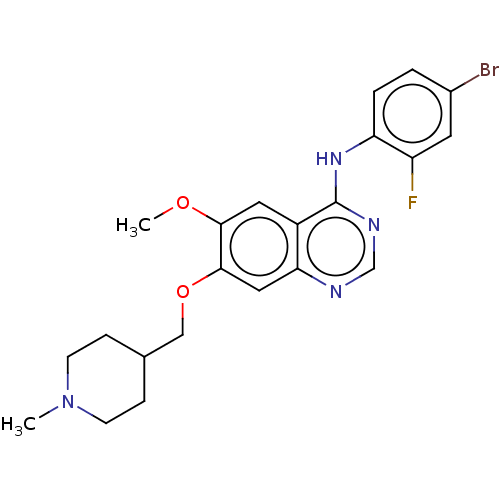
(CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...)Show SMILES COc1cc2c(Nc3ccc(Br)cc3F)ncnc2cc1OCC1CCN(C)CC1 Show InChI InChI=1S/C22H24BrFN4O2/c1-28-7-5-14(6-8-28)12-30-21-11-19-16(10-20(21)29-2)22(26-13-25-19)27-18-4-3-15(23)9-17(18)24/h3-4,9-11,13-14H,5-8,12H2,1-2H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human EGFR cytoplasmic domain (669 to 1210 residues) expressed in baculovirus expression system preincubated for ... |
Eur J Med Chem 125: 245-254 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.039
BindingDB Entry DOI: 10.7270/Q2QJ7K9C |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50345908
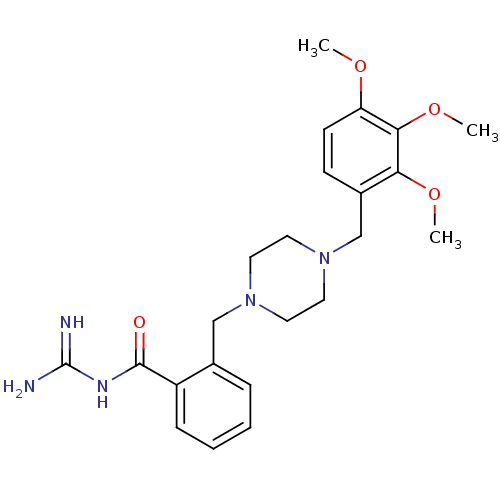
(CHEMBL1783849 | N-(diaminomethylene)-2-((4-(2,3,4-...)Show SMILES COc1ccc(CN2CCN(Cc3ccccc3C(=O)NC(N)=N)CC2)c(OC)c1OC Show InChI InChI=1S/C23H31N5O4/c1-30-19-9-8-17(20(31-2)21(19)32-3)15-28-12-10-27(11-13-28)14-16-6-4-5-7-18(16)22(29)26-23(24)25/h4-9H,10-15H2,1-3H3,(H4,24,25,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1-mediated rat platelet swelling |
Bioorg Med Chem Lett 19: 3283-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.079
BindingDB Entry DOI: 10.7270/Q2BR8SJ1 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50297576
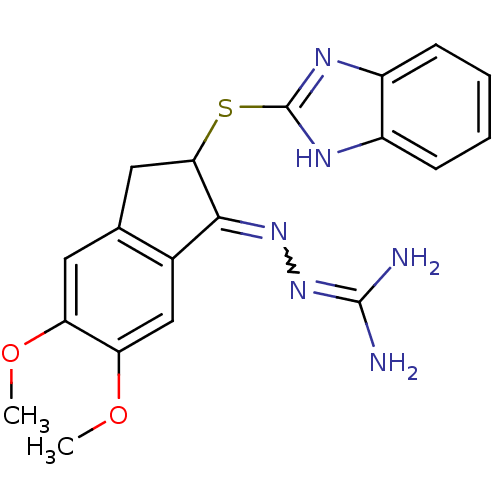
(CHEMBL561142 | N''-[2-(1H-benzimidazol-2-ylthio)-5...)Show SMILES COc1cc2CC(Sc3nc4ccccc4[nH]3)C(=NN=C(N)N)c2cc1OC |w:18.20,(28.87,-5.3,;30.2,-4.53,;30.2,-2.98,;28.87,-2.21,;28.87,-.68,;27.73,.34,;28.35,1.74,;27.58,3.08,;26.04,3.08,;25.13,4.34,;23.65,3.85,;22.3,4.62,;20.98,3.85,;20.98,2.3,;22.31,1.53,;23.65,2.3,;25.13,1.82,;29.88,1.59,;30.91,2.74,;32.42,2.42,;33.45,3.57,;34.96,3.26,;32.97,5.04,;30.21,.08,;31.53,-.68,;31.54,-2.21,;32.87,-2.98,;32.88,-4.52,)| Show InChI InChI=1S/C19H20N6O2S/c1-26-14-7-10-8-16(28-19-22-12-5-3-4-6-13(12)23-19)17(24-25-18(20)21)11(10)9-15(14)27-2/h3-7,9,16H,8H2,1-2H3,(H,22,23)(H4,20,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.2 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat platelet-rich plasma by optical swelling assay |
Eur J Med Chem 44: 3771-6 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.036
BindingDB Entry DOI: 10.7270/Q2474BS7 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50345914
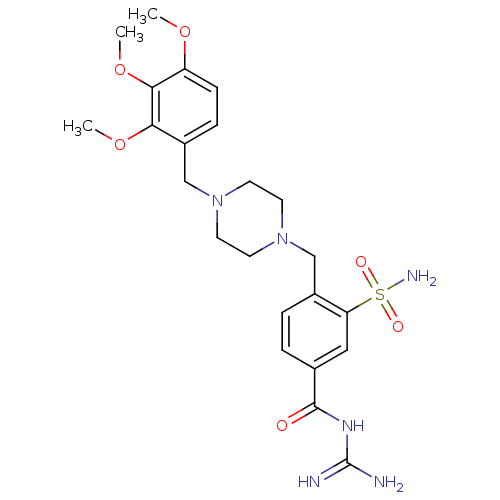
(CHEMBL1783830 | N-(diaminomethylene)-3-sulfamoyl-4...)Show SMILES COc1ccc(CN2CCN(Cc3ccc(cc3S(N)(=O)=O)C(=O)NC(N)=N)CC2)c(OC)c1OC Show InChI InChI=1S/C23H32N6O6S/c1-33-18-7-6-17(20(34-2)21(18)35-3)14-29-10-8-28(9-11-29)13-16-5-4-15(22(30)27-23(24)25)12-19(16)36(26,31)32/h4-7,12H,8-11,13-14H2,1-3H3,(H2,26,31,32)(H4,24,25,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1-mediated rat platelet swelling |
Bioorg Med Chem Lett 19: 3283-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.079
BindingDB Entry DOI: 10.7270/Q2BR8SJ1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50142838
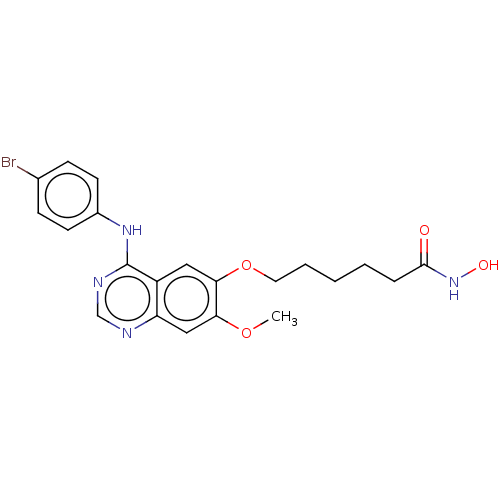
(CHEMBL3758697)Show SMILES COc1cc2ncnc(Nc3ccc(Br)cc3)c2cc1OCCCCCC(=O)NO Show InChI InChI=1S/C21H23BrN4O4/c1-29-18-12-17-16(11-19(18)30-10-4-2-3-5-20(27)26-28)21(24-13-23-17)25-15-8-6-14(22)7-9-15/h6-9,11-13,28H,2-5,10H2,1H3,(H,26,27)(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorom... |
Eur J Med Chem 109: 1-12 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.033
BindingDB Entry DOI: 10.7270/Q2377BJX |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Rattus norvegicus) | BDBM50297569
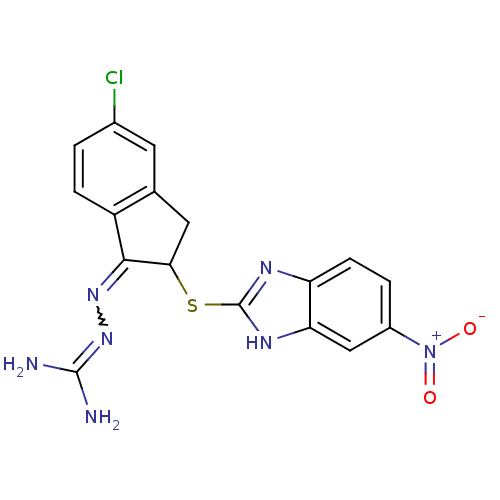
(CHEMBL563763 | N''-{5-chloro-2-[(5-nitro-1H-benzim...)Show SMILES NC(N)=NN=C1C(Cc2cc(Cl)ccc12)Sc1nc2ccc(cc2[nH]1)[N+]([O-])=O |w:4.3,(6.38,-34.14,;4.87,-33.82,;4.39,-32.36,;3.84,-34.97,;2.33,-34.66,;1.3,-35.8,;-.23,-35.65,;-.86,-37.05,;.29,-38.08,;.29,-39.61,;1.62,-40.38,;1.62,-41.92,;2.95,-39.61,;2.95,-38.08,;1.63,-37.31,;-1,-34.32,;-2.54,-34.32,;-3.45,-35.58,;-4.93,-35.1,;-6.27,-35.87,;-7.6,-35.1,;-7.6,-33.55,;-6.28,-32.78,;-4.93,-33.54,;-3.46,-33.06,;-8.94,-32.78,;-8.95,-31.24,;-10.28,-33.55,)| Show InChI InChI=1S/C17H14ClN7O2S/c18-9-1-3-11-8(5-9)6-14(15(11)23-24-16(19)20)28-17-21-12-4-2-10(25(26)27)7-13(12)22-17/h1-5,7,14H,6H2,(H,21,22)(H4,19,20,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.2 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of NHE1 in Sprague-Dawley rat platelet-rich plasma by optical swelling assay |
Eur J Med Chem 44: 3771-6 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.036
BindingDB Entry DOI: 10.7270/Q2474BS7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data