

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50174361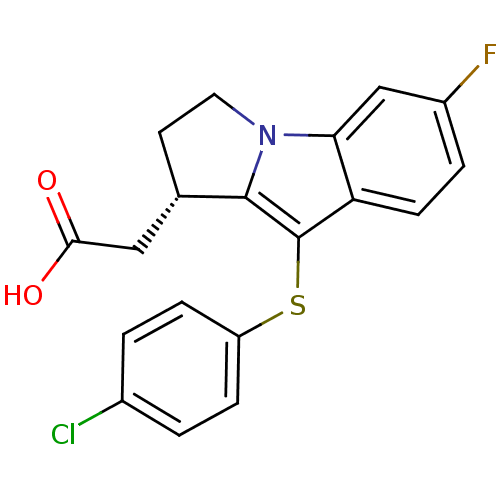 (CHEMBL370606 | L-888607 | [(S)-9-(4-Chloro-phenyls...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd. Curated by ChEMBL | Assay Description Binding affinity towards human CRTH2 receptor expressed in CHO cells | J Med Chem 48: 6174-7 (2005) Article DOI: 10.1021/jm050519b BindingDB Entry DOI: 10.7270/Q2KK9B9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | CHEMBL5291138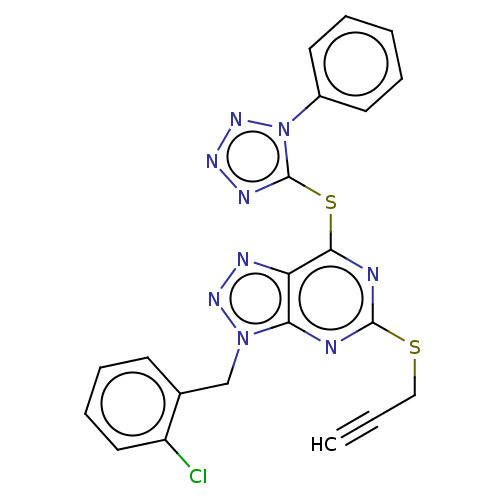 | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Mean functional activity against human H3 receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50174354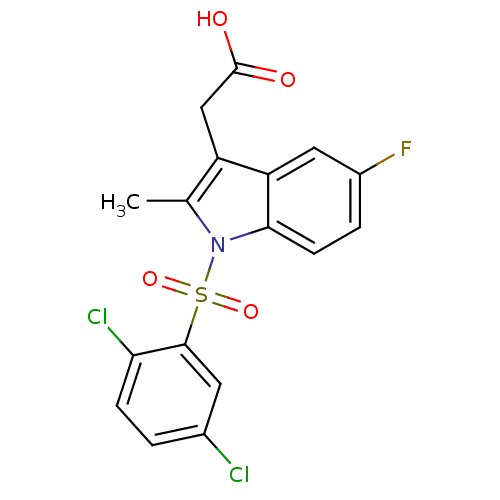 (CHEMBL373294 | [1-(2,5-Dichloro-benzenesulfonyl)-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd. Curated by ChEMBL | Assay Description Binding affinity towards human CRTH2 receptor expressed in CHO cells | J Med Chem 48: 6174-7 (2005) Article DOI: 10.1021/jm050519b BindingDB Entry DOI: 10.7270/Q2KK9B9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50174357 (CHEMBL199040 | [1-(4-Chloro-benzenesulfonyl)-5-flu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd. Curated by ChEMBL | Assay Description Binding affinity towards human CRTH2 receptor expressed in CHO cells | J Med Chem 48: 6174-7 (2005) Article DOI: 10.1021/jm050519b BindingDB Entry DOI: 10.7270/Q2KK9B9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346870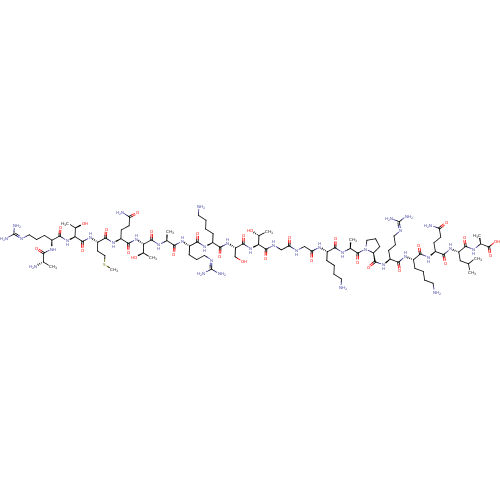 (CHEMBL1797647) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Blocking activity was assessed by antagonism of (-)-noradrenaline induced contraction of rat prostatic vas deferens (alpha1A adrenoceptor) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity (agonistic) towards human CRTH2 receptor expressed in CHO cells; range 15 to 25 nM | J Med Chem 48: 6174-7 (2005) Article DOI: 10.1021/jm050519b BindingDB Entry DOI: 10.7270/Q2KK9B9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50346870 (CHEMBL1797647) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Mean functional activity against human H3 receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50174351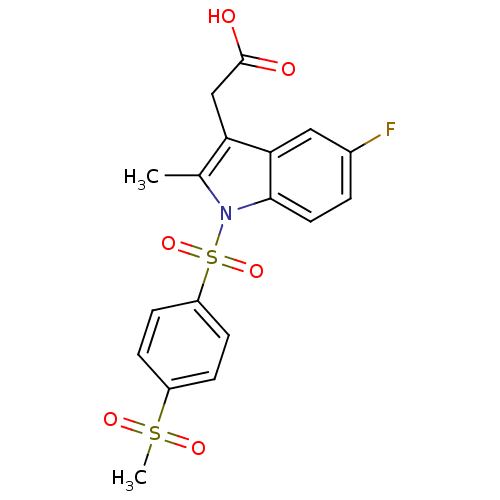 (CHEMBL196707 | [5-Fluoro-1-(4-methanesulfonyl-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd. Curated by ChEMBL | Assay Description Binding affinity towards human CRTH2 receptor expressed in CHO cells | J Med Chem 48: 6174-7 (2005) Article DOI: 10.1021/jm050519b BindingDB Entry DOI: 10.7270/Q2KK9B9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50552232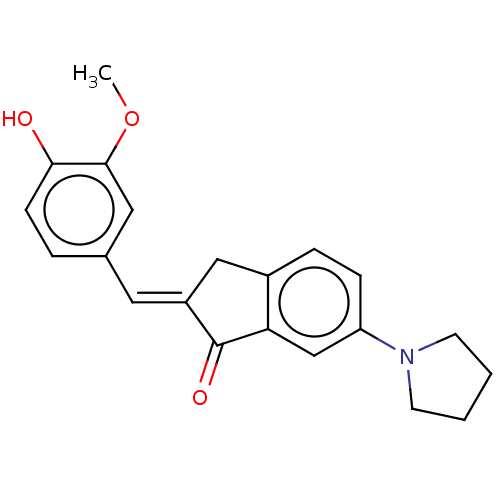 (CHEMBL4776624) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed inhibition of hCES2A in human liver microsome assessed as reduction in fluorescein diacetate hydrolysis preincubated for 10 mins followed by su... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112856 BindingDB Entry DOI: 10.7270/Q2NZ8CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50174352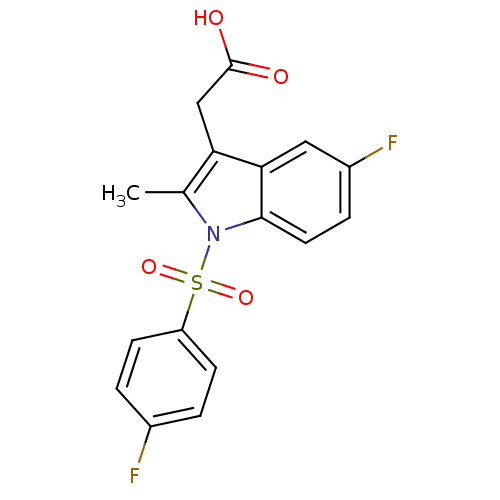 (CHEMBL370257 | [5-Fluoro-1-(4-fluoro-benzenesulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd. Curated by ChEMBL | Assay Description Binding affinity towards human CRTH2 receptor expressed in CHO cells | J Med Chem 48: 6174-7 (2005) Article DOI: 10.1021/jm050519b BindingDB Entry DOI: 10.7270/Q2KK9B9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50586363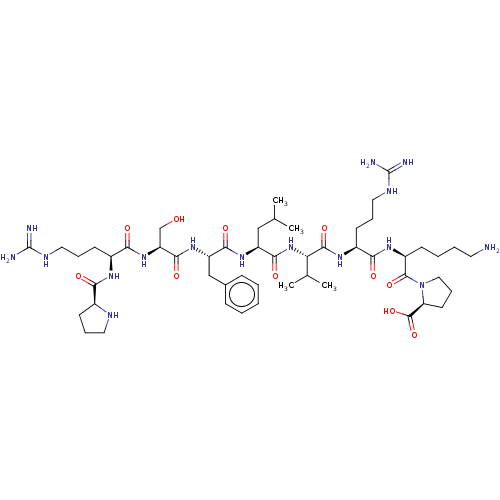 (CHEMBL5079374) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Blocking activity was assessed by antagonism of (-)-noradrenaline induced contraction of rat prostatic vas deferens (alpha1A adrenoceptor) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | CHEMBL5281080 | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Mean functional activity against human H3 receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | CHEMBL1255711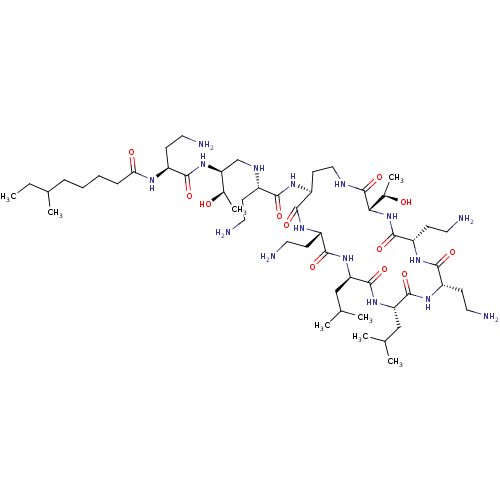 | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Mean functional activity against human H3 receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | CHEMBL5287743 | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Blocking activity was assessed by antagonism of (-)-phenylephrine induced contraction of rat spleen (alpha1B adrenoceptor) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50151161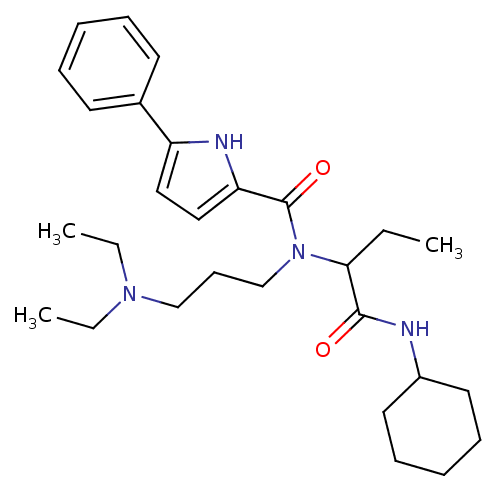 (5-Phenyl-1H-pyrrole-2-carboxylic acid (1-cyclohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research Curated by ChEMBL | Assay Description Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin | J Med Chem 47: 4286-90 (2004) Article DOI: 10.1021/jm040103i BindingDB Entry DOI: 10.7270/Q2CR5SV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50174353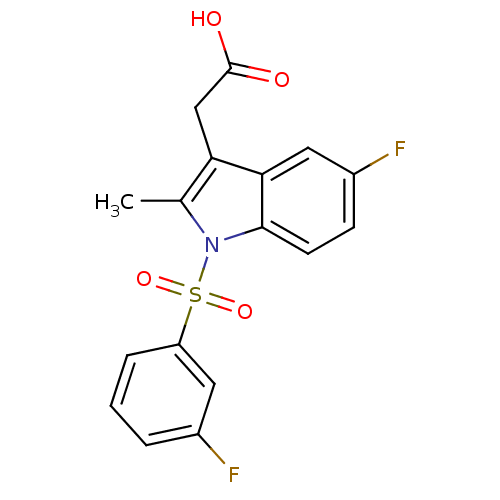 (CHEMBL196617 | [5-Fluoro-1-(3-fluoro-benzenesulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd. Curated by ChEMBL | Assay Description Binding affinity towards human CRTH2 receptor expressed in CHO cells | J Med Chem 48: 6174-7 (2005) Article DOI: 10.1021/jm050519b BindingDB Entry DOI: 10.7270/Q2KK9B9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50174358 ((1-Benzenesulfonyl-5-fluoro-2-methyl-1H-indol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd. Curated by ChEMBL | Assay Description Binding affinity towards human CRTH2 receptor expressed in CHO cells | J Med Chem 48: 6174-7 (2005) Article DOI: 10.1021/jm050519b BindingDB Entry DOI: 10.7270/Q2KK9B9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50151159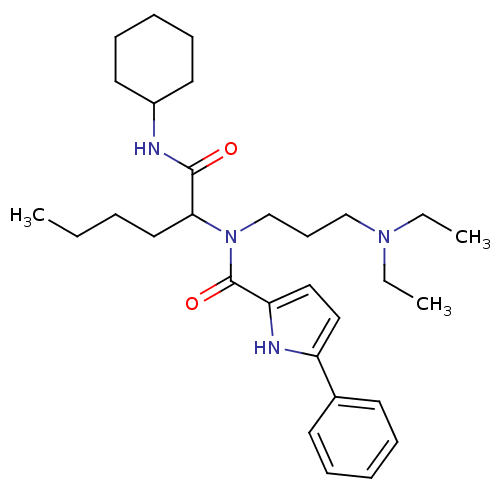 (5-Phenyl-1H-pyrrole-2-carboxylic acid (1-cyclohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research Curated by ChEMBL | Assay Description Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin | J Med Chem 47: 4286-90 (2004) Article DOI: 10.1021/jm040103i BindingDB Entry DOI: 10.7270/Q2CR5SV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50174360 (CHEMBL364299 | [5-Fluoro-1-(4-methoxy-benzenesulfo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 299 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd. Curated by ChEMBL | Assay Description Binding affinity towards human CRTH2 receptor expressed in CHO cells | J Med Chem 48: 6174-7 (2005) Article DOI: 10.1021/jm050519b BindingDB Entry DOI: 10.7270/Q2KK9B9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50568522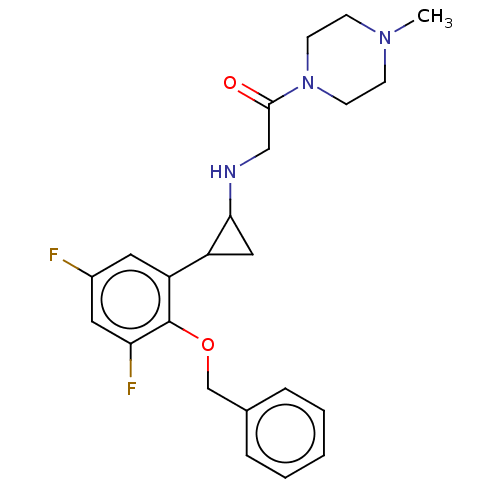 (CHEMBL4864352) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human LSD1 (172 to 833 residues) using Lys4-dimethylated H3 (1-20) peptide as substrate incubated for 30 mins by peroxidase-coupled ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50174356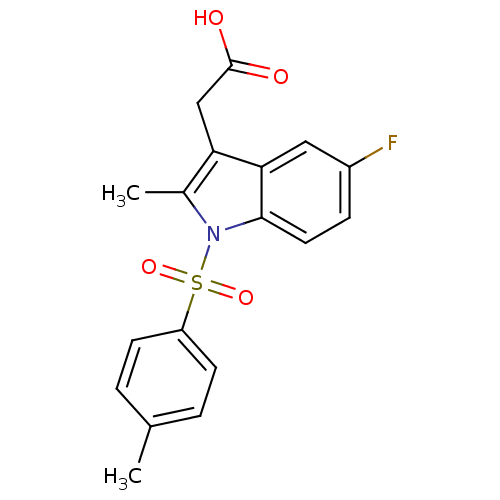 (CHEMBL193753 | [5-Fluoro-2-methyl-1-(toluene-4-sul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 457 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd. Curated by ChEMBL | Assay Description Binding affinity towards human CRTH2 receptor expressed in CHO cells | J Med Chem 48: 6174-7 (2005) Article DOI: 10.1021/jm050519b BindingDB Entry DOI: 10.7270/Q2KK9B9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50181669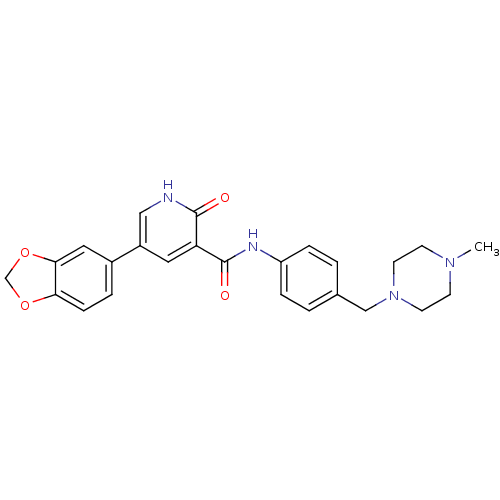 (5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation Curated by ChEMBL | Assay Description Inhibitory activity against ALK | J Med Chem 49: 1006-15 (2006) Article DOI: 10.1021/jm050824x BindingDB Entry DOI: 10.7270/Q2H994T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50346586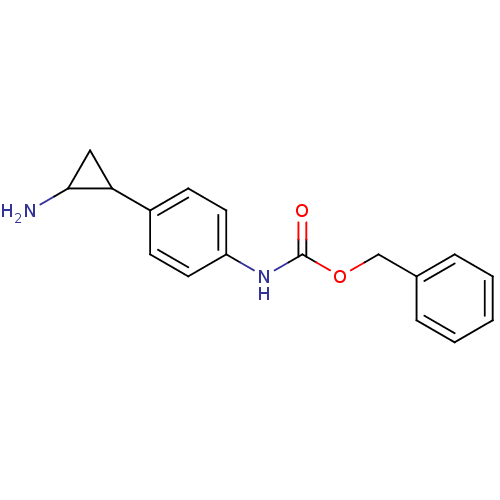 (CHEMBL1795981 | US8765820, 5a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MAO-A expressed in Pichia pastoris using kynuramine as substrate by peroxidase-coupled method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50568521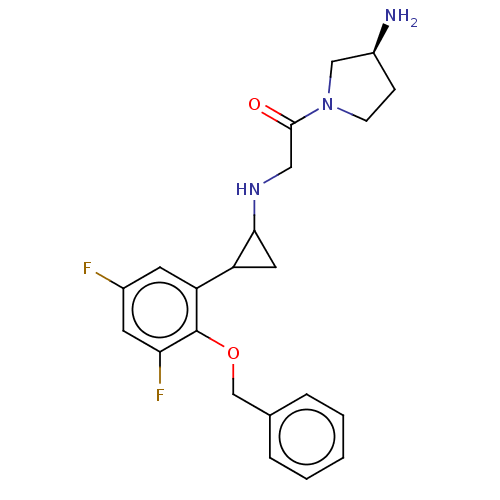 (CHEMBL4878787) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human LSD1 (172 to 833 residues) using Lys4-dimethylated H3 (1-20) peptide as substrate incubated for 30 mins by peroxidase-coupled ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346585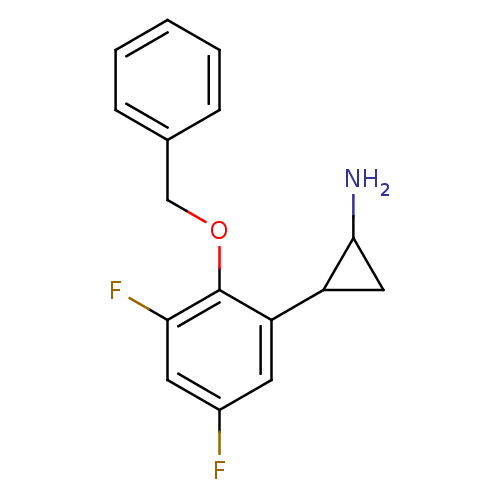 (CHEMBL1795980 | S2101) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hexahistidine-tagged human LSD1 (172 to 833 residues) expressed in Escherichia coli Rosetta (DE3) cells using H3K4me2 peptide as substr... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50174359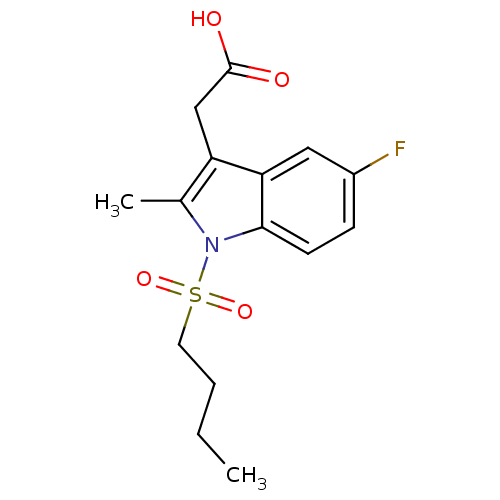 (CHEMBL194918 | [1-(Butane-1-sulfonyl)-5-fluoro-2-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 634 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd. Curated by ChEMBL | Assay Description Binding affinity towards human CRTH2 receptor expressed in CHO cells | J Med Chem 48: 6174-7 (2005) Article DOI: 10.1021/jm050519b BindingDB Entry DOI: 10.7270/Q2KK9B9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50151163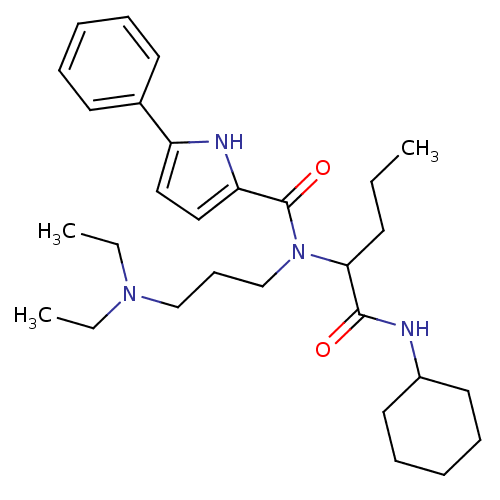 (5-Phenyl-1H-pyrrole-2-carboxylic acid (1-cyclohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research Curated by ChEMBL | Assay Description Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin | J Med Chem 47: 4286-90 (2004) Article DOI: 10.1021/jm040103i BindingDB Entry DOI: 10.7270/Q2CR5SV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346585 (CHEMBL1795980 | S2101) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human LSD1 (172 to 833 residues) using Lys4-dimethylated H3 (1-20) peptide as substrate incubated for 30 mins by peroxidase-coupled ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50151168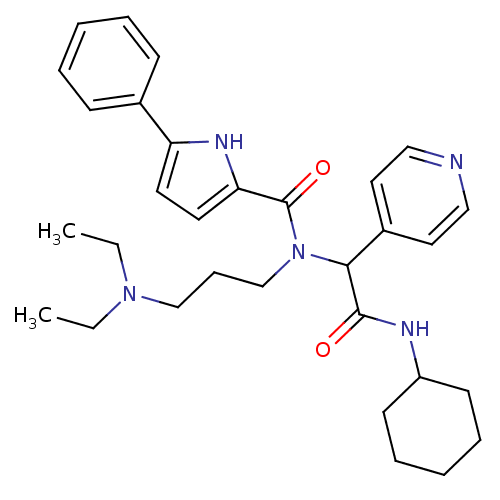 (5-Phenyl-1H-pyrrole-2-carboxylic acid (cyclohexylc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research Curated by ChEMBL | Assay Description Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin | J Med Chem 47: 4286-90 (2004) Article DOI: 10.1021/jm040103i BindingDB Entry DOI: 10.7270/Q2CR5SV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50181675 (5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation Curated by ChEMBL | Assay Description Inhibitory activity against ALK | J Med Chem 49: 1006-15 (2006) Article DOI: 10.1021/jm050824x BindingDB Entry DOI: 10.7270/Q2H994T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50346863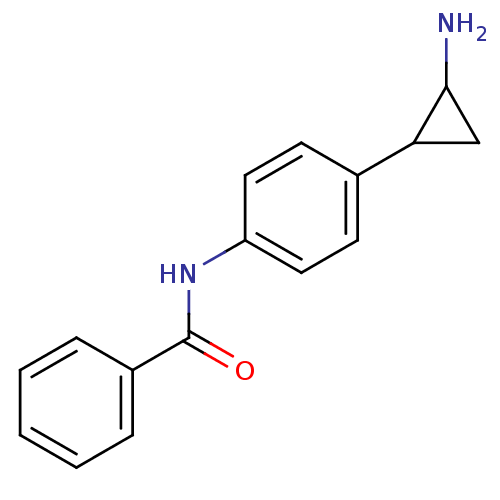 (CHEMBL1797640 | US8765820, 5b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human LSD1/CoREST expressed in Escherichia coli using histone H3 peptide monomethylated at Lys4 as substrate by peroxidase-coupled assa... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50151160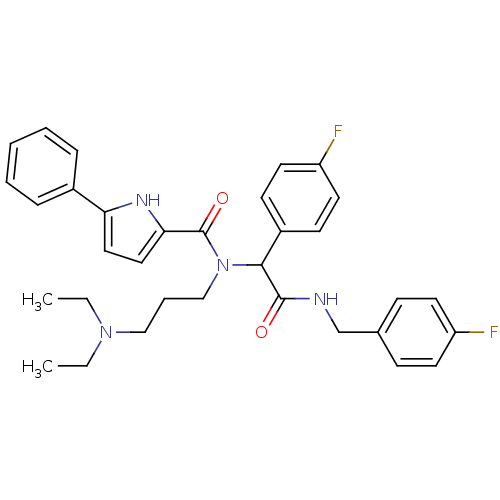 (5-Phenyl-1H-pyrrole-2-carboxylic acid (3-diethylam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research Curated by ChEMBL | Assay Description Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin | J Med Chem 47: 4286-90 (2004) Article DOI: 10.1021/jm040103i BindingDB Entry DOI: 10.7270/Q2CR5SV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50151169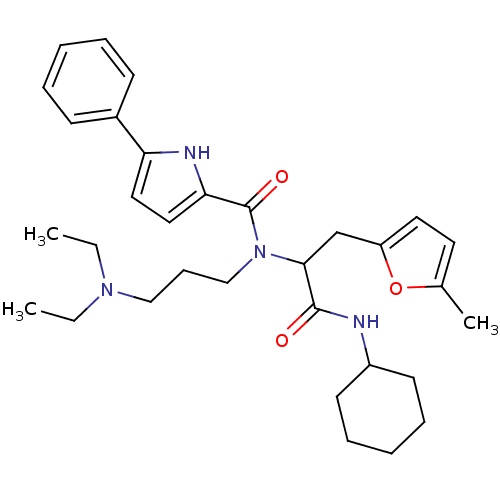 (5-Phenyl-1H-pyrrole-2-carboxylic acid [1-cyclohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research Curated by ChEMBL | Assay Description Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin | J Med Chem 47: 4286-90 (2004) Article DOI: 10.1021/jm040103i BindingDB Entry DOI: 10.7270/Q2CR5SV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50151158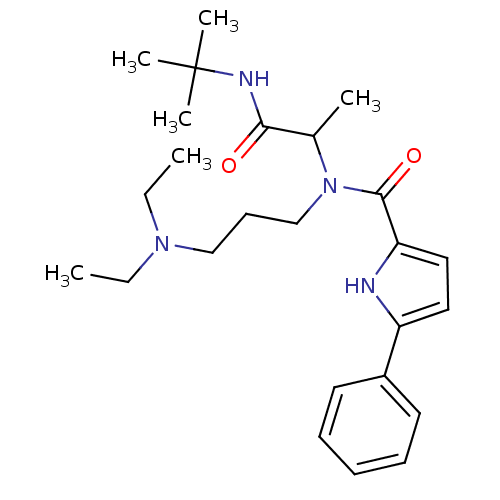 (5-Phenyl-1H-pyrrole-2-carboxylic acid (1-tert-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research Curated by ChEMBL | Assay Description Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin | J Med Chem 47: 4286-90 (2004) Article DOI: 10.1021/jm040103i BindingDB Entry DOI: 10.7270/Q2CR5SV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50346864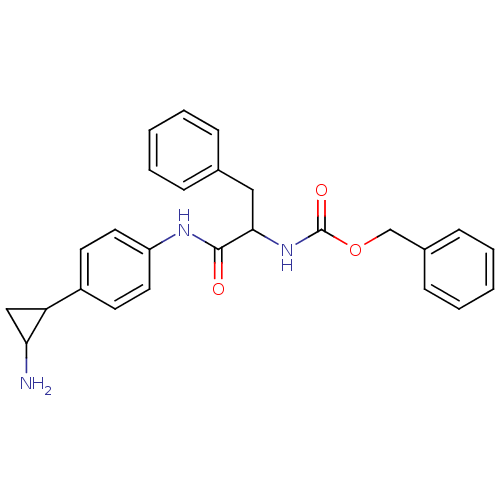 (CHEMBL1797641 | CHEMBL3104337 | US8765820, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MAO-A expressed in Pichia pastoris using kynuramine as substrate by peroxidase-coupled method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50151167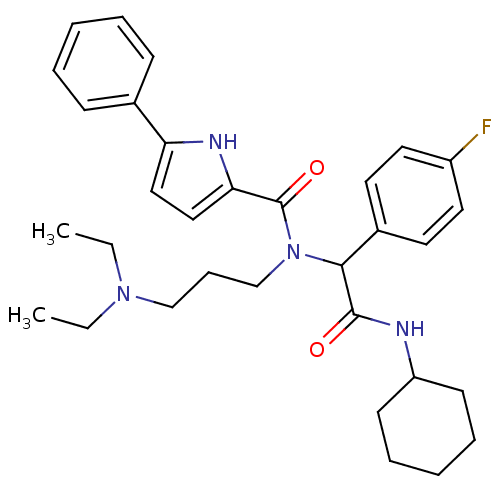 (5-Phenyl-1H-pyrrole-2-carboxylic acid [cyclohexylc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research Curated by ChEMBL | Assay Description Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin | J Med Chem 47: 4286-90 (2004) Article DOI: 10.1021/jm040103i BindingDB Entry DOI: 10.7270/Q2CR5SV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50346864 (CHEMBL1797641 | CHEMBL3104337 | US8765820, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human LSD1/CoREST expressed in Escherichia coli using histone H3 peptide monomethylated at Lys4 as substrate by peroxidase-coupled assa... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50151164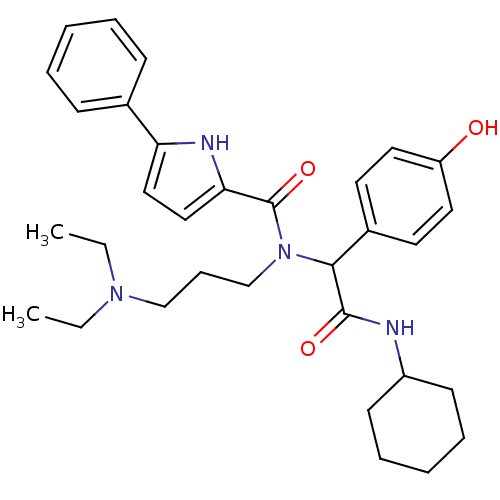 (5-Phenyl-1H-pyrrole-2-carboxylic acid [cyclohexylc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research Curated by ChEMBL | Assay Description Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin | J Med Chem 47: 4286-90 (2004) Article DOI: 10.1021/jm040103i BindingDB Entry DOI: 10.7270/Q2CR5SV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50151157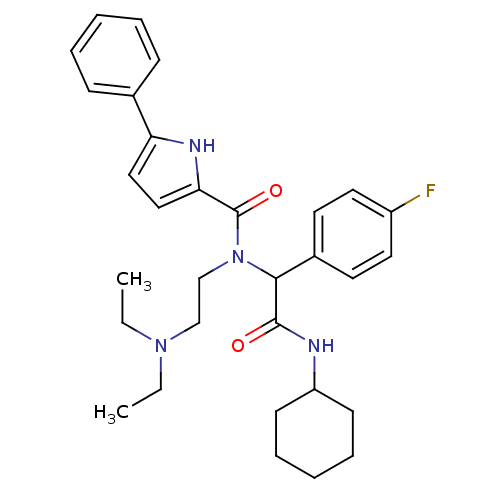 (5-Phenyl-1H-pyrrole-2-carboxylic acid [cyclohexylc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research Curated by ChEMBL | Assay Description Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin | J Med Chem 47: 4286-90 (2004) Article DOI: 10.1021/jm040103i BindingDB Entry DOI: 10.7270/Q2CR5SV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50017698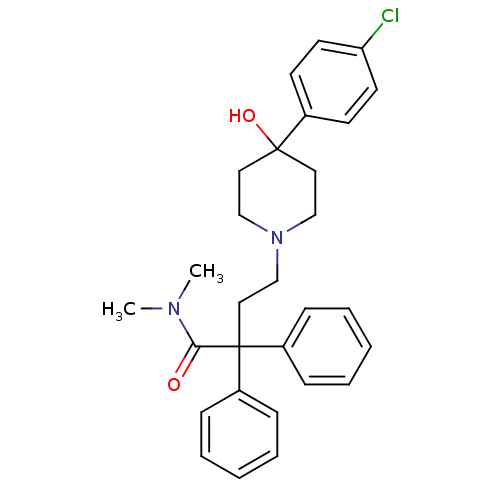 (4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-N,N...) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CES2A using 4-methylumbelliferone as a substrate | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112856 BindingDB Entry DOI: 10.7270/Q2NZ8CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50158869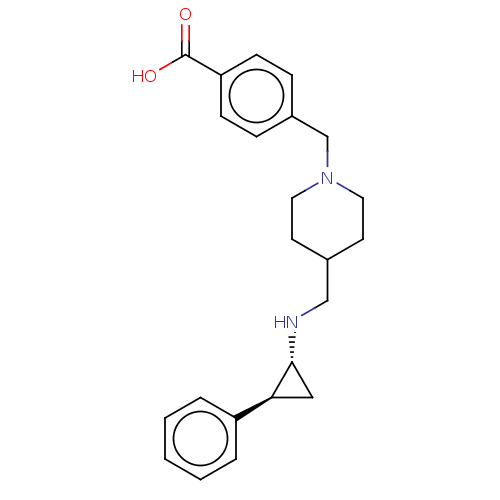 (CHEMBL3786182 | US10836743, Compound GSK-2879552 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of LSD1 (unknown origin) using H3K4(diMe) peptide as substrate measured after 60 mins by amplex red dye based HRP-coupled assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50174354 (CHEMBL373294 | [1-(2,5-Dichloro-benzenesulfonyl)-5...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd. Curated by ChEMBL | Assay Description Binding affinity towards human DP receptor expressed in CHO cells | J Med Chem 48: 6174-7 (2005) Article DOI: 10.1021/jm050519b BindingDB Entry DOI: 10.7270/Q2KK9B9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50346586 (CHEMBL1795981 | US8765820, 5a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human LSD1/CoREST expressed in Escherichia coli using histone H3 peptide monomethylated at Lys4 as substrate by peroxidase-coupled assa... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50151165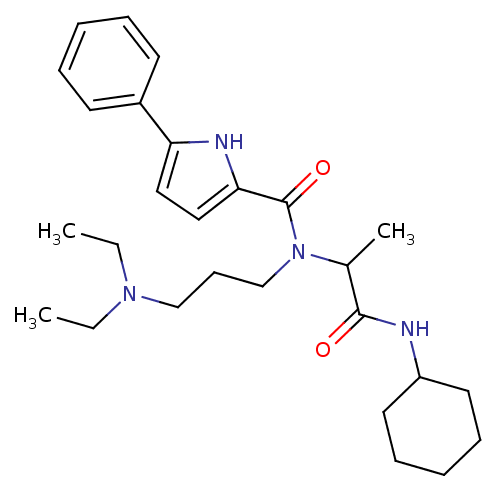 (5-Phenyl-1H-pyrrole-2-carboxylic acid (1-cyclohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research Curated by ChEMBL | Assay Description Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin | J Med Chem 47: 4286-90 (2004) Article DOI: 10.1021/jm040103i BindingDB Entry DOI: 10.7270/Q2CR5SV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50151170 (CHEMBL185887 | N-[Cyclohexylcarbamoyl-(4-fluoro-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research Curated by ChEMBL | Assay Description Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin | J Med Chem 47: 4286-90 (2004) Article DOI: 10.1021/jm040103i BindingDB Entry DOI: 10.7270/Q2CR5SV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | CHEMBL5274037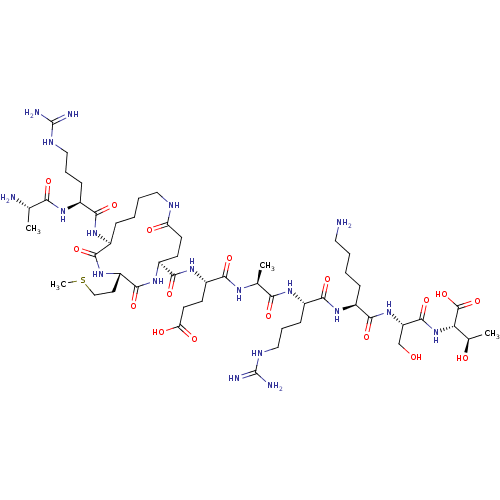 | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Mean functional activity against human H3 receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50346863 (CHEMBL1797640 | US8765820, 5b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MAO-A expressed in Pichia pastoris using kynuramine as substrate by peroxidase-coupled method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346866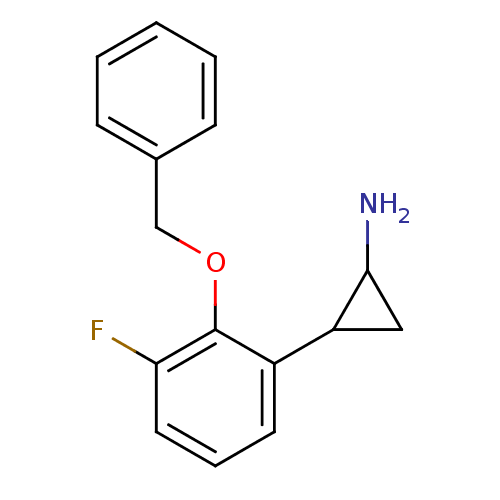 (CHEMBL1797643 | S1201) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hexahistidine-tagged human LSD1 (172 to 833 residues) expressed in Escherichia coli Rosetta (DE3) cells using H3K4me2 peptide as substr... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50151166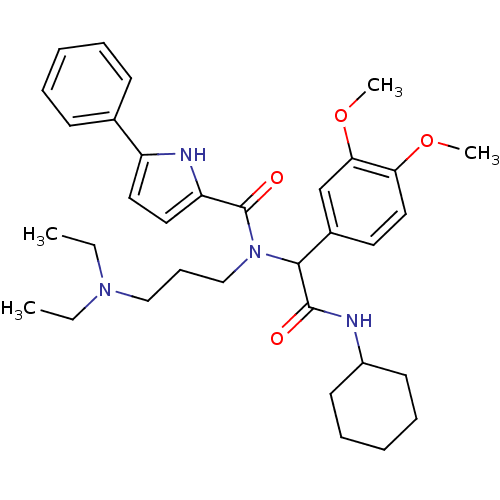 (5-Phenyl-1H-pyrrole-2-carboxylic acid [cyclohexylc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research Curated by ChEMBL | Assay Description Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin | J Med Chem 47: 4286-90 (2004) Article DOI: 10.1021/jm040103i BindingDB Entry DOI: 10.7270/Q2CR5SV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50174355 (CHEMBL194019 | [1-(1,2-Dimethyl-1H-imidazole-4-sul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd. Curated by ChEMBL | Assay Description Binding affinity towards human CRTH2 receptor expressed in CHO cells | J Med Chem 48: 6174-7 (2005) Article DOI: 10.1021/jm050519b BindingDB Entry DOI: 10.7270/Q2KK9B9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1398 total ) | Next | Last >> |