 Found 467 hits with Last Name = 'yamada' and Initial = 's'
Found 467 hits with Last Name = 'yamada' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22904

((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...)Show InChI InChI=1S/C6H11N3/c1-5(7)2-6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9)/t5-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of Histamine H3 receptor |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290098

((R)-3-Cyclohexylmethyl-1,8,8-trimethyl-3-aza-bicyc...)Show SMILES CC1(C)C2CC[C@@]1(C)CN(CC1CCCCC1)C2 |THB:10:9:1:5.4| Show InChI InChI=1S/C17H31N/c1-16(2)15-9-10-17(16,3)13-18(12-15)11-14-7-5-4-6-8-14/h14-15H,4-13H2,1-3H3/t15?,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290099

((S)-3-(4-Cyclohexyl-butyl)-1,8,8-trimethyl-3-aza-b...)Show SMILES CC1(C)C2CC[C@]1(C)CN(CCCCC1CCCCC1)C2 |THB:10:9:1:5.4| Show InChI InChI=1S/C20H37N/c1-19(2)18-12-13-20(19,3)16-21(15-18)14-8-7-11-17-9-5-4-6-10-17/h17-18H,4-16H2,1-3H3/t18?,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127610
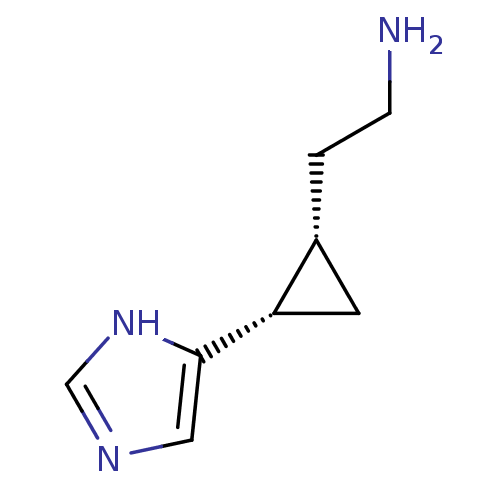
((1S,2S)-2-(2-aminoethyl)-1-(1H-imidazol-4-yl)cyclo...)Show InChI InChI=1S/C8H13N3/c9-2-1-6-3-7(6)8-4-10-5-11-8/h4-7H,1-3,9H2,(H,10,11)/t6-,7+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H3 receptor |
Bioorg Med Chem 18: 1076-82 (2010)
Article DOI: 10.1016/j.bmc.2009.12.046
BindingDB Entry DOI: 10.7270/Q21V5F35 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50127610

((1S,2S)-2-(2-aminoethyl)-1-(1H-imidazol-4-yl)cyclo...)Show InChI InChI=1S/C8H13N3/c9-2-1-6-3-7(6)8-4-10-5-11-8/h4-7H,1-3,9H2,(H,10,11)/t6-,7+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290100

((S)-3-Cyclohexylmethyl-1,8,8-trimethyl-3-aza-bicyc...)Show SMILES CC1(C)C2CC[C@]1(C)CN(CC1CCCCC1)C2 |THB:10:9:1:5.4| Show InChI InChI=1S/C17H31N/c1-16(2)15-9-10-17(16,3)13-18(12-15)11-14-7-5-4-6-8-14/h14-15H,4-13H2,1-3H3/t15?,17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290095

((R)-3-(2-Cyclohexyl-ethyl)-1,8,8-trimethyl-3-aza-b...)Show SMILES CC1(C)C2CC[C@@]1(C)CN(CCC1CCCCC1)C2 |THB:10:9:1:5.4| Show InChI InChI=1S/C18H33N/c1-17(2)16-9-11-18(17,3)14-19(13-16)12-10-15-7-5-4-6-8-15/h15-16H,4-14H2,1-3H3/t16?,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50165008

((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...)Show InChI InChI=1S/C22H31NO/c1-16(2)23(17(3)4)14-13-20(19-9-7-6-8-10-19)21-15-18(5)11-12-22(21)24/h6-12,15-17,20,24H,13-14H2,1-5H3/t20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from muscarinic receptor M1 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method |
J Med Chem 61: 4020-4029 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00041
BindingDB Entry DOI: 10.7270/Q29Z97H3 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290096

((S)-3-(2-Cyclopentyl-ethyl)-1,8,8-trimethyl-3-aza-...)Show SMILES CC1(C)C2CC[C@]1(C)CN(CCC1CCCC1)C2 |THB:10:9:1:5.4| Show InChI InChI=1S/C17H31N/c1-16(2)15-8-10-17(16,3)13-18(12-15)11-9-14-6-4-5-7-14/h14-15H,4-13H2,1-3H3/t15?,17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290094

((S)-3-(2-Cyclohexyl-ethyl)-1,8,8-trimethyl-3-aza-b...)Show SMILES CC1(C)C2CC[C@]1(C)CN(CCC1CCCCC1)C2 |THB:10:9:1:5.4| Show InChI InChI=1S/C18H33N/c1-17(2)16-9-11-18(17,3)14-19(13-16)12-10-15-7-5-4-6-8-15/h15-16H,4-14H2,1-3H3/t16?,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(RAT) | BDBM50165008

((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...)Show InChI InChI=1S/C22H31NO/c1-16(2)23(17(3)4)14-13-20(19-9-7-6-8-10-19)21-15-18(5)11-12-22(21)24/h6-12,15-17,20,24H,13-14H2,1-5H3/t20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from muscarinic receptor M5 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method |
J Med Chem 61: 4020-4029 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00041
BindingDB Entry DOI: 10.7270/Q29Z97H3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50127604
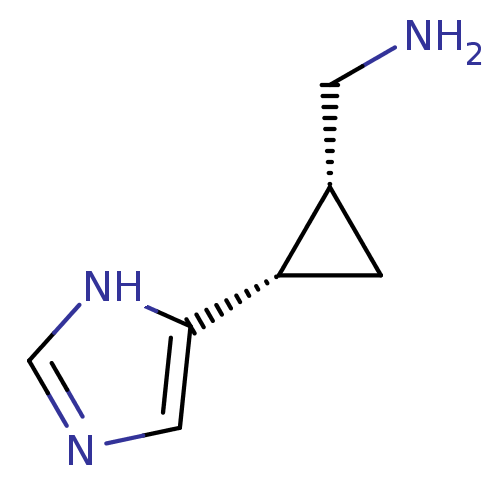
(C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...)Show InChI InChI=1S/C7H11N3/c8-2-5-1-6(5)7-3-9-4-10-7/h3-6H,1-2,8H2,(H,9,10)/t5-,6-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290101

((S)-3-(3-Cyclohexyl-propyl)-1,8,8-trimethyl-3-aza-...)Show SMILES CC1(C)C2CC[C@]1(C)CN(CCCC1CCCCC1)C2 |THB:10:9:1:5.4| Show InChI InChI=1S/C19H35N/c1-18(2)17-11-12-19(18,3)15-20(14-17)13-7-10-16-8-5-4-6-9-16/h16-17H,4-15H2,1-3H3/t17?,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50165008

((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...)Show InChI InChI=1S/C22H31NO/c1-16(2)23(17(3)4)14-13-20(19-9-7-6-8-10-19)21-15-18(5)11-12-22(21)24/h6-12,15-17,20,24H,13-14H2,1-5H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from muscarinic receptor M2 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method |
J Med Chem 61: 4020-4029 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00041
BindingDB Entry DOI: 10.7270/Q29Z97H3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290092

((S)-3-(2-Cycloheptyl-ethyl)-1,8,8-trimethyl-3-aza-...)Show SMILES CC1(C)C2CC[C@]1(C)CN(CCC1CCCCCC1)C2 |THB:10:9:1:5.4| Show InChI InChI=1S/C19H35N/c1-18(2)17-10-12-19(18,3)15-20(14-17)13-11-16-8-6-4-5-7-9-16/h16-17H,4-15H2,1-3H3/t17?,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290093

((S)-3-(2-Cyclooctyl-ethyl)-1,8,8-trimethyl-3-aza-b...)Show SMILES CC1(C)C2CC[C@]1(C)CN(CCC1CCCCCCC1)C2 |THB:10:9:1:5.4| Show InChI InChI=1S/C20H37N/c1-19(2)18-11-13-20(19,3)16-21(15-18)14-12-17-9-7-5-4-6-8-10-17/h17-18H,4-16H2,1-3H3/t18?,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290097
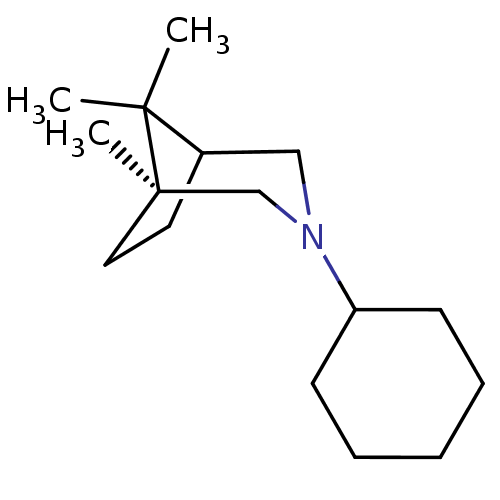
((S)-3-Cyclohexyl-1,8,8-trimethyl-3-aza-bicyclo[3.2...)Show SMILES CC1(C)C2CC[C@]1(C)CN(C2)C1CCCCC1 |THB:11:9:1:5.4| Show InChI InChI=1S/C16H29N/c1-15(2)13-9-10-16(15,3)12-17(11-13)14-7-5-4-6-8-14/h13-14H,4-12H2,1-3H3/t13?,16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50165008

((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...)Show InChI InChI=1S/C22H31NO/c1-16(2)23(17(3)4)14-13-20(19-9-7-6-8-10-19)21-15-18(5)11-12-22(21)24/h6-12,15-17,20,24H,13-14H2,1-5H3/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from muscarinic receptor M4 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method |
J Med Chem 61: 4020-4029 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00041
BindingDB Entry DOI: 10.7270/Q29Z97H3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50194208
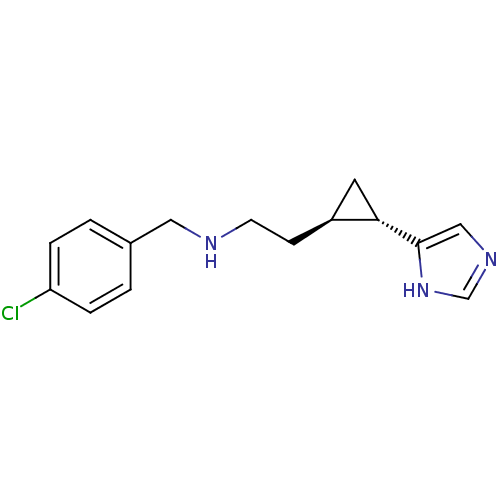
((1S,2R)-trans-2-[2-(4-chlorobenzylamino)ethyl]-1-(...)Show InChI InChI=1S/C15H18ClN3/c16-13-3-1-11(2-4-13)8-17-6-5-12-7-14(12)15-9-18-10-19-15/h1-4,9-10,12,14,17H,5-8H2,(H,18,19)/t12-,14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methyl-histamine from human histamine H3 receptor expressed in human HEK293-EBNA cells |
J Med Chem 53: 3585-93 (2010)
Article DOI: 10.1021/jm901848b
BindingDB Entry DOI: 10.7270/Q2MP53DM |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165008

((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...)Show InChI InChI=1S/C22H31NO/c1-16(2)23(17(3)4)14-13-20(19-9-7-6-8-10-19)21-15-18(5)11-12-22(21)24/h6-12,15-17,20,24H,13-14H2,1-5H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from muscarinic receptor M3 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method |
J Med Chem 61: 4020-4029 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00041
BindingDB Entry DOI: 10.7270/Q29Z97H3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50194208

((1S,2R)-trans-2-[2-(4-chlorobenzylamino)ethyl]-1-(...)Show InChI InChI=1S/C15H18ClN3/c16-13-3-1-11(2-4-13)8-17-6-5-12-7-14(12)15-9-18-10-19-15/h1-4,9-10,12,14,17H,5-8H2,(H,18,19)/t12-,14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine form human H3 receptor |
J Med Chem 49: 5587-96 (2006)
Article DOI: 10.1021/jm0603318
BindingDB Entry DOI: 10.7270/Q2MW2GRK |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290091

((S)-3-(2-Adamantan-2-yl-ethyl)-1,8,8-trimethyl-3-a...)Show SMILES CC1(C)C2CC[C@]1(C)CN(CCC1C3CC4CC(C3)CC1C4)C2 |wD:6.7,TLB:11:12:14:17.18.16,THB:16:15:12:17.18.19,16:17:12:15.14.21,10:9:1:5.4,11:12:15.14.21:17.18.19,19:20:14:17.18.16,19:17:14:20.12.21,(-7.25,2.8,;-5.75,2.79,;-6.14,4.25,;-4.39,2.21,;-4,.76,;-4.93,-.34,;-4.93,1.16,;-6.43,1.16,;-2.69,2.13,;-1.35,1.44,;-.27,.4,;1.17,.82,;2.25,-.23,;2.88,-1.98,;2.25,-3.46,;3.36,-4.49,;5,-4.47,;5.66,-2.93,;4.41,-2.03,;5.18,-1.24,;3.56,-1.24,;2.87,-2.78,;-2.32,3.17,)| Show InChI InChI=1S/C22H37N/c1-21(2)19-4-6-22(21,3)14-23(13-19)7-5-20-17-9-15-8-16(11-17)12-18(20)10-15/h15-20H,4-14H2,1-3H3/t15?,16?,17?,18?,19?,20?,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50127605
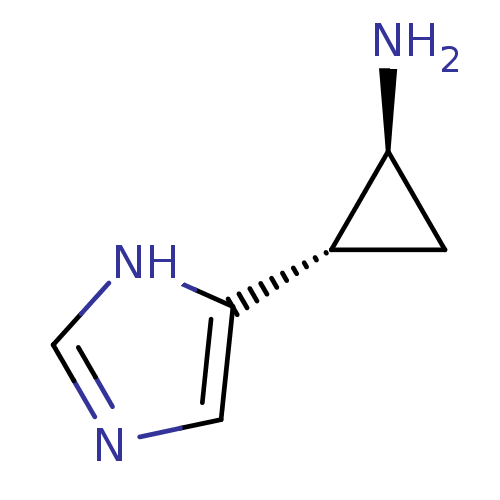
((1S,2S)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...)Show InChI InChI=1S/C6H9N3/c7-5-1-4(5)6-2-8-3-9-6/h2-5H,1,7H2,(H,8,9)/t4-,5-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50374004
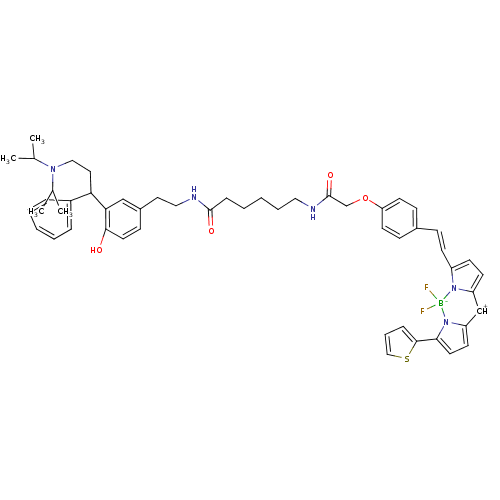
(CHEMBL271108)Show SMILES CC(C)N(CCC(c1ccccc1)c1cc(CCNC(=O)CCCCCNC(=O)COc2ccc([CH+]\C=c3/ccc4=Cc5ccc(-c6cccs6)n5[B-](F)(F)n34)cc2)ccc1O)C(C)C |t:41| Show InChI InChI=1S/C52H60BF2N5O4S/c1-37(2)58(38(3)4)32-29-46(41-12-7-5-8-13-41)47-34-40(19-27-49(47)61)28-31-57-51(62)15-9-6-10-30-56-52(63)36-64-45-24-17-39(18-25-45)16-20-42-21-22-43-35-44-23-26-48(50-14-11-33-65-50)60(44)53(54,55)59(42)43/h5,7-8,11-14,16-27,33-35,37-38,46,61H,6,9-10,15,28-32,36H2,1-4H3,(H,56,63)(H,57,62)/b42-20+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from muscarinic receptor M1 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method |
J Med Chem 61: 4020-4029 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00041
BindingDB Entry DOI: 10.7270/Q29Z97H3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50127608
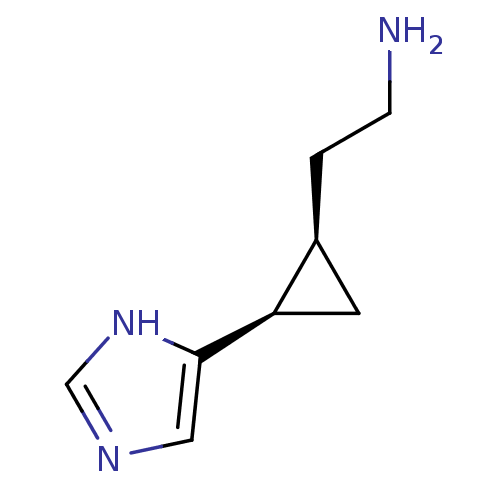
(2-[2-(1H-Imidazol-4-yl)-cyclopropyl]-ethylamine | ...)Show InChI InChI=1S/C8H13N3/c9-2-1-6-3-7(6)8-4-10-5-11-8/h4-7H,1-3,9H2,(H,10,11)/t6-,7+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 5.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]YM-09151-2 from human dopamine D2S receptor in membrane suspensions by liquid scintillation counter |
Bioorg Med Chem 16: 8875-81 (2008)
Article DOI: 10.1016/j.bmc.2008.08.061
BindingDB Entry DOI: 10.7270/Q2WQ03M7 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50194202
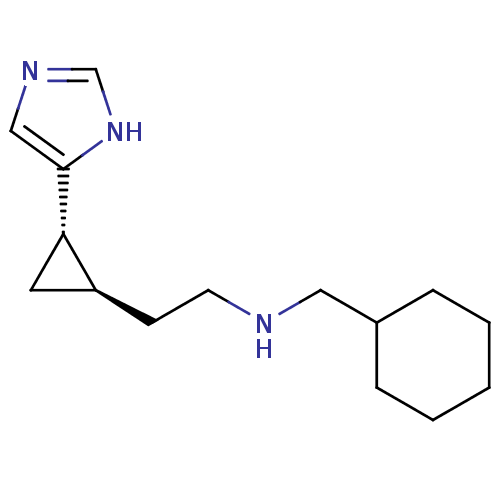
((1S,2R)-trans-2-[2-(cyclohexylmethylamino)ethyl]-1...)Show InChI InChI=1S/C15H25N3/c1-2-4-12(5-3-1)9-16-7-6-13-8-14(13)15-10-17-11-18-15/h10-14,16H,1-9H2,(H,17,18)/t13-,14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine form human H3 receptor |
J Med Chem 49: 5587-96 (2006)
Article DOI: 10.1021/jm0603318
BindingDB Entry DOI: 10.7270/Q2MW2GRK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50374004

(CHEMBL271108)Show SMILES CC(C)N(CCC(c1ccccc1)c1cc(CCNC(=O)CCCCCNC(=O)COc2ccc([CH+]\C=c3/ccc4=Cc5ccc(-c6cccs6)n5[B-](F)(F)n34)cc2)ccc1O)C(C)C |t:41| Show InChI InChI=1S/C52H60BF2N5O4S/c1-37(2)58(38(3)4)32-29-46(41-12-7-5-8-13-41)47-34-40(19-27-49(47)61)28-31-57-51(62)15-9-6-10-30-56-52(63)36-64-45-24-17-39(18-25-45)16-20-42-21-22-43-35-44-23-26-48(50-14-11-33-65-50)60(44)53(54,55)59(42)43/h5,7-8,11-14,16-27,33-35,37-38,46,61H,6,9-10,15,28-32,36H2,1-4H3,(H,56,63)(H,57,62)/b42-20+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from muscarinic receptor M4 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method |
J Med Chem 61: 4020-4029 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00041
BindingDB Entry DOI: 10.7270/Q29Z97H3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50318106

((1S,2R)-2-[(1R)-1-Ethyl-2-(4-chlorobenzylamino)eth...)Show SMILES CC[C@@H](CNCc1ccc(Cl)cc1)[C@H]1C[C@@H]1c1cnc[nH]1 |r| Show InChI InChI=1S/C17H22ClN3/c1-2-13(15-7-16(15)17-10-20-11-21-17)9-19-8-12-3-5-14(18)6-4-12/h3-6,10-11,13,15-16,19H,2,7-9H2,1H3,(H,20,21)/t13-,15+,16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methyl-histamine from human histamine H3 receptor expressed in human HEK293-EBNA cells |
J Med Chem 53: 3585-93 (2010)
Article DOI: 10.1021/jm901848b
BindingDB Entry DOI: 10.7270/Q2MP53DM |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50194205
((1R,2S)-trans-2-[2-(4-chlorobenzylamino)ethyl]-1-(...)Show InChI InChI=1S/C15H18ClN3/c16-13-3-1-11(2-4-13)8-17-6-5-12-7-14(12)15-9-18-10-19-15/h1-4,9-10,12,14,17H,5-8H2,(H,18,19)/t12-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine form human H4 receptor |
J Med Chem 49: 5587-96 (2006)
Article DOI: 10.1021/jm0603318
BindingDB Entry DOI: 10.7270/Q2MW2GRK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50194203
((1R,2R)-cis-2-[2-(4-chlorobenzylamino)ethyl]-1-(1H...)Show InChI InChI=1S/C15H18ClN3/c16-13-3-1-11(2-4-13)8-17-6-5-12-7-14(12)15-9-18-10-19-15/h1-4,9-10,12,14,17H,5-8H2,(H,18,19)/t12-,14+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methyl-histamine from human histamine H3 receptor expressed in human HEK293-EBNA cells |
J Med Chem 53: 3585-93 (2010)
Article DOI: 10.1021/jm901848b
BindingDB Entry DOI: 10.7270/Q2MP53DM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50194205

((1R,2S)-trans-2-[2-(4-chlorobenzylamino)ethyl]-1-(...)Show InChI InChI=1S/C15H18ClN3/c16-13-3-1-11(2-4-13)8-17-6-5-12-7-14(12)15-9-18-10-19-15/h1-4,9-10,12,14,17H,5-8H2,(H,18,19)/t12-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine form human H3 receptor |
J Med Chem 49: 5587-96 (2006)
Article DOI: 10.1021/jm0603318
BindingDB Entry DOI: 10.7270/Q2MW2GRK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50374004

(CHEMBL271108)Show SMILES CC(C)N(CCC(c1ccccc1)c1cc(CCNC(=O)CCCCCNC(=O)COc2ccc([CH+]\C=c3/ccc4=Cc5ccc(-c6cccs6)n5[B-](F)(F)n34)cc2)ccc1O)C(C)C |t:41| Show InChI InChI=1S/C52H60BF2N5O4S/c1-37(2)58(38(3)4)32-29-46(41-12-7-5-8-13-41)47-34-40(19-27-49(47)61)28-31-57-51(62)15-9-6-10-30-56-52(63)36-64-45-24-17-39(18-25-45)16-20-42-21-22-43-35-44-23-26-48(50-14-11-33-65-50)60(44)53(54,55)59(42)43/h5,7-8,11-14,16-27,33-35,37-38,46,61H,6,9-10,15,28-32,36H2,1-4H3,(H,56,63)(H,57,62)/b42-20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from muscarinic receptor M2 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method |
J Med Chem 61: 4020-4029 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00041
BindingDB Entry DOI: 10.7270/Q29Z97H3 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50374004

(CHEMBL271108)Show SMILES CC(C)N(CCC(c1ccccc1)c1cc(CCNC(=O)CCCCCNC(=O)COc2ccc([CH+]\C=c3/ccc4=Cc5ccc(-c6cccs6)n5[B-](F)(F)n34)cc2)ccc1O)C(C)C |t:41| Show InChI InChI=1S/C52H60BF2N5O4S/c1-37(2)58(38(3)4)32-29-46(41-12-7-5-8-13-41)47-34-40(19-27-49(47)61)28-31-57-51(62)15-9-6-10-30-56-52(63)36-64-45-24-17-39(18-25-45)16-20-42-21-22-43-35-44-23-26-48(50-14-11-33-65-50)60(44)53(54,55)59(42)43/h5,7-8,11-14,16-27,33-35,37-38,46,61H,6,9-10,15,28-32,36H2,1-4H3,(H,56,63)(H,57,62)/b42-20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from muscarinic receptor M3 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method |
J Med Chem 61: 4020-4029 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00041
BindingDB Entry DOI: 10.7270/Q29Z97H3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50127603
(C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...)Show InChI InChI=1S/C7H11N3/c8-2-5-1-6(5)7-3-9-4-10-7/h3-6H,1-2,8H2,(H,9,10)/t5-,6+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50194203

((1R,2R)-cis-2-[2-(4-chlorobenzylamino)ethyl]-1-(1H...)Show InChI InChI=1S/C15H18ClN3/c16-13-3-1-11(2-4-13)8-17-6-5-12-7-14(12)15-9-18-10-19-15/h1-4,9-10,12,14,17H,5-8H2,(H,18,19)/t12-,14+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine form human H3 receptor |
J Med Chem 49: 5587-96 (2006)
Article DOI: 10.1021/jm0603318
BindingDB Entry DOI: 10.7270/Q2MW2GRK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(RAT) | BDBM50374004

(CHEMBL271108)Show SMILES CC(C)N(CCC(c1ccccc1)c1cc(CCNC(=O)CCCCCNC(=O)COc2ccc([CH+]\C=c3/ccc4=Cc5ccc(-c6cccs6)n5[B-](F)(F)n34)cc2)ccc1O)C(C)C |t:41| Show InChI InChI=1S/C52H60BF2N5O4S/c1-37(2)58(38(3)4)32-29-46(41-12-7-5-8-13-41)47-34-40(19-27-49(47)61)28-31-57-51(62)15-9-6-10-30-56-52(63)36-64-45-24-17-39(18-25-45)16-20-42-21-22-43-35-44-23-26-48(50-14-11-33-65-50)60(44)53(54,55)59(42)43/h5,7-8,11-14,16-27,33-35,37-38,46,61H,6,9-10,15,28-32,36H2,1-4H3,(H,56,63)(H,57,62)/b42-20+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from muscarinic receptor M5 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method |
J Med Chem 61: 4020-4029 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00041
BindingDB Entry DOI: 10.7270/Q29Z97H3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50318103

((1R,2S)-2-[(1R)-1-Ethyl-2-(4-chlorobenzylamino)eth...)Show SMILES CC[C@@H](CNCc1ccc(Cl)cc1)[C@@H]1C[C@H]1c1cnc[nH]1 |r| Show InChI InChI=1S/C17H22ClN3/c1-2-13(15-7-16(15)17-10-20-11-21-17)9-19-8-12-3-5-14(18)6-4-12/h3-6,10-11,13,15-16,19H,2,7-9H2,1H3,(H,20,21)/t13-,15-,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methyl-histamine from human histamine H3 receptor expressed in human HEK293-EBNA cells |
J Med Chem 53: 3585-93 (2010)
Article DOI: 10.1021/jm901848b
BindingDB Entry DOI: 10.7270/Q2MP53DM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50194211
((1S,2S)-cis-2-[2-(cyclohexylmethylamino)ethyl]-1-(...)Show InChI InChI=1S/C15H25N3/c1-2-4-12(5-3-1)9-16-7-6-13-8-14(13)15-10-17-11-18-15/h10-14,16H,1-9H2,(H,17,18)/t13-,14+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine form human H3 receptor |
J Med Chem 49: 5587-96 (2006)
Article DOI: 10.1021/jm0603318
BindingDB Entry DOI: 10.7270/Q2MW2GRK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50127609
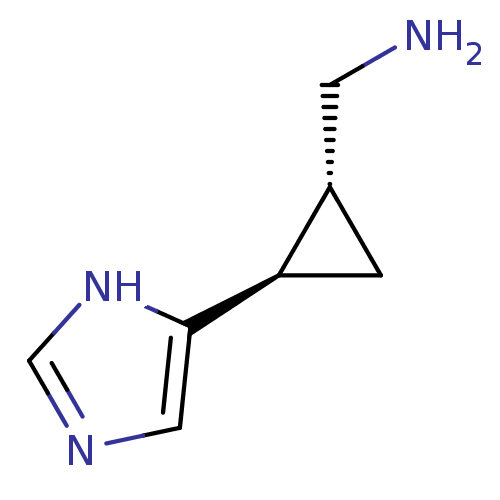
(C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...)Show InChI InChI=1S/C7H11N3/c8-2-5-1-6(5)7-3-9-4-10-7/h3-6H,1-2,8H2,(H,9,10)/t5-,6+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM81982
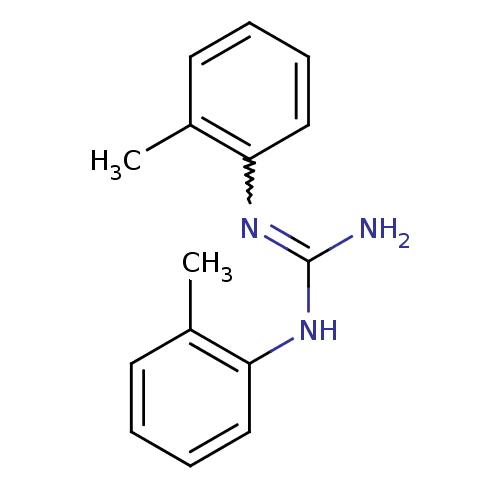
(CAS_97-39-2 | DITOLYLGUANIDINE | DTG | Di-o-tolylg...)Show InChI InChI=1S/C15H17N3/c1-11-7-3-5-9-13(11)17-15(16)18-14-10-6-4-8-12(14)2/h3-10H,1-2H3,(H3,16,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50194206

((1R,2S)-trans-2-[2-(cyclohexylmethylamino)ethyl]-1...)Show InChI InChI=1S/C15H25N3/c1-2-4-12(5-3-1)9-16-7-6-13-8-14(13)15-10-17-11-18-15/h10-14,16H,1-9H2,(H,17,18)/t13-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine form human H4 receptor |
J Med Chem 49: 5587-96 (2006)
Article DOI: 10.1021/jm0603318
BindingDB Entry DOI: 10.7270/Q2MW2GRK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50194209
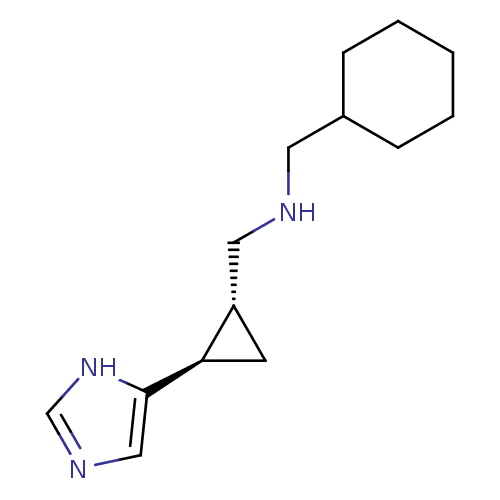
((1R,2R)-trans-2-(cyclohexylmethylamino)methyl-1-(1...)Show InChI InChI=1S/C14H23N3/c1-2-4-11(5-3-1)7-15-8-12-6-13(12)14-9-16-10-17-14/h9-13,15H,1-8H2,(H,16,17)/t12-,13+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine form human H3 receptor |
J Med Chem 49: 5587-96 (2006)
Article DOI: 10.1021/jm0603318
BindingDB Entry DOI: 10.7270/Q2MW2GRK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50194206

((1R,2S)-trans-2-[2-(cyclohexylmethylamino)ethyl]-1...)Show InChI InChI=1S/C15H25N3/c1-2-4-12(5-3-1)9-16-7-6-13-8-14(13)15-10-17-11-18-15/h10-14,16H,1-9H2,(H,17,18)/t13-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine form human H3 receptor |
J Med Chem 49: 5587-96 (2006)
Article DOI: 10.1021/jm0603318
BindingDB Entry DOI: 10.7270/Q2MW2GRK |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50194208

((1S,2R)-trans-2-[2-(4-chlorobenzylamino)ethyl]-1-(...)Show InChI InChI=1S/C15H18ClN3/c16-13-3-1-11(2-4-13)8-17-6-5-12-7-14(12)15-9-18-10-19-15/h1-4,9-10,12,14,17H,5-8H2,(H,18,19)/t12-,14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine form human H4 receptor |
J Med Chem 49: 5587-96 (2006)
Article DOI: 10.1021/jm0603318
BindingDB Entry DOI: 10.7270/Q2MW2GRK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50310163
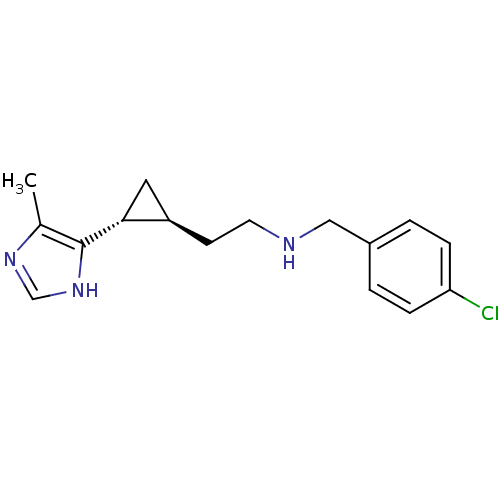
((1R,2S)-2-[2-(4-Chlorobenzylamino)ethyl]-1-(5(4)-m...)Show SMILES Cc1nc[nH]c1[C@@H]1C[C@H]1CCNCc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C16H20ClN3/c1-11-16(20-10-19-11)15-8-13(15)6-7-18-9-12-2-4-14(17)5-3-12/h2-5,10,13,15,18H,6-9H2,1H3,(H,19,20)/t13-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylahistamine from human histamine H3 receptor |
Bioorg Med Chem 18: 1076-82 (2010)
Article DOI: 10.1016/j.bmc.2009.12.046
BindingDB Entry DOI: 10.7270/Q21V5F35 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50127607
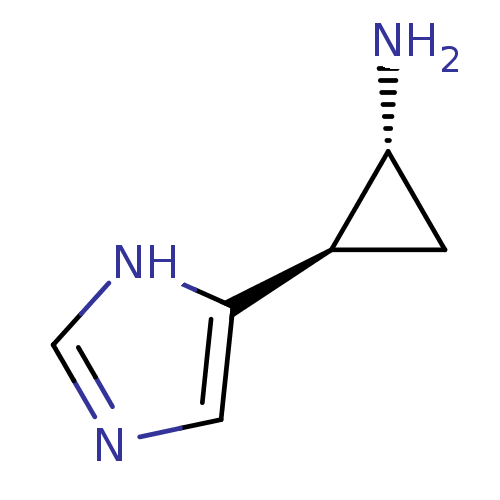
((1R,2R)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...)Show InChI InChI=1S/C6H9N3/c7-5-1-4(5)6-2-8-3-9-6/h2-5H,1,7H2,(H,8,9)/t4-,5-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50194212
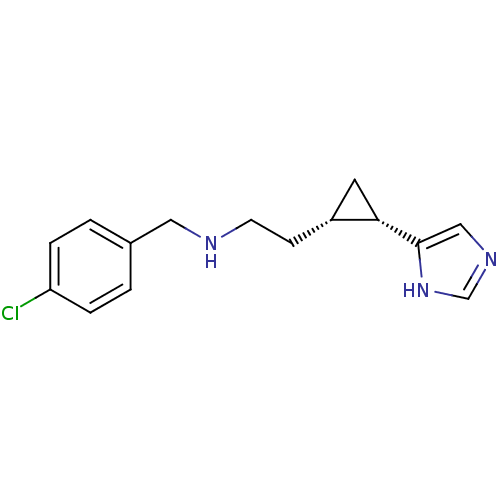
((1S,2S)-cis-2-[2-(4-chlorobenzylamino)ethyl]-1-(1H...)Show InChI InChI=1S/C15H18ClN3/c16-13-3-1-11(2-4-13)8-17-6-5-12-7-14(12)15-9-18-10-19-15/h1-4,9-10,12,14,17H,5-8H2,(H,18,19)/t12-,14+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine form human H3 receptor |
J Med Chem 49: 5587-96 (2006)
Article DOI: 10.1021/jm0603318
BindingDB Entry DOI: 10.7270/Q2MW2GRK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data