 Found 416 hits with Last Name = 'yamazaki' and Initial = 'h'
Found 416 hits with Last Name = 'yamazaki' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429605

(CHEMBL2334538)Show SMILES COc1ccc(cc1)C(=O)O[C@H]1C[C@H]2[C@](C)(COC(C)=O)[C@H](CC[C@]2(C)[C@H]2[C@@H](O)c3c(O[C@]12C)cc(oc3=O)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C37H41NO11/c1-20(39)45-19-36(4)27-17-29(48-33(42)22-9-11-24(44-6)12-10-22)37(5)32(35(27,3)14-13-28(36)46-21(2)40)31(41)30-26(49-37)16-25(47-34(30)43)23-8-7-15-38-18-23/h7-12,15-16,18,27-29,31-32,41H,13-14,17,19H2,1-6H3/t27-,28+,29+,31+,32-,35+,36+,37-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429604
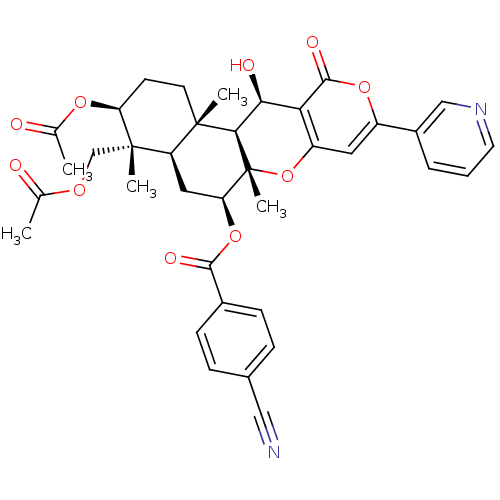
(CHEMBL2334539)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(cc1)C#N)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C37H38N2O10/c1-20(40)45-19-36(4)27-16-29(48-33(43)23-10-8-22(17-38)9-11-23)37(5)32(35(27,3)13-12-28(36)46-21(2)41)31(42)30-26(49-37)15-25(47-34(30)44)24-7-6-14-39-18-24/h6-11,14-15,18,27-29,31-32,42H,12-13,16,19H2,1-5H3/t27-,28+,29+,31+,32-,35+,36+,37-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429604

(CHEMBL2334539)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(cc1)C#N)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C37H38N2O10/c1-20(40)45-19-36(4)27-16-29(48-33(43)23-10-8-22(17-38)9-11-23)37(5)32(35(27,3)13-12-28(36)46-21(2)41)31(42)30-26(49-37)15-25(47-34(30)44)24-7-6-14-39-18-24/h6-11,14-15,18,27-29,31-32,42H,12-13,16,19H2,1-5H3/t27-,28+,29+,31+,32-,35+,36+,37-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) |
Bioorg Med Chem Lett 23: 3798-801 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.075
BindingDB Entry DOI: 10.7270/Q2WD41Z9 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429610

(CHEMBL2334222)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(C)cc1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C37H41NO10/c1-20-9-11-23(12-10-20)33(42)47-29-17-27-35(4,14-13-28(45-22(3)40)36(27,5)19-44-21(2)39)32-31(41)30-26(48-37(29,32)6)16-25(46-34(30)43)24-8-7-15-38-18-24/h7-12,15-16,18,27-29,31-32,41H,13-14,17,19H2,1-6H3/t27-,28+,29+,31+,32-,35+,36+,37-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429603

(CHEMBL2334540)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1cccc(F)c1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38FNO10/c1-19(39)44-18-35(4)26-16-28(47-32(42)21-8-6-10-23(37)14-21)36(5)31(34(26,3)12-11-27(35)45-20(2)40)30(41)29-25(48-36)15-24(46-33(29)43)22-9-7-13-38-17-22/h6-10,13-15,17,26-28,30-31,41H,11-12,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429601

(CHEMBL2333808)Show SMILES CCc1ccc(cc1)C(=O)O[C@H]1C[C@H]2[C@](C)(COC(C)=O)[C@H](CC[C@]2(C)[C@H]2[C@@H](O)c3c(O[C@]12C)cc(oc3=O)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C38H43NO10/c1-7-23-10-12-24(13-11-23)34(43)48-30-18-28-36(4,15-14-29(46-22(3)41)37(28,5)20-45-21(2)40)33-32(42)31-27(49-38(30,33)6)17-26(47-35(31)44)25-9-8-16-39-19-25/h8-13,16-17,19,28-30,32-33,42H,7,14-15,18,20H2,1-6H3/t28-,29+,30+,32+,33-,36+,37+,38-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429602

(CHEMBL2334541)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(Cl)cc1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38ClNO10/c1-19(39)44-18-35(4)26-16-28(47-32(42)21-8-10-23(37)11-9-21)36(5)31(34(26,3)13-12-27(35)45-20(2)40)30(41)29-25(48-36)15-24(46-33(29)43)22-7-6-14-38-17-22/h6-11,14-15,17,26-28,30-31,41H,12-13,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429600

(CHEMBL2333809)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1cccc(Br)c1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38BrNO10/c1-19(39)44-18-35(4)26-16-28(47-32(42)21-8-6-10-23(37)14-21)36(5)31(34(26,3)12-11-27(35)45-20(2)40)30(41)29-25(48-36)15-24(46-33(29)43)22-9-7-13-38-17-22/h6-10,13-15,17,26-28,30-31,41H,11-12,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM85330

(CAS_68647 | NSC_68647 | ONDANSETRON | Ondansetron ...)Show InChI InChI=1S/C18H19N3O/c1-12-19-9-10-21(12)11-13-7-8-16-17(18(13)22)14-5-3-4-6-15(14)20(16)2/h3-6,9-10,13H,7-8,11H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 receptor in rat cortical membrane using [3H]GR-65630 as radioligand |
Bioorg Med Chem Lett 8: 619-24 (1999)
BindingDB Entry DOI: 10.7270/Q25Q4WN6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50366571

(CHEMBL1788261)Show SMILES CCN1CCN(C)C[C@@H](C1)NC(=O)c1cc(Cl)c(NC)cc1OC |r| Show InChI InChI=1S/C17H27ClN4O2/c1-5-22-7-6-21(3)10-12(11-22)20-17(23)13-8-14(18)15(19-2)9-16(13)24-4/h8-9,12,19H,5-7,10-11H2,1-4H3,(H,20,23)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 receptor in rat cortical membrane using [3H]GR-65630 as radioligand |
Bioorg Med Chem Lett 8: 619-24 (1999)
BindingDB Entry DOI: 10.7270/Q25Q4WN6 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429599
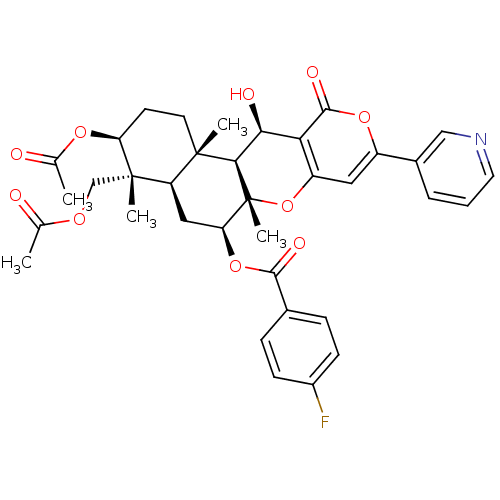
(CHEMBL2333810)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(F)cc1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38FNO10/c1-19(39)44-18-35(4)26-16-28(47-32(42)21-8-10-23(37)11-9-21)36(5)31(34(26,3)13-12-27(35)45-20(2)40)30(41)29-25(48-36)15-24(46-33(29)43)22-7-6-14-38-17-22/h6-11,14-15,17,26-28,30-31,41H,12-13,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50069286

(5-Chloro-4-ethylamino-N-(1-ethyl-4-methyl-[1,4]dia...)Show InChI InChI=1S/C18H29ClN4O2/c1-5-20-16-10-17(25-4)14(9-15(16)19)18(24)21-13-11-22(3)7-8-23(6-2)12-13/h9-10,13,20H,5-8,11-12H2,1-4H3,(H,21,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 receptor in rat cortical membrane using [3H]GR-65630 as radioligand |
Bioorg Med Chem Lett 8: 619-24 (1999)
BindingDB Entry DOI: 10.7270/Q25Q4WN6 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429598

(CHEMBL2333811)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(Br)cc1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38BrNO10/c1-19(39)44-18-35(4)26-16-28(47-32(42)21-8-10-23(37)11-9-21)36(5)31(34(26,3)13-12-27(35)45-20(2)40)30(41)29-25(48-36)15-24(46-33(29)43)22-7-6-14-38-17-22/h6-11,14-15,17,26-28,30-31,41H,12-13,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50112041
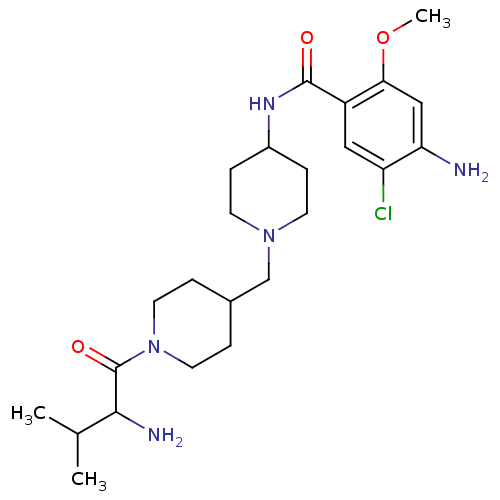
(4-Amino-N-{1-[1-(2-amino-3-methyl-butyryl)-piperid...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CCN(CC2CCN(CC2)C(=O)C(N)C(C)C)CC1 Show InChI InChI=1S/C24H38ClN5O3/c1-15(2)22(27)24(32)30-10-4-16(5-11-30)14-29-8-6-17(7-9-29)28-23(31)18-12-19(25)20(26)13-21(18)33-3/h12-13,15-17,22H,4-11,14,26-27H2,1-3H3,(H,28,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand |
Bioorg Med Chem Lett 12: 967-70 (2002)
BindingDB Entry DOI: 10.7270/Q2RF5TB7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50241107

(1-(3-(4-(5-chloro-2-oxo-2,3-dihydrobenzo[d]imidazo...)Show SMILES Clc1ccc2n(C3CCN(CCCn4c5ccccc5[nH]c4=O)CC3)c(=O)[nH]c2c1 Show InChI InChI=1S/C22H24ClN5O2/c23-15-6-7-20-18(14-15)25-22(30)28(20)16-8-12-26(13-9-16)10-3-11-27-19-5-2-1-4-17(19)24-21(27)29/h1-2,4-7,14,16H,3,8-13H2,(H,24,29)(H,25,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against dopamine D2 receptor in rat brain synaptic membrane using [3H]-spiperone as radioligand |
Bioorg Med Chem Lett 8: 619-24 (1999)
BindingDB Entry DOI: 10.7270/Q25Q4WN6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50112039
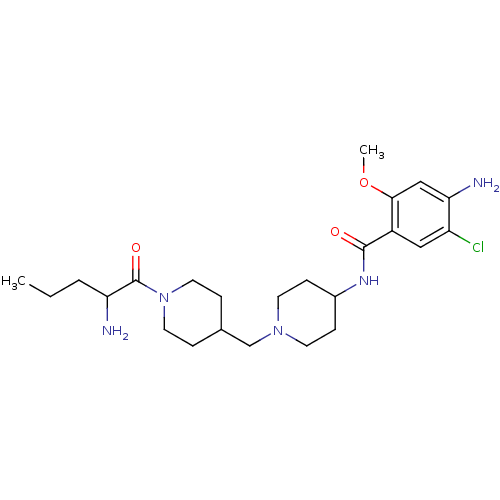
(4-Amino-N-{1-[1-(2-amino-pentanoyl)-piperidin-4-yl...)Show SMILES CCCC(N)C(=O)N1CCC(CN2CCC(CC2)NC(=O)c2cc(Cl)c(N)cc2OC)CC1 Show InChI InChI=1S/C24H38ClN5O3/c1-3-4-20(26)24(32)30-11-5-16(6-12-30)15-29-9-7-17(8-10-29)28-23(31)18-13-19(25)21(27)14-22(18)33-2/h13-14,16-17,20H,3-12,15,26-27H2,1-2H3,(H,28,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand |
Bioorg Med Chem Lett 12: 967-70 (2002)
BindingDB Entry DOI: 10.7270/Q2RF5TB7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50112038

(4-Amino-N-{1-[1-(2-amino-2-methyl-propionyl)-piper...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CCN(CC2CCN(CC2)C(=O)C(C)(C)N)CC1 Show InChI InChI=1S/C23H36ClN5O3/c1-23(2,26)22(31)29-10-4-15(5-11-29)14-28-8-6-16(7-9-28)27-21(30)17-12-18(24)19(25)13-20(17)32-3/h12-13,15-16H,4-11,14,25-26H2,1-3H3,(H,27,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand |
Bioorg Med Chem Lett 12: 967-70 (2002)
BindingDB Entry DOI: 10.7270/Q2RF5TB7 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429597
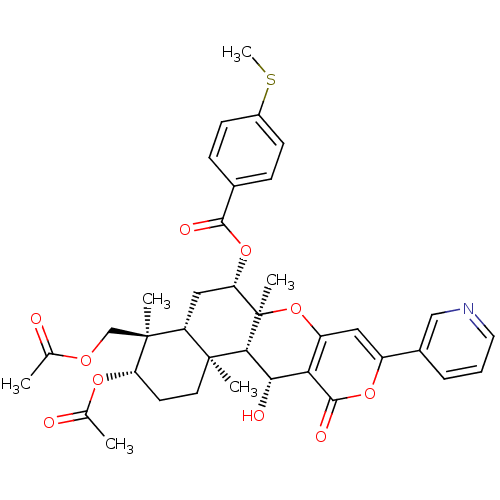
(CHEMBL2333812)Show SMILES CSc1ccc(cc1)C(=O)O[C@H]1C[C@H]2[C@](C)(COC(C)=O)[C@H](CC[C@]2(C)[C@H]2[C@@H](O)c3c(O[C@]12C)cc(oc3=O)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C37H41NO10S/c1-20(39)44-19-36(4)27-17-29(47-33(42)22-9-11-24(49-6)12-10-22)37(5)32(35(27,3)14-13-28(36)45-21(2)40)31(41)30-26(48-37)16-25(46-34(30)43)23-8-7-15-38-18-23/h7-12,15-16,18,27-29,31-32,41H,13-14,17,19H2,1-6H3/t27-,28+,29+,31+,32-,35+,36+,37-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50070570
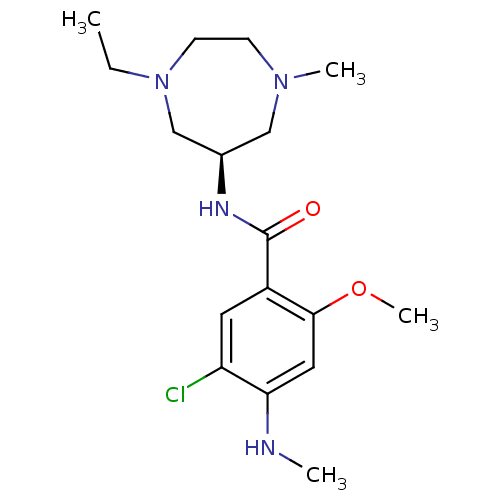
(5-Chloro-N-((R)-1-ethyl-4-methyl-[1,4]diazepan-6-y...)Show SMILES CCN1CCN(C)C[C@H](C1)NC(=O)c1cc(Cl)c(NC)cc1OC Show InChI InChI=1S/C17H27ClN4O2/c1-5-22-7-6-21(3)10-12(11-22)20-17(23)13-8-14(18)15(19-2)9-16(13)24-4/h8-9,12,19H,5-7,10-11H2,1-4H3,(H,20,23)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 receptor in rat cortical membrane using [3H]GR-65630 as radioligand |
Bioorg Med Chem Lett 8: 619-24 (1999)
BindingDB Entry DOI: 10.7270/Q25Q4WN6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50112046
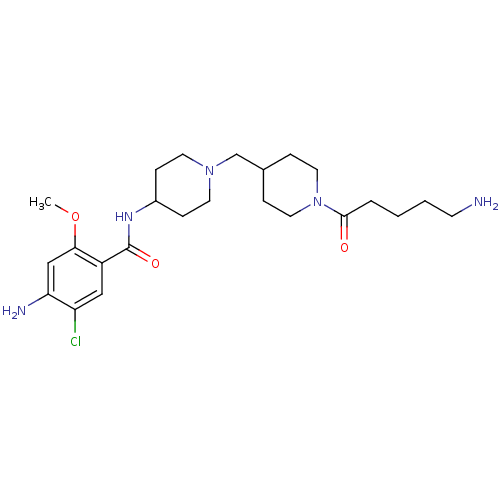
(4-Amino-N-{1-[1-(5-amino-pentanoyl)-piperidin-4-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CCN(CC2CCN(CC2)C(=O)CCCCN)CC1 Show InChI InChI=1S/C24H38ClN5O3/c1-33-22-15-21(27)20(25)14-19(22)24(32)28-18-7-10-29(11-8-18)16-17-5-12-30(13-6-17)23(31)4-2-3-9-26/h14-15,17-18H,2-13,16,26-27H2,1H3,(H,28,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand |
Bioorg Med Chem Lett 12: 967-70 (2002)
BindingDB Entry DOI: 10.7270/Q2RF5TB7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50112042
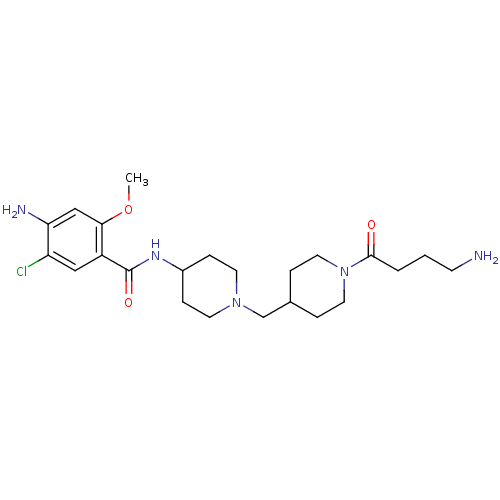
(4-Amino-N-{1-[1-(4-amino-butyryl)-piperidin-4-ylme...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CCN(CC2CCN(CC2)C(=O)CCCN)CC1 Show InChI InChI=1S/C23H36ClN5O3/c1-32-21-14-20(26)19(24)13-18(21)23(31)27-17-6-9-28(10-7-17)15-16-4-11-29(12-5-16)22(30)3-2-8-25/h13-14,16-17H,2-12,15,25-26H2,1H3,(H,27,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand |
Bioorg Med Chem Lett 12: 967-70 (2002)
BindingDB Entry DOI: 10.7270/Q2RF5TB7 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429596
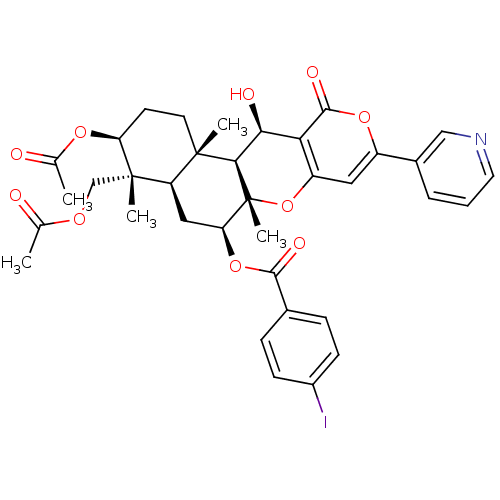
(CHEMBL2333813)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(I)cc1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38INO10/c1-19(39)44-18-35(4)26-16-28(47-32(42)21-8-10-23(37)11-9-21)36(5)31(34(26,3)13-12-27(35)45-20(2)40)30(41)29-25(48-36)15-24(46-33(29)43)22-7-6-14-38-17-22/h6-11,14-15,17,26-28,30-31,41H,12-13,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50450877

(CHEMBL2093035)Show SMILES CCN1CCCC[C@@H](C1)NC(=O)c1cc(Cl)c(NC)cc1OC |r| Show InChI InChI=1S/C17H26ClN3O2/c1-4-21-8-6-5-7-12(11-21)20-17(22)13-9-14(18)15(19-2)10-16(13)23-3/h9-10,12,19H,4-8,11H2,1-3H3,(H,20,22)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 receptor in rat cortical membrane using [3H]GR-65630 as radioligand |
Bioorg Med Chem Lett 8: 619-24 (1999)
BindingDB Entry DOI: 10.7270/Q25Q4WN6 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50366569

(CHEMBL1788262)Show SMILES CCN1CCCC[C@H](C1)NC(=O)c1cc(Cl)c(NC)cc1OC |r| Show InChI InChI=1S/C17H26ClN3O2/c1-4-21-8-6-5-7-12(11-21)20-17(22)13-9-14(18)15(19-2)10-16(13)23-3/h9-10,12,19H,4-8,11H2,1-3H3,(H,20,22)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against dopamine D2 receptor in rat brain synaptic membrane using [3H]-spiperone as radioligand |
Bioorg Med Chem Lett 8: 619-24 (1999)
BindingDB Entry DOI: 10.7270/Q25Q4WN6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50069288
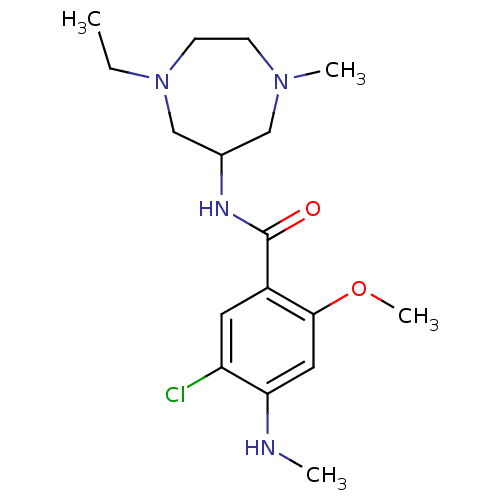
(5-Chloro-N-(1-ethyl-4-methyl-[1,4]diazepan-6-yl)-2...)Show InChI InChI=1S/C17H27ClN4O2/c1-5-22-7-6-21(3)10-12(11-22)20-17(23)13-8-14(18)15(19-2)9-16(13)24-4/h8-9,12,19H,5-7,10-11H2,1-4H3,(H,20,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 receptor in rat cortical membrane using [3H]GR-65630 as radioligand |
Bioorg Med Chem Lett 8: 619-24 (1999)
BindingDB Entry DOI: 10.7270/Q25Q4WN6 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429577

(CHEMBL2334212)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(Br)c(F)c1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H37BrFNO10/c1-18(40)45-17-35(4)26-15-28(48-32(43)20-8-9-22(37)23(38)13-20)36(5)31(34(26,3)11-10-27(35)46-19(2)41)30(42)29-25(49-36)14-24(47-33(29)44)21-7-6-12-39-16-21/h6-9,12-14,16,26-28,30-31,42H,10-11,15,17H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429595
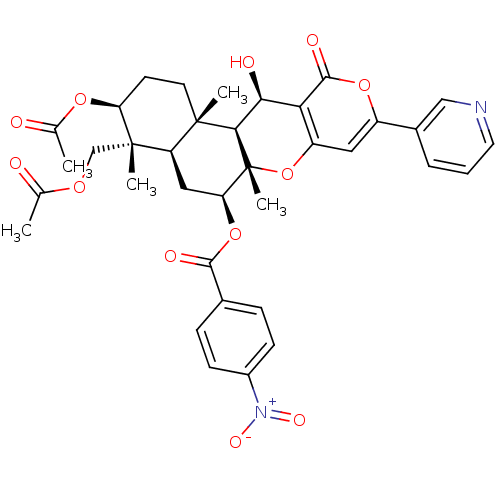
(CHEMBL2334194)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(cc1)[N+]([O-])=O)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38N2O12/c1-19(39)46-18-35(4)26-16-28(49-32(42)21-8-10-23(11-9-21)38(44)45)36(5)31(34(26,3)13-12-27(35)47-20(2)40)30(41)29-25(50-36)15-24(48-33(29)43)22-7-6-14-37-17-22/h6-11,14-15,17,26-28,30-31,41H,12-13,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429594
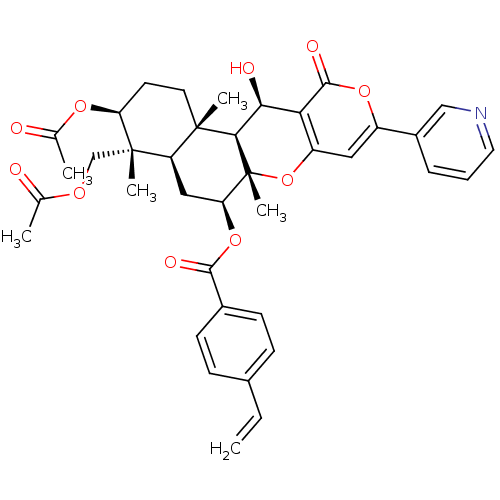
(CHEMBL2334195)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(C=C)cc1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C38H41NO10/c1-7-23-10-12-24(13-11-23)34(43)48-30-18-28-36(4,15-14-29(46-22(3)41)37(28,5)20-45-21(2)40)33-32(42)31-27(49-38(30,33)6)17-26(47-35(31)44)25-9-8-16-39-19-25/h7-13,16-17,19,28-30,32-33,42H,1,14-15,18,20H2,2-6H3/t28-,29+,30+,32+,33-,36+,37+,38-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429593
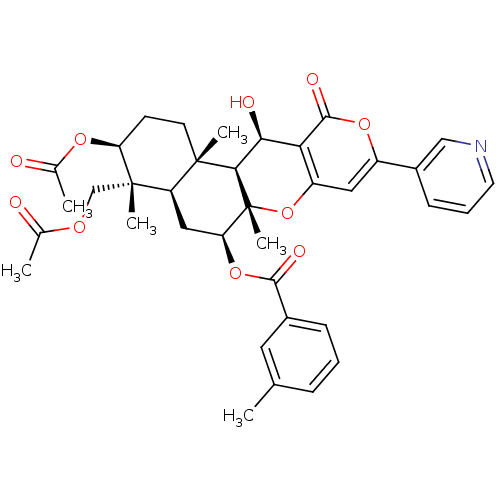
(CHEMBL2334196)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1cccc(C)c1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C37H41NO10/c1-20-9-7-10-23(15-20)33(42)47-29-17-27-35(4,13-12-28(45-22(3)40)36(27,5)19-44-21(2)39)32-31(41)30-26(48-37(29,32)6)16-25(46-34(30)43)24-11-8-14-38-18-24/h7-11,14-16,18,27-29,31-32,41H,12-13,17,19H2,1-6H3/t27-,28+,29+,31+,32-,35+,36+,37-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429590

(CHEMBL2334199)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(cc1)N=[N+]=[N-])[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38N4O10/c1-19(41)46-18-35(4)26-16-28(49-32(44)21-8-10-23(11-9-21)39-40-37)36(5)31(34(26,3)13-12-27(35)47-20(2)42)30(43)29-25(50-36)15-24(48-33(29)45)22-7-6-14-38-17-22/h6-11,14-15,17,26-28,30-31,43H,12-13,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429592
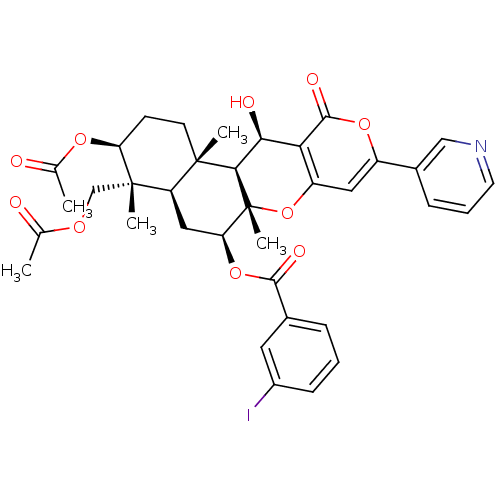
(CHEMBL2334197)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1cccc(I)c1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38INO10/c1-19(39)44-18-35(4)26-16-28(47-32(42)21-8-6-10-23(37)14-21)36(5)31(34(26,3)12-11-27(35)45-20(2)40)30(41)29-25(48-36)15-24(46-33(29)43)22-9-7-13-38-17-22/h6-10,13-15,17,26-28,30-31,41H,11-12,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429591

(CHEMBL2334198)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccccc1I)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38INO10/c1-19(39)44-18-35(4)26-16-28(47-32(42)22-10-6-7-11-23(22)37)36(5)31(34(26,3)13-12-27(35)45-20(2)40)30(41)29-25(48-36)15-24(46-33(29)43)21-9-8-14-38-17-21/h6-11,14-15,17,26-28,30-31,41H,12-13,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50112045
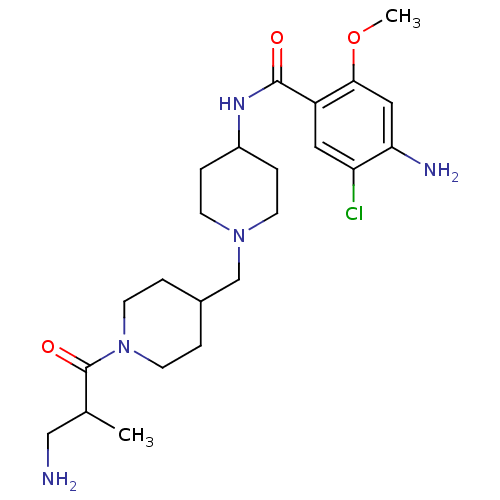
(4-Amino-N-{1-[1-(3-amino-2-methyl-propionyl)-piper...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CCN(CC2CCN(CC2)C(=O)C(C)CN)CC1 Show InChI InChI=1S/C23H36ClN5O3/c1-15(13-25)23(31)29-9-3-16(4-10-29)14-28-7-5-17(6-8-28)27-22(30)18-11-19(24)20(26)12-21(18)32-2/h11-12,15-17H,3-10,13-14,25-26H2,1-2H3,(H,27,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand |
Bioorg Med Chem Lett 12: 967-70 (2002)
BindingDB Entry DOI: 10.7270/Q2RF5TB7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50112031
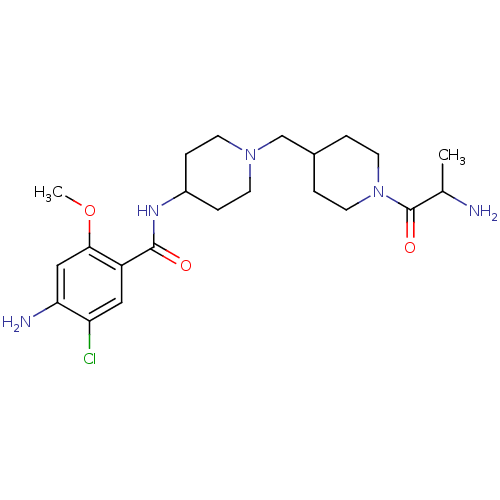
(4-Amino-N-{1-[1-(2-amino-propionyl)-piperidin-4-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CCN(CC2CCN(CC2)C(=O)C(C)N)CC1 Show InChI InChI=1S/C22H34ClN5O3/c1-14(24)22(30)28-9-3-15(4-10-28)13-27-7-5-16(6-8-27)26-21(29)17-11-18(23)19(25)12-20(17)31-2/h11-12,14-16H,3-10,13,24-25H2,1-2H3,(H,26,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand |
Bioorg Med Chem Lett 12: 967-70 (2002)
BindingDB Entry DOI: 10.7270/Q2RF5TB7 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50433214
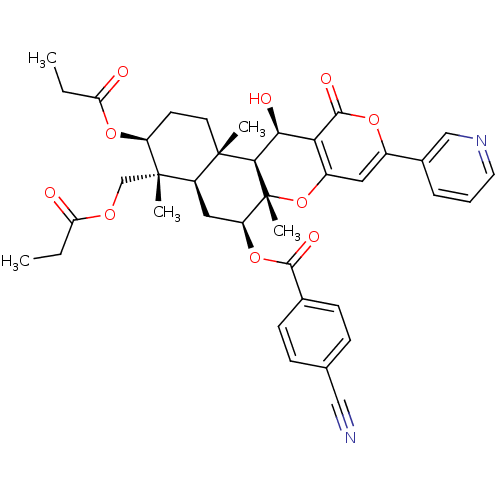
(CHEMBL2375701)Show SMILES CCC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(cc1)C#N)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(=O)CC |r| Show InChI InChI=1S/C39H42N2O10/c1-6-30(42)47-21-38(4)27-18-29(50-35(45)23-12-10-22(19-40)11-13-23)39(5)34(37(27,3)15-14-28(38)49-31(43)7-2)33(44)32-26(51-39)17-25(48-36(32)46)24-9-8-16-41-20-24/h8-13,16-17,20,27-29,33-34,44H,6-7,14-15,18,21H2,1-5H3/t27-,28+,29+,33+,34-,37+,38+,39-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) |
Bioorg Med Chem Lett 23: 2659-62 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.088
BindingDB Entry DOI: 10.7270/Q20866P3 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429576
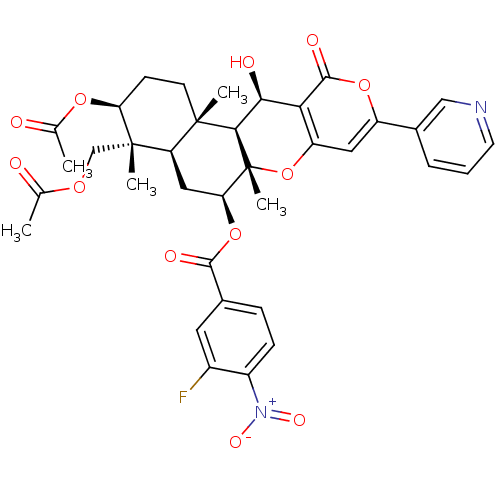
(CHEMBL2334213)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(c(F)c1)[N+]([O-])=O)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H37FN2O12/c1-18(40)47-17-35(4)26-15-28(50-32(43)20-8-9-23(39(45)46)22(37)13-20)36(5)31(34(26,3)11-10-27(35)48-19(2)41)30(42)29-25(51-36)14-24(49-33(29)44)21-7-6-12-38-16-21/h6-9,12-14,16,26-28,30-31,42H,10-11,15,17H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50436344
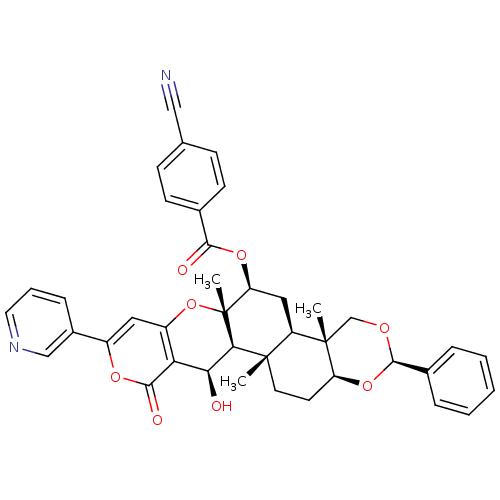
(CHEMBL2398815)Show SMILES C[C@]12CC[C@@H]3O[C@@H](OC[C@@]3(C)[C@@H]1C[C@H](OC(=O)c1ccc(cc1)C#N)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)c1ccccc1 |r| Show InChI InChI=1S/C40H38N2O8/c1-38-16-15-30-39(2,22-46-37(49-30)25-8-5-4-6-9-25)29(38)19-31(48-35(44)24-13-11-23(20-41)12-14-24)40(3)34(38)33(43)32-28(50-40)18-27(47-36(32)45)26-10-7-17-42-21-26/h4-14,17-18,21,29-31,33-34,37,43H,15-16,19,22H2,1-3H3/t29-,30+,31+,33+,34-,37-,38+,39+,40-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) |
Bioorg Med Chem Lett 23: 3798-801 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.075
BindingDB Entry DOI: 10.7270/Q2WD41Z9 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429575

(CHEMBL2334214)Show SMILES COc1ccc(cc1F)C(=O)O[C@H]1C[C@H]2[C@](C)(COC(C)=O)[C@H](CC[C@]2(C)[C@H]2[C@@H](O)c3c(O[C@]12C)cc(oc3=O)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C37H40FNO11/c1-19(40)46-18-36(4)27-16-29(49-33(43)21-9-10-24(45-6)23(38)14-21)37(5)32(35(27,3)12-11-28(36)47-20(2)41)31(42)30-26(50-37)15-25(48-34(30)44)22-8-7-13-39-17-22/h7-10,13-15,17,27-29,31-32,42H,11-12,16,18H2,1-6H3/t27-,28+,29+,31+,32-,35+,36+,37-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429574

(CHEMBL2334215)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(C)c(F)c1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C37H40FNO10/c1-19-9-10-22(14-24(19)38)33(43)48-29-16-27-35(4,12-11-28(46-21(3)41)36(27,5)18-45-20(2)40)32-31(42)30-26(49-37(29,32)6)15-25(47-34(30)44)23-8-7-13-39-17-23/h7-10,13-15,17,27-29,31-32,42H,11-12,16,18H2,1-6H3/t27-,28+,29+,31+,32-,35+,36+,37-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50112029
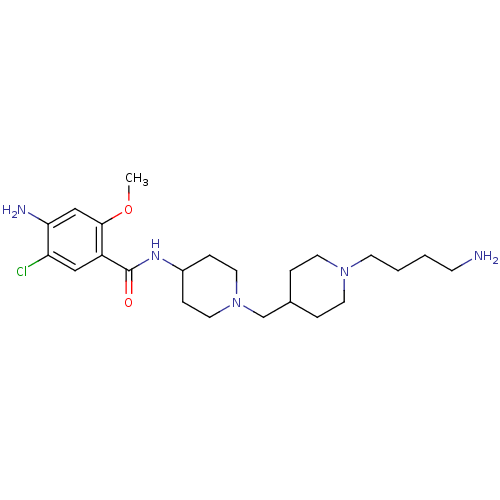
(4-Amino-N-{1-[1-(4-amino-butyl)-piperidin-4-ylmeth...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CCN(CC2CCN(CCCCN)CC2)CC1 Show InChI InChI=1S/C23H38ClN5O2/c1-31-22-15-21(26)20(24)14-19(22)23(30)27-18-6-12-29(13-7-18)16-17-4-10-28(11-5-17)9-3-2-8-25/h14-15,17-18H,2-13,16,25-26H2,1H3,(H,27,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand |
Bioorg Med Chem Lett 12: 967-70 (2002)
BindingDB Entry DOI: 10.7270/Q2RF5TB7 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50433213

(CHEMBL2375702)Show SMILES CC(C)C(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(cc1)C#N)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(=O)C(C)C |r| Show InChI InChI=1S/C41H46N2O10/c1-22(2)35(45)49-21-40(6)29-18-31(52-37(47)25-12-10-24(19-42)11-13-25)41(7)34(39(29,5)15-14-30(40)51-36(46)23(3)4)33(44)32-28(53-41)17-27(50-38(32)48)26-9-8-16-43-20-26/h8-13,16-17,20,22-23,29-31,33-34,44H,14-15,18,21H2,1-7H3/t29-,30+,31+,33+,34-,39+,40+,41-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) |
Bioorg Med Chem Lett 23: 2659-62 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.088
BindingDB Entry DOI: 10.7270/Q20866P3 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50436347

(CHEMBL2398812)Show SMILES Cc1ccc([C@@H]2OC[C@]3(C)[C@H](CC[C@@]4(C)[C@H]3C[C@H](OC(=O)c3ccc(cc3)C#N)[C@@]3(C)Oc5cc(oc(=O)c5[C@H](O)[C@H]43)-c3cccnc3)O2)c(C)c1 |r| Show InChI InChI=1S/C42H42N2O8/c1-23-8-13-28(24(2)17-23)39-48-22-41(4)31-19-33(50-37(46)26-11-9-25(20-43)10-12-26)42(5)36(40(31,3)15-14-32(41)51-39)35(45)34-30(52-42)18-29(49-38(34)47)27-7-6-16-44-21-27/h6-13,16-18,21,31-33,35-36,39,45H,14-15,19,22H2,1-5H3/t31-,32+,33+,35+,36-,39-,40+,41+,42-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) |
Bioorg Med Chem Lett 23: 3798-801 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.075
BindingDB Entry DOI: 10.7270/Q2WD41Z9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50069287

(5-Chloro-N-(1-ethyl-azepan-3-yl)-2-methoxy-4-methy...)Show InChI InChI=1S/C17H26ClN3O2/c1-4-21-8-6-5-7-12(11-21)20-17(22)13-9-14(18)15(19-2)10-16(13)23-3/h9-10,12,19H,4-8,11H2,1-3H3,(H,20,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 receptor in rat cortical membrane using [3H]GR-65630 as radioligand |
Bioorg Med Chem Lett 8: 619-24 (1999)
BindingDB Entry DOI: 10.7270/Q25Q4WN6 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50436348
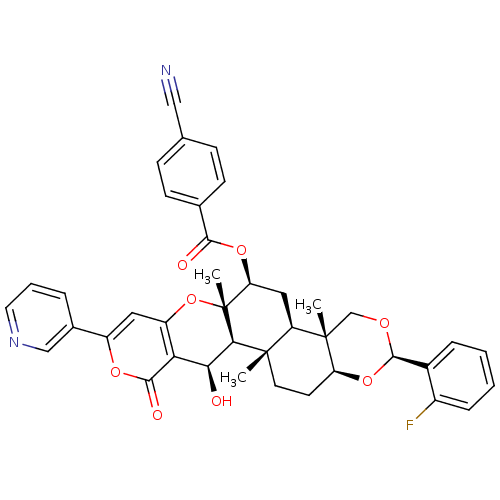
(CHEMBL2398811)Show SMILES C[C@]12CC[C@@H]3O[C@@H](OC[C@@]3(C)[C@@H]1C[C@H](OC(=O)c1ccc(cc1)C#N)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)c1ccccc1F |r| Show InChI InChI=1S/C40H37FN2O8/c1-38-15-14-30-39(2,21-47-37(50-30)25-8-4-5-9-26(25)41)29(38)18-31(49-35(45)23-12-10-22(19-42)11-13-23)40(3)34(38)33(44)32-28(51-40)17-27(48-36(32)46)24-7-6-16-43-20-24/h4-13,16-17,20,29-31,33-34,37,44H,14-15,18,21H2,1-3H3/t29-,30+,31+,33+,34-,37-,38+,39+,40-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) |
Bioorg Med Chem Lett 23: 3798-801 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.075
BindingDB Entry DOI: 10.7270/Q2WD41Z9 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50436343
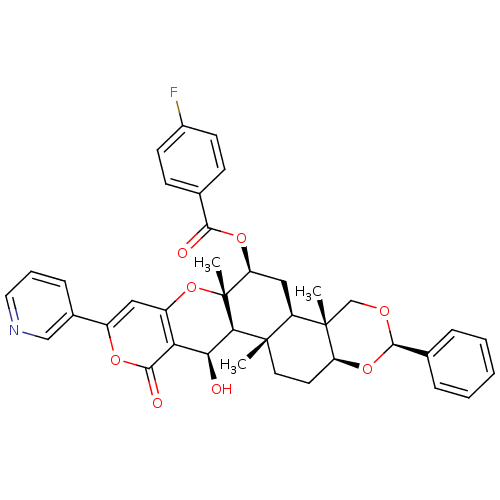
(CHEMBL2398816)Show SMILES C[C@@]12CO[C@H](O[C@H]1CC[C@@]1(C)[C@H]2C[C@H](OC(=O)c2ccc(F)cc2)[C@@]2(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]12)-c1cccnc1)c1ccccc1 |r| Show InChI InChI=1S/C39H38FNO8/c1-37-16-15-29-38(2,21-45-36(48-29)23-8-5-4-6-9-23)28(37)19-30(47-34(43)22-11-13-25(40)14-12-22)39(3)33(37)32(42)31-27(49-39)18-26(46-35(31)44)24-10-7-17-41-20-24/h4-14,17-18,20,28-30,32-33,36,42H,15-16,19,21H2,1-3H3/t28-,29+,30+,32+,33-,36-,37+,38+,39-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) |
Bioorg Med Chem Lett 23: 3798-801 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.075
BindingDB Entry DOI: 10.7270/Q2WD41Z9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50450876
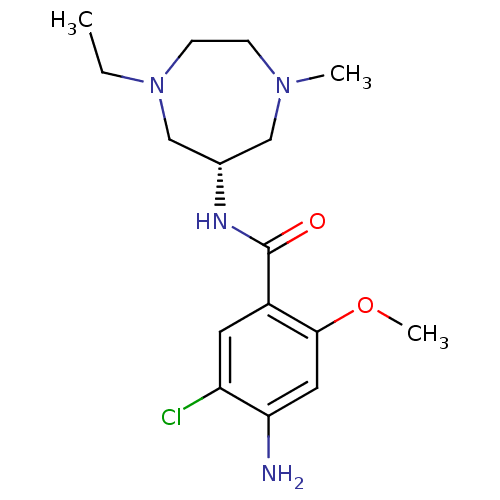
(CHEMBL2093034)Show SMILES CCN1CCN(C)C[C@@H](C1)NC(=O)c1cc(Cl)c(N)cc1OC |r| Show InChI InChI=1S/C16H25ClN4O2/c1-4-21-6-5-20(2)9-11(10-21)19-16(22)12-7-13(17)14(18)8-15(12)23-3/h7-8,11H,4-6,9-10,18H2,1-3H3,(H,19,22)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 receptor in rat cortical membrane using [3H]GR-65630 as radioligand |
Bioorg Med Chem Lett 8: 619-24 (1999)
BindingDB Entry DOI: 10.7270/Q25Q4WN6 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429573
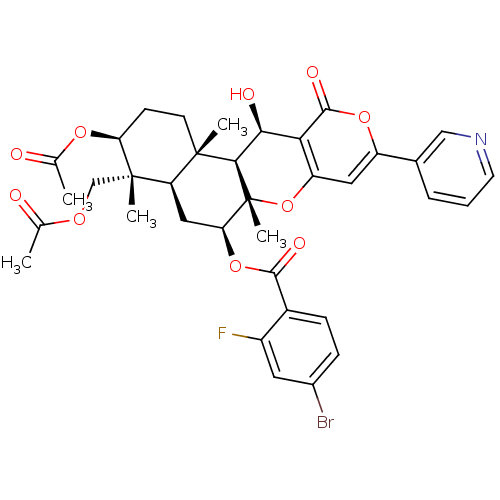
(CHEMBL2334216)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(Br)cc1F)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H37BrFNO10/c1-18(40)45-17-35(4)26-15-28(48-32(43)22-9-8-21(37)13-23(22)38)36(5)31(34(26,3)11-10-27(35)46-19(2)41)30(42)29-25(49-36)14-24(47-33(29)44)20-7-6-12-39-16-20/h6-9,12-14,16,26-28,30-31,42H,10-11,15,17H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429589

(CHEMBL2334200)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1cccc(Cl)c1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38ClNO10/c1-19(39)44-18-35(4)26-16-28(47-32(42)21-8-6-10-23(37)14-21)36(5)31(34(26,3)12-11-27(35)45-20(2)40)30(41)29-25(48-36)15-24(46-33(29)43)22-9-7-13-38-17-22/h6-10,13-15,17,26-28,30-31,41H,11-12,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50436346

(CHEMBL2398813)Show SMILES Cc1cccc(C)c1[C@@H]1OC[C@]2(C)[C@H](CC[C@@]3(C)[C@H]2C[C@H](OC(=O)c2ccc(cc2)C#N)[C@@]2(C)Oc4cc(oc(=O)c4[C@H](O)[C@H]32)-c2cccnc2)O1 |r| Show InChI InChI=1S/C42H42N2O8/c1-23-8-6-9-24(2)33(23)39-48-22-41(4)30-19-32(50-37(46)26-13-11-25(20-43)12-14-26)42(5)36(40(30,3)16-15-31(41)51-39)35(45)34-29(52-42)18-28(49-38(34)47)27-10-7-17-44-21-27/h6-14,17-18,21,30-32,35-36,39,45H,15-16,19,22H2,1-5H3/t30-,31+,32+,35+,36-,39-,40+,41+,42-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) |
Bioorg Med Chem Lett 23: 3798-801 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.075
BindingDB Entry DOI: 10.7270/Q2WD41Z9 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429572

(CHEMBL2334217)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(C#N)c(F)c1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C37H37FN2O10/c1-19(41)46-18-36(4)27-15-29(49-33(44)21-8-9-22(16-39)24(38)13-21)37(5)32(35(27,3)11-10-28(36)47-20(2)42)31(43)30-26(50-37)14-25(48-34(30)45)23-7-6-12-40-17-23/h6-9,12-14,17,27-29,31-32,43H,10-11,15,18H2,1-5H3/t27-,28+,29+,31+,32-,35+,36+,37-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data