 Found 14 hits with Last Name = 'yan' and Initial = 'jf'
Found 14 hits with Last Name = 'yan' and Initial = 'jf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM35440
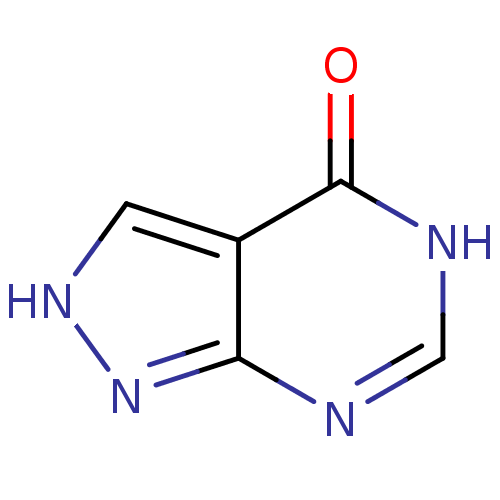
(ALLOPURINOL | MLS000069453 | SMR000059083 | cid_20...)Show InChI InChI=1S/C5H4N4O/c10-5-3-1-8-9-4(3)6-2-7-5/h1-2H,(H2,6,7,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of XOD |
J Nat Prod 72: 1198-201 (2009)
Article DOI: 10.1021/np800643n
BindingDB Entry DOI: 10.7270/Q200027K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50433190
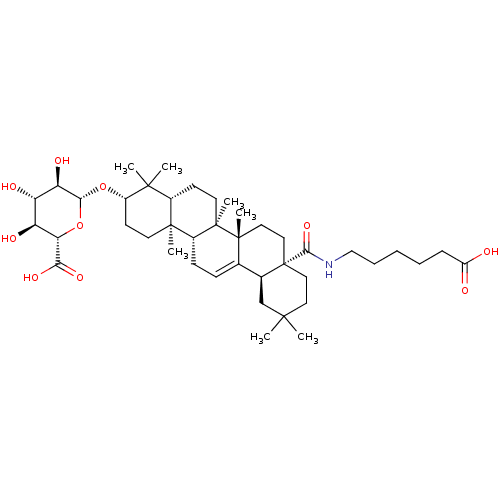
(CHEMBL2375455)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O[C@@H]6O[C@@H]([C@@H](O)[C@H](O)[C@H]6O)C(O)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(=O)NCCCCCC(O)=O |r,c:10| Show InChI InChI=1S/C42H67NO10/c1-37(2)18-20-42(36(51)43-22-10-8-9-11-29(44)45)21-19-40(6)24(25(42)23-37)12-13-27-39(5)16-15-28(38(3,4)26(39)14-17-41(27,40)7)52-35-32(48)30(46)31(47)33(53-35)34(49)50/h12,25-28,30-33,35,46-48H,8-11,13-23H2,1-7H3,(H,43,51)(H,44,45)(H,49,50)/t25-,26-,27+,28-,30-,31-,32+,33-,35+,39-,40+,41+,42-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwest University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) expressed in Escherichia coli expression system using p-nitrophenyl phosphate as substrate assessed as release o... |
Eur J Med Chem 63: 511-22 (2013)
Article DOI: 10.1016/j.ejmech.2013.03.001
BindingDB Entry DOI: 10.7270/Q27S7Q59 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50433193
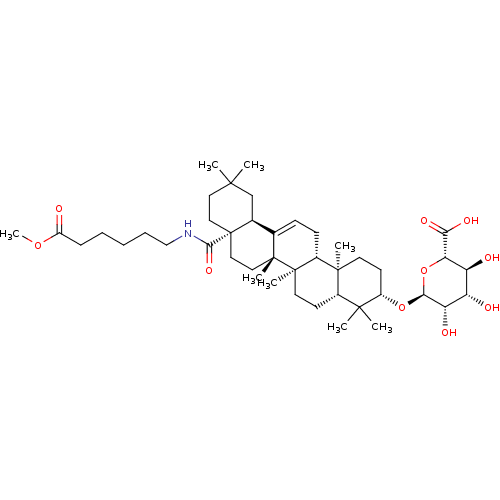
(CHEMBL2375471)Show SMILES COC(=O)CCCCCNC(=O)[C@]12CCC(C)(C)C[C@H]1C1=CC[C@@H]3[C@@]4(C)CC[C@H](O[C@H]5O[C@@H]([C@@H](O)[C@H](O)[C@@H]5O)C(O)=O)C(C)(C)[C@@H]4CC[C@@]3(C)[C@]1(C)CC2 |r,t:21| Show InChI InChI=1S/C43H69NO10/c1-38(2)19-21-43(37(51)44-23-11-9-10-12-30(45)52-8)22-20-41(6)25(26(43)24-38)13-14-28-40(5)17-16-29(39(3,4)27(40)15-18-42(28,41)7)53-36-33(48)31(46)32(47)34(54-36)35(49)50/h13,26-29,31-34,36,46-48H,9-12,14-24H2,1-8H3,(H,44,51)(H,49,50)/t26-,27-,28+,29-,31-,32-,33-,34-,36-,40-,41+,42+,43-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwest University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) expressed in Escherichia coli expression system using p-nitrophenyl phosphate as substrate assessed as release o... |
Eur J Med Chem 63: 511-22 (2013)
Article DOI: 10.1016/j.ejmech.2013.03.001
BindingDB Entry DOI: 10.7270/Q27S7Q59 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM4375
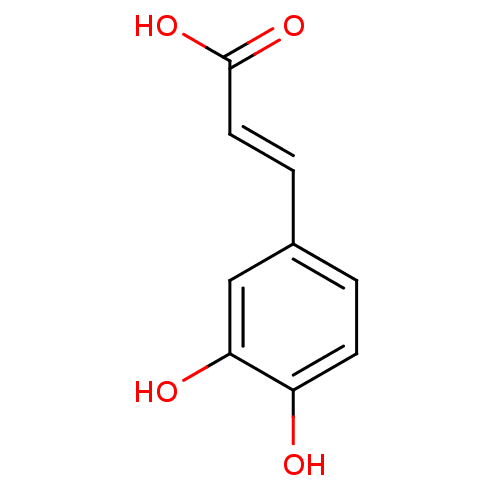
((2E)-3-(3,4-dihydroxyphenyl)prop-2-enoic acid | (2...)Show InChI InChI=1S/C9H8O4/c10-7-3-1-6(5-8(7)11)2-4-9(12)13/h1-5,10-11H,(H,12,13)/b4-2+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 10 mins |
J Nat Prod 72: 1198-201 (2009)
Article DOI: 10.1021/np800643n
BindingDB Entry DOI: 10.7270/Q200027K |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50310442
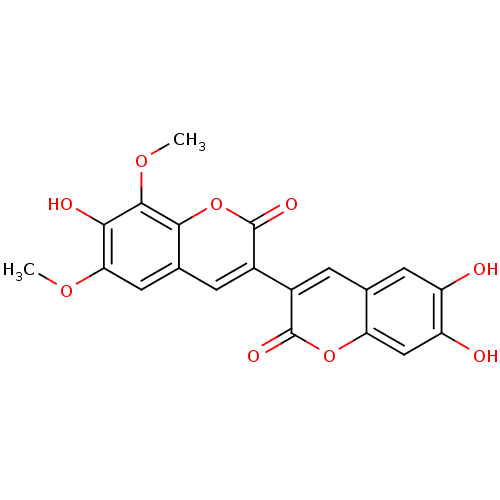
(CHEMBL1085430 | arteminorin C)Show SMILES COc1cc2cc(-c3cc4cc(O)c(O)cc4oc3=O)c(=O)oc2c(OC)c1O Show InChI InChI=1S/C20H14O9/c1-26-15-6-9-4-11(20(25)29-17(9)18(27-2)16(15)23)10-3-8-5-12(21)13(22)7-14(8)28-19(10)24/h3-7,21-23H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of XOD |
J Nat Prod 72: 1198-201 (2009)
Article DOI: 10.1021/np800643n
BindingDB Entry DOI: 10.7270/Q200027K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50433177
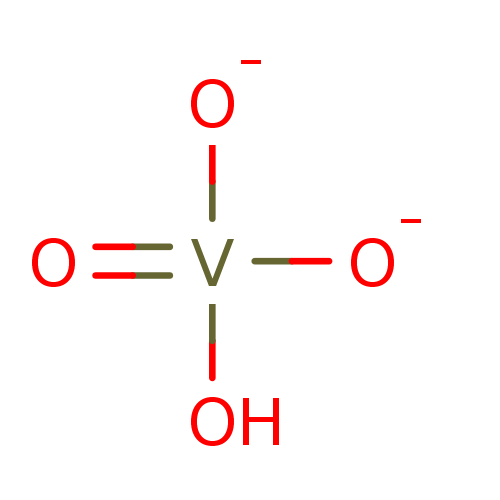
(SODIUM ORTHOVANADATE)Show InChI InChI=1S/H2O.3O.V/h1H2;;;;/q;;2*-1;+1/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwest University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) expressed in Escherichia coli expression system using p-nitrophenyl phosphate as substrate assessed as release o... |
Eur J Med Chem 63: 511-22 (2013)
Article DOI: 10.1016/j.ejmech.2013.03.001
BindingDB Entry DOI: 10.7270/Q27S7Q59 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50433191
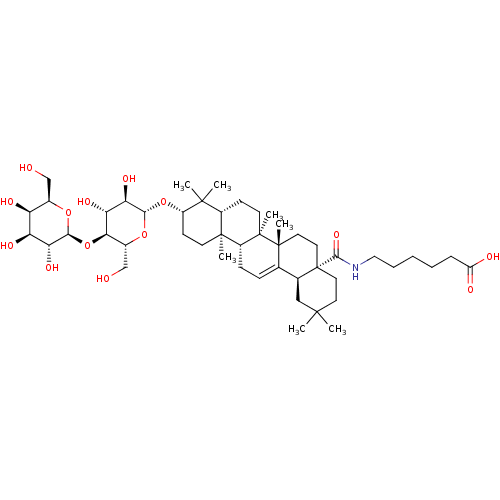
(CHEMBL2375454)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O[C@@H]6O[C@H](CO)[C@@H](O[C@@H]7O[C@H](CO)[C@H](O)[C@H](O)[C@H]7O)[C@H](O)[C@H]6O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(=O)NCCCCCC(O)=O |r,c:10| Show InChI InChI=1S/C48H79NO14/c1-43(2)18-20-48(42(59)49-22-10-8-9-11-33(52)53)21-19-46(6)26(27(48)23-43)12-13-31-45(5)16-15-32(44(3,4)30(45)14-17-47(31,46)7)62-40-38(58)36(56)39(29(25-51)61-40)63-41-37(57)35(55)34(54)28(24-50)60-41/h12,27-32,34-41,50-51,54-58H,8-11,13-25H2,1-7H3,(H,49,59)(H,52,53)/t27-,28+,29+,30-,31+,32-,34-,35-,36+,37+,38+,39+,40-,41-,45-,46+,47+,48-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwest University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) expressed in Escherichia coli expression system using p-nitrophenyl phosphate as substrate assessed as release o... |
Eur J Med Chem 63: 511-22 (2013)
Article DOI: 10.1016/j.ejmech.2013.03.001
BindingDB Entry DOI: 10.7270/Q27S7Q59 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM7459

(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...)Show InChI InChI=1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of XOD |
J Nat Prod 72: 1198-201 (2009)
Article DOI: 10.1021/np800643n
BindingDB Entry DOI: 10.7270/Q200027K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50433192
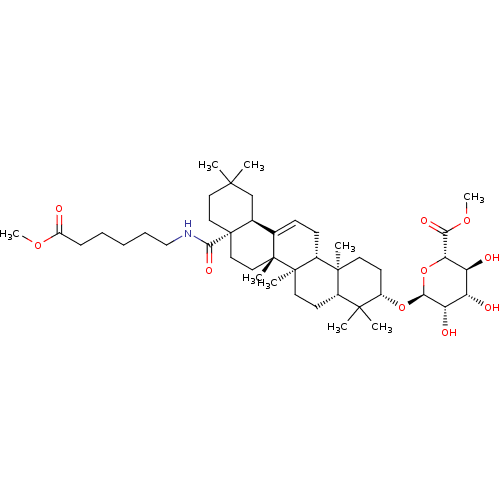
(CHEMBL2375448)Show SMILES COC(=O)CCCCCNC(=O)[C@]12CCC(C)(C)C[C@H]1C1=CC[C@@H]3[C@@]4(C)CC[C@H](O[C@H]5O[C@@H]([C@@H](O)[C@H](O)[C@@H]5O)C(=O)OC)C(C)(C)[C@@H]4CC[C@@]3(C)[C@]1(C)CC2 |r,t:21| Show InChI InChI=1S/C44H71NO10/c1-39(2)20-22-44(38(51)45-24-12-10-11-13-31(46)52-8)23-21-42(6)26(27(44)25-39)14-15-29-41(5)18-17-30(40(3,4)28(41)16-19-43(29,42)7)54-37-34(49)32(47)33(48)35(55-37)36(50)53-9/h14,27-30,32-35,37,47-49H,10-13,15-25H2,1-9H3,(H,45,51)/t27-,28-,29+,30-,32-,33-,34-,35-,37-,41-,42+,43+,44-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwest University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) expressed in Escherichia coli expression system using p-nitrophenyl phosphate as substrate assessed as release o... |
Eur J Med Chem 63: 511-22 (2013)
Article DOI: 10.1016/j.ejmech.2013.03.001
BindingDB Entry DOI: 10.7270/Q27S7Q59 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50433189
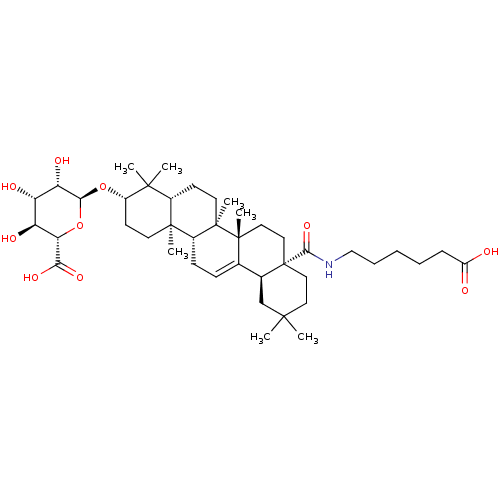
(CHEMBL2375456)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O[C@H]6O[C@@H]([C@@H](O)[C@H](O)[C@@H]6O)C(O)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(=O)NCCCCCC(O)=O |r,c:10| Show InChI InChI=1S/C42H67NO10/c1-37(2)18-20-42(36(51)43-22-10-8-9-11-29(44)45)21-19-40(6)24(25(42)23-37)12-13-27-39(5)16-15-28(38(3,4)26(39)14-17-41(27,40)7)52-35-32(48)30(46)31(47)33(53-35)34(49)50/h12,25-28,30-33,35,46-48H,8-11,13-23H2,1-7H3,(H,43,51)(H,44,45)(H,49,50)/t25-,26-,27+,28-,30-,31-,32-,33-,35-,39-,40+,41+,42-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwest University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) expressed in Escherichia coli expression system using p-nitrophenyl phosphate as substrate assessed as release o... |
Eur J Med Chem 63: 511-22 (2013)
Article DOI: 10.1016/j.ejmech.2013.03.001
BindingDB Entry DOI: 10.7270/Q27S7Q59 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha/delta/gamma
(Homo sapiens (Human)) | BDBM50484848
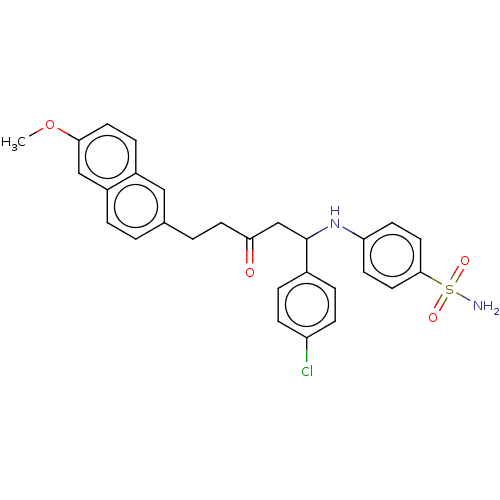
(CHEMBL1956027)Show SMILES COc1ccc2cc(CCC(=O)CC(Nc3ccc(cc3)S(N)(=O)=O)c3ccc(Cl)cc3)ccc2c1 Show InChI InChI=1S/C28H27ClN2O4S/c1-35-26-13-7-21-16-19(2-4-22(21)17-26)3-12-25(32)18-28(20-5-8-23(29)9-6-20)31-24-10-14-27(15-11-24)36(30,33)34/h2,4-11,13-17,28,31H,3,12,18H2,1H3,(H2,30,33,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a |
Southwest University
Curated by ChEMBL
| Assay Description
Activation of PPAR in human HepG2 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 2119-30 (2012)
Article DOI: 10.1016/j.bmc.2012.01.028
BindingDB Entry DOI: 10.7270/Q26H4M86 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha/delta/gamma
(Homo sapiens (Human)) | BDBM50030474
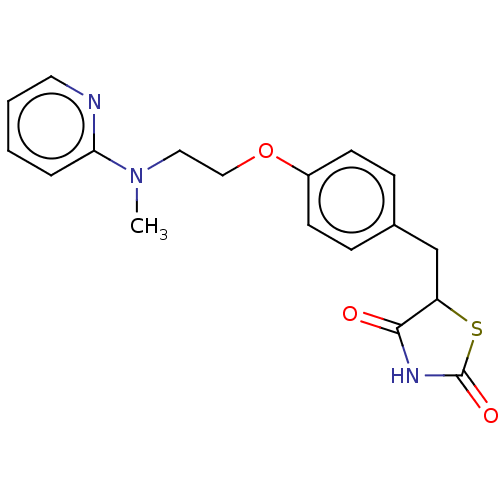
(Avandamet | Avandaryl | Avandia | BRL-49653 | CHEB...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,15H,10-12H2,1H3,(H,20,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
Southwest University
Curated by ChEMBL
| Assay Description
Activation of PPAR in human HepG2 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 2119-30 (2012)
Article DOI: 10.1016/j.bmc.2012.01.028
BindingDB Entry DOI: 10.7270/Q26H4M86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha/delta/gamma
(Homo sapiens (Human)) | BDBM50484849
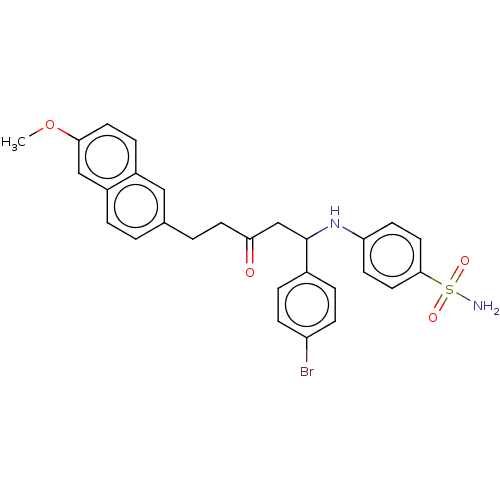
(CHEMBL1956029)Show SMILES COc1ccc2cc(CCC(=O)CC(Nc3ccc(cc3)S(N)(=O)=O)c3ccc(Br)cc3)ccc2c1 Show InChI InChI=1S/C28H27BrN2O4S/c1-35-26-13-7-21-16-19(2-4-22(21)17-26)3-12-25(32)18-28(20-5-8-23(29)9-6-20)31-24-10-14-27(15-11-24)36(30,33)34/h2,4-11,13-17,28,31H,3,12,18H2,1H3,(H2,30,33,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a |
Southwest University
Curated by ChEMBL
| Assay Description
Activation of PPAR in human HepG2 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 2119-30 (2012)
Article DOI: 10.1016/j.bmc.2012.01.028
BindingDB Entry DOI: 10.7270/Q26H4M86 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha/delta/gamma
(Homo sapiens (Human)) | BDBM50484850
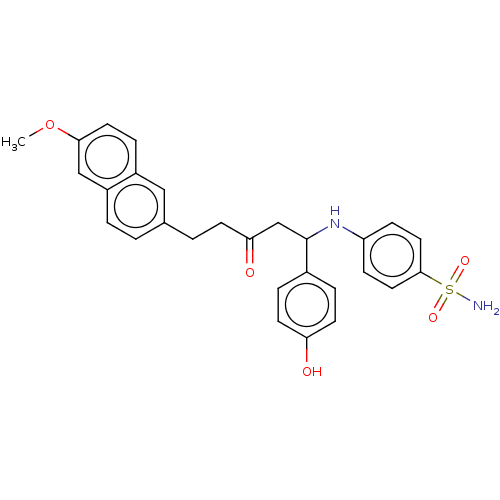
(CHEMBL1956035)Show SMILES COc1ccc2cc(CCC(=O)CC(Nc3ccc(cc3)S(N)(=O)=O)c3ccc(O)cc3)ccc2c1 Show InChI InChI=1S/C28H28N2O5S/c1-35-26-13-7-21-16-19(2-4-22(21)17-26)3-10-25(32)18-28(20-5-11-24(31)12-6-20)30-23-8-14-27(15-9-23)36(29,33)34/h2,4-9,11-17,28,30-31H,3,10,18H2,1H3,(H2,29,33,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a |
Southwest University
Curated by ChEMBL
| Assay Description
Activation of PPAR in human HepG2 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 2119-30 (2012)
Article DOI: 10.1016/j.bmc.2012.01.028
BindingDB Entry DOI: 10.7270/Q26H4M86 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data