

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50253328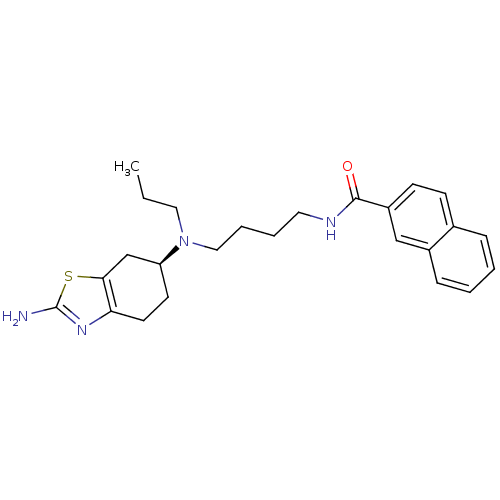 ((S)-N-(4-((2-amino-4,5,6,7-tetrahydrobenzo[d]thiaz...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]PD128907 from dopamine D3 receptor in Sprague-Dawley rat ventral striatum | J Med Chem 51: 5905-8 (2008) Article DOI: 10.1021/jm800471h BindingDB Entry DOI: 10.7270/Q2FN161H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50459819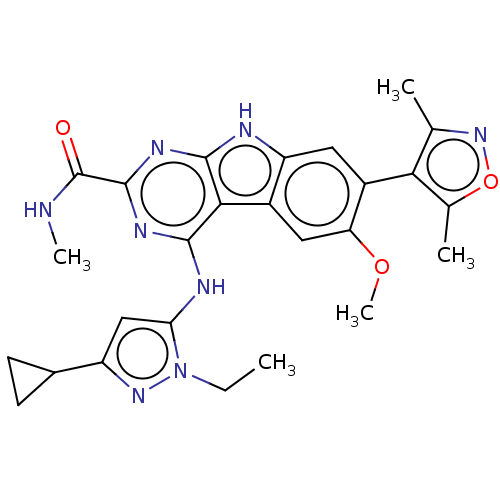 (CHEMBL4228445) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant N-terminal His6-tagged BRD3 bromodomain 1 (24 to 144 residues) (unknown origin) expressed in ... | J Med Chem 61: 462-481 (2018) Article DOI: 10.1021/acs.jmedchem.6b01816 BindingDB Entry DOI: 10.7270/Q2CV4MD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50181840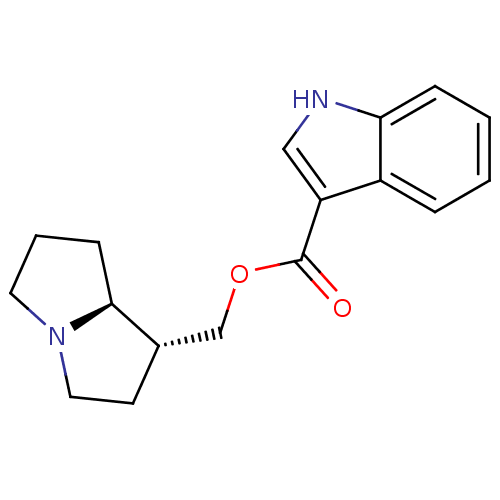 ((1R,7aS)-hexahydro-1H-pyrrolizin-1-ylmethyl 1-Meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50181844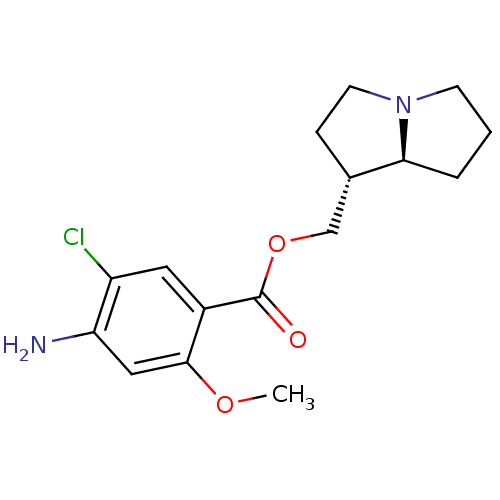 ((1R,7aS)-hexahydro-1H-pyrrolizin-1-ylmethyl 4-amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50459819 (CHEMBL4228445) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant N-terminal His6-tagged BRD3 bromodomain 2 (306 to 417residues) (unknown origin) expressed in ... | J Med Chem 61: 462-481 (2018) Article DOI: 10.1021/acs.jmedchem.6b01816 BindingDB Entry DOI: 10.7270/Q2CV4MD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50556826 (CHEMBL4745863) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [Glp65, Nle75, Tyr77][125I]-apelin13 from human APJ receptor expressed in HEK293 cells incubated for 1 hr by gamma counting based rad... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01913 BindingDB Entry DOI: 10.7270/Q2BG2SNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50368604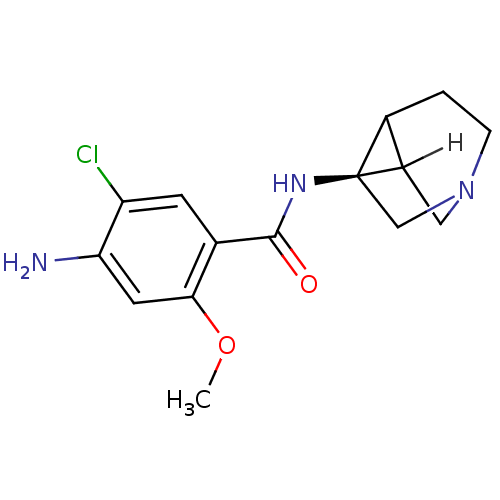 (CHEMBL1907770) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]GR-65630 binding to 5-hydroxytryptamine 3 receptor | J Med Chem 35: 1486-9 (1992) BindingDB Entry DOI: 10.7270/Q2CC11B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50255183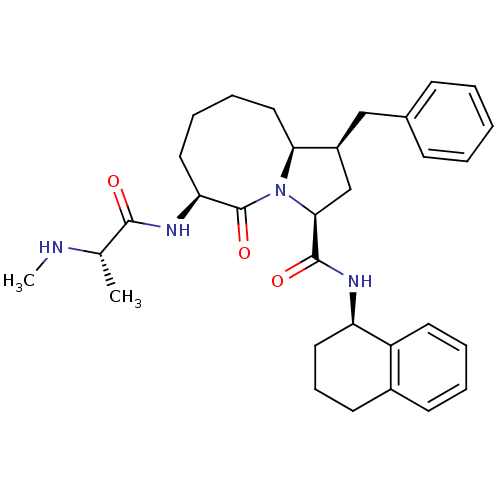 ((1S,3S,6S,10aS)-1-benzyl-6-((S)-2-(methylamino)pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of fluorescent SM5F peptide from His-tagged human cIAP2 BIR3 domain expressed in Escherichia coli BL21(DE3) cells by fluorescence polari... | J Med Chem 52: 593-6 (2009) Article DOI: 10.1021/jm801101z BindingDB Entry DOI: 10.7270/Q2Z03816 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50556827 (CHEMBL4748838) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [Glp65, Nle75, Tyr77][125I]-apelin13 from human APJ receptor expressed in HEK293 cells incubated for 1 hr by gamma counting based rad... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01913 BindingDB Entry DOI: 10.7270/Q2BG2SNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50459819 (CHEMBL4228445) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant N-terminal His6-tagged BRD2 bromodomain 2 (349 to 460 residues) (unknown origin) expressed in... | J Med Chem 61: 462-481 (2018) Article DOI: 10.1021/acs.jmedchem.6b01816 BindingDB Entry DOI: 10.7270/Q2CV4MD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50315206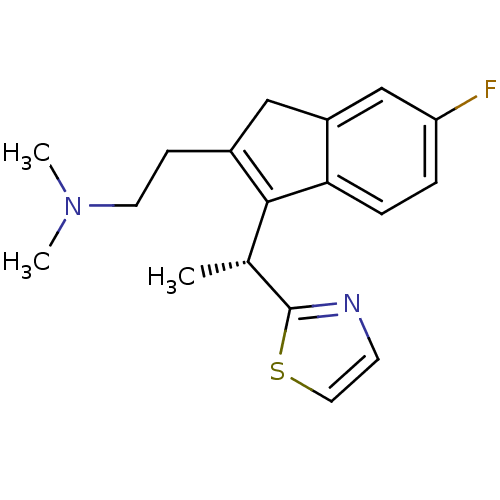 ((R)-2-(6-fluoro-3-(1-(thiazol-2-yl)ethyl)-1H-inden...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Binding affinity at histamine H1 receptor | Bioorg Med Chem Lett 20: 2629-33 (2010) Article DOI: 10.1016/j.bmcl.2010.02.055 BindingDB Entry DOI: 10.7270/Q2NG4QSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50559603 (CHEMBL4787458) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to recombinant human S1P5 receptor expressed in Chem-1 cell membrane by 33P-SIP binding assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00631 BindingDB Entry DOI: 10.7270/Q2D79G4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50181845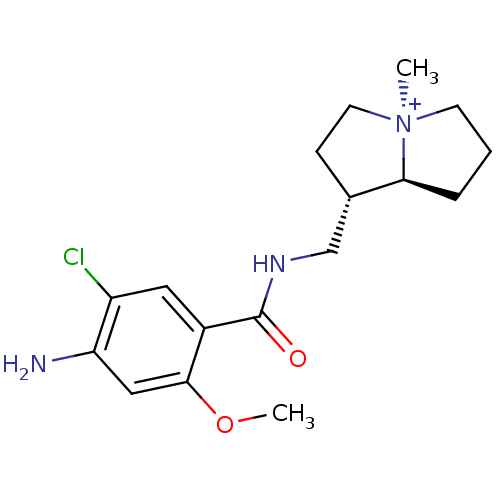 ((1S,7aS)-1-{[(4-amino-5-chloro-2-methoxybenzoyl)am...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50255183 ((1S,3S,6S,10aS)-1-benzyl-6-((S)-2-(methylamino)pro...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of fluorescent SM5F peptide from His-tagged human cIAP1 BIR3 domain expressed in Escherichia coli BL21(DE3) cells by fluorescence polari... | J Med Chem 52: 593-6 (2009) Article DOI: 10.1021/jm801101z BindingDB Entry DOI: 10.7270/Q2Z03816 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50463297 (CHEMBL4246433) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human MOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 26: 4254-4263 (2018) Article DOI: 10.1016/j.bmc.2018.07.020 BindingDB Entry DOI: 10.7270/Q28W3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50463294 (CHEMBL4249256) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 26: 4254-4263 (2018) Article DOI: 10.1016/j.bmc.2018.07.020 BindingDB Entry DOI: 10.7270/Q28W3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50393632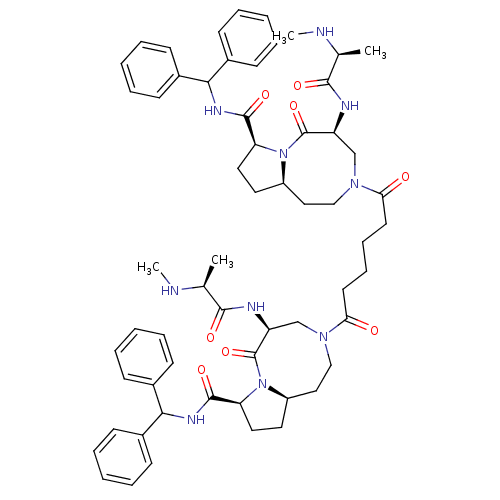 (CHEMBL2158601) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to BIR3 domain of cIAP1 by fluorescence polarization assay | J Med Chem 55: 106-14 (2012) Article DOI: 10.1021/jm201072x BindingDB Entry DOI: 10.7270/Q2BG2Q3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50253393 (CHEMBL495327 | Naphthalene-2-carboxylic acid (4-{2...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]PD128907 from dopamine D3 receptor in Sprague-Dawley rat ventral striatum | J Med Chem 51: 5905-8 (2008) Article DOI: 10.1021/jm800471h BindingDB Entry DOI: 10.7270/Q2FN161H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50181837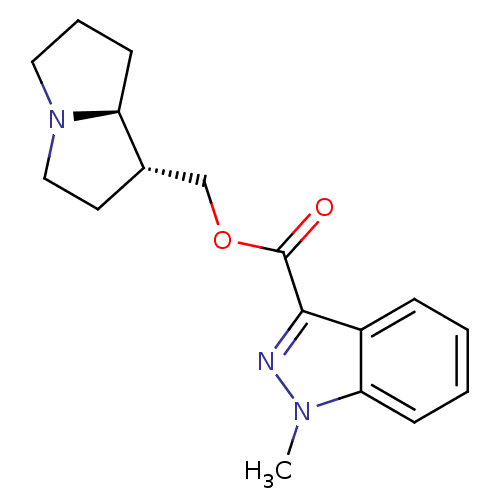 ((1S,7aR)-hexahydro-1H-pyrrolizin-1-ylmethyl 1-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50253473 (CHEMBL492883 | N-(4-{2-[((S)-2-Amino-4,5,6,7-tetra...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]PD128907 from dopamine D3 receptor in Sprague-Dawley rat ventral striatum | J Med Chem 51: 5905-8 (2008) Article DOI: 10.1021/jm800471h BindingDB Entry DOI: 10.7270/Q2FN161H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50556825 (CHEMBL4787784) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [Glp65, Nle75, Tyr77][125I]-apelin13 from human APJ receptor expressed in HEK293 cells incubated for 1 hr by gamma counting based rad... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01913 BindingDB Entry DOI: 10.7270/Q2BG2SNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50453769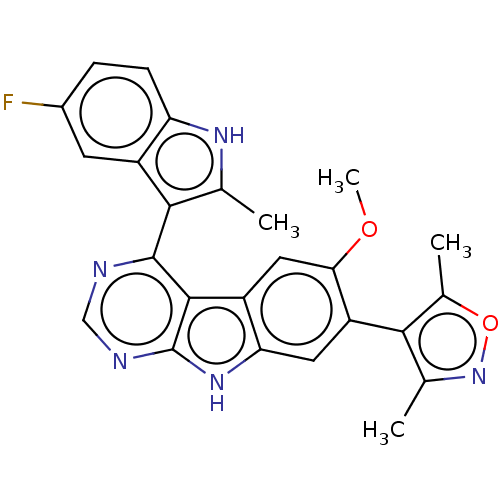 (CHEMBL4214978) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 DE... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50459819 (CHEMBL4228445) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) (unknown origin) expressed in ... | J Med Chem 61: 462-481 (2018) Article DOI: 10.1021/acs.jmedchem.6b01816 BindingDB Entry DOI: 10.7270/Q2CV4MD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50453810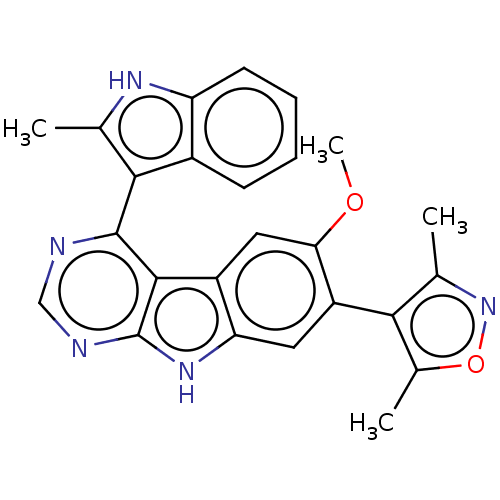 (CHEMBL4208405) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 DE... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50453807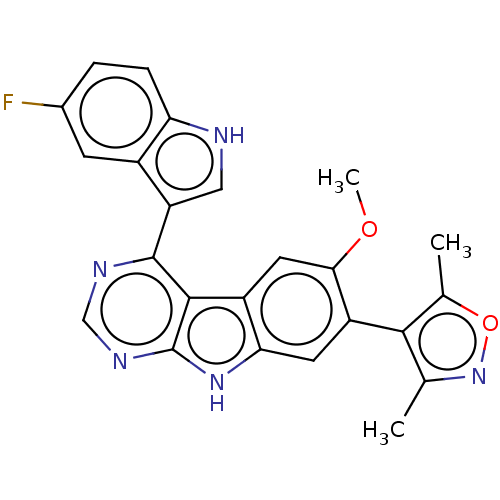 (CHEMBL4211497) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 DE... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50366704 (CHEMBL4166630) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM179480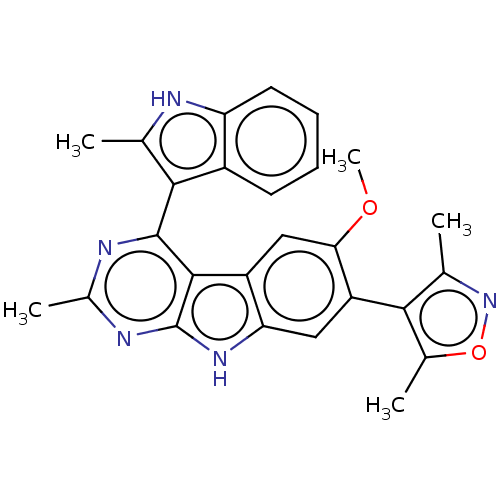 (US9675697, Cpd. No. 68) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 2 (333 to 460 residues) expressed in Rosetta2 D... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50343522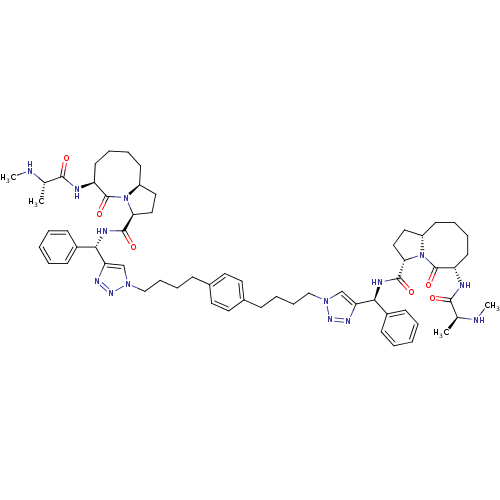 ((S,3S,3'S,6S,6'S,10aS,10a'S)-N,N'-((1S,1'S)-(1,1'-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to BIR3 domain of cIAP1 by fluorescence polarization assay | J Med Chem 55: 106-14 (2012) Article DOI: 10.1021/jm201072x BindingDB Entry DOI: 10.7270/Q2BG2Q3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50253472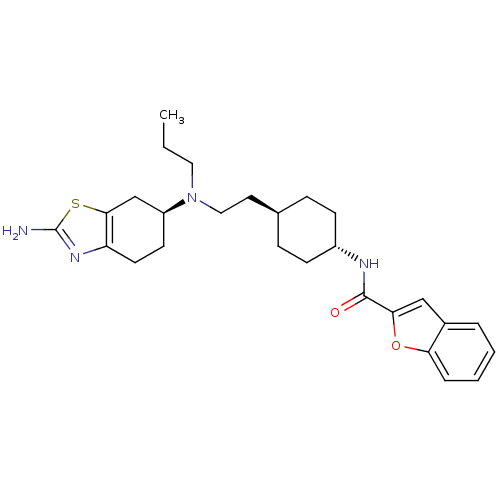 (Benzofuran-2-carboxylic acid (4-{2-[((S)-2-amino-4...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]PD128907 from dopamine D3 receptor in Sprague-Dawley rat ventral striatum | J Med Chem 51: 5905-8 (2008) Article DOI: 10.1021/jm800471h BindingDB Entry DOI: 10.7270/Q2FN161H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50384584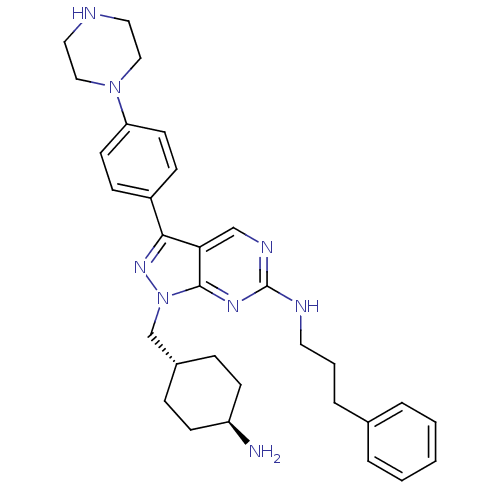 (CHEMBL2036807 | US9744172, Compound UNC607A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation | ACS Med Chem Lett 3: 129-134 (2012) Article DOI: 10.1021/ml200239k BindingDB Entry DOI: 10.7270/Q2F76DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50463297 (CHEMBL4246433) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 26: 4254-4263 (2018) Article DOI: 10.1016/j.bmc.2018.07.020 BindingDB Entry DOI: 10.7270/Q28W3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50556828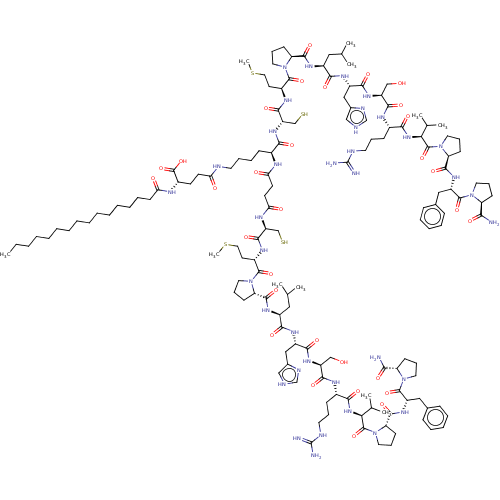 (CHEMBL4798771) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [Glp65, Nle75, Tyr77][125I]-apelin13 from human APJ receptor expressed in HEK293 cells incubated for 1 hr by gamma counting based rad... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01913 BindingDB Entry DOI: 10.7270/Q2BG2SNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50166450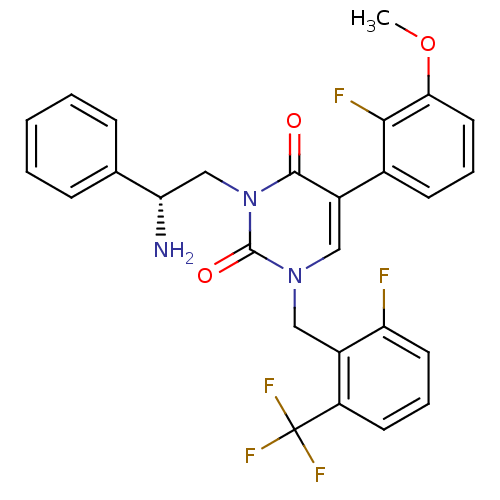 ((R)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-3-(2-am...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Pro-N-Et-GnRH from human cloned GnRH receptor expressed in HEK cells | Bioorg Med Chem Lett 18: 4503-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.059 BindingDB Entry DOI: 10.7270/Q2QR4WZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 [326-398] (Homo sapiens (Human)) | BDBM179480 (US9675697, Cpd. No. 68) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.650 | -52.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description Fluorescence Polarization (FP) competitive binding studies (see above) were carried out using the FAM labeled fluorescent probe Cpd. No. 350 to deter... | US Patent US9675697 (2017) BindingDB Entry DOI: 10.7270/Q2CC0XV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM26223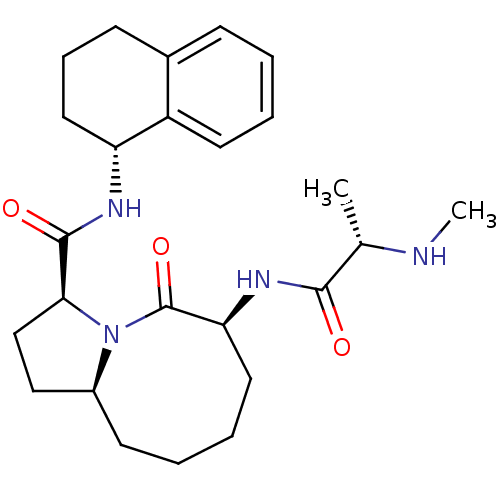 ((3S,6S,10aS)-6-[(2S)-2-(methylamino)propanamido]-5...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of fluorescent SM5F peptide from His-tagged human cIAP1 BIR3 domain expressed in Escherichia coli BL21(DE3) cells by fluorescence polari... | J Med Chem 52: 593-6 (2009) Article DOI: 10.1021/jm801101z BindingDB Entry DOI: 10.7270/Q2Z03816 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50181842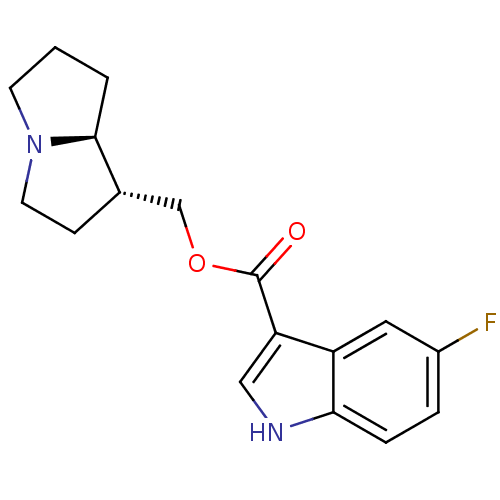 ((1R,7aS)-hexahydro-1H-pyrrolizin-1-ylmethyl 5-Fluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50393631 (CHEMBL2158600) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to BIR3 domain of cIAP1 by fluorescence polarization assay | J Med Chem 55: 106-14 (2012) Article DOI: 10.1021/jm201072x BindingDB Entry DOI: 10.7270/Q2BG2Q3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50366716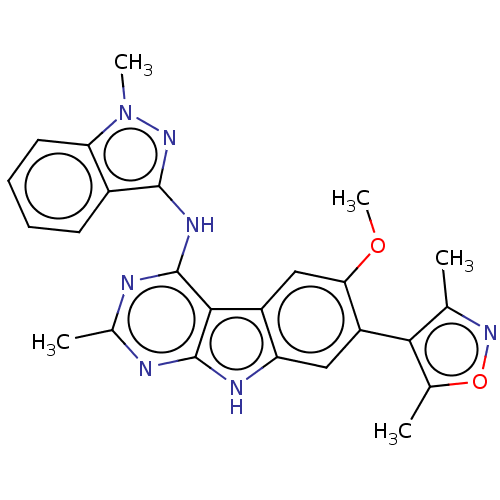 (CHEMBL4174669) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50453810 (CHEMBL4208405) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 2 (333 to 460 residues) expressed in Rosetta2 D... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50315205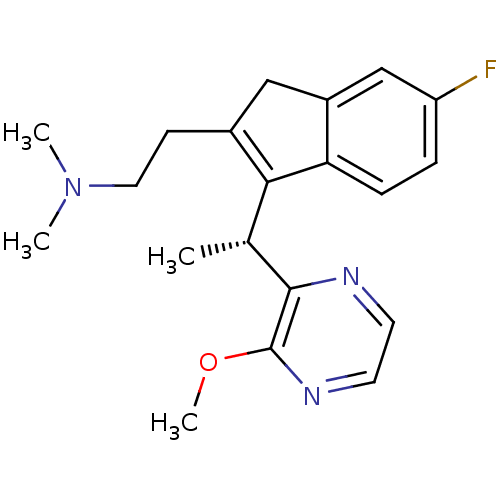 ((R)-2-(6-fluoro-3-(1-(3-methoxypyrazin-2-yl)ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Binding affinity at histamine H1 receptor | Bioorg Med Chem Lett 20: 2629-33 (2010) Article DOI: 10.1016/j.bmcl.2010.02.055 BindingDB Entry DOI: 10.7270/Q2NG4QSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50315205 ((R)-2-(6-fluoro-3-(1-(3-methoxypyrazin-2-yl)ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Inhibition of histamine H1 receptor | Bioorg Med Chem Lett 20: 5874-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.117 BindingDB Entry DOI: 10.7270/Q26W9BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50384583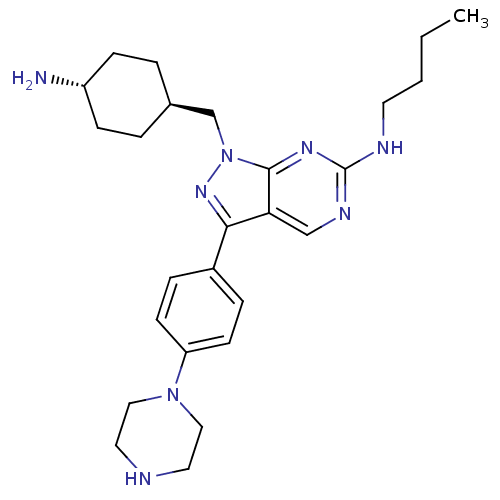 (CHEMBL2036806) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation | ACS Med Chem Lett 3: 129-134 (2012) Article DOI: 10.1021/ml200239k BindingDB Entry DOI: 10.7270/Q2F76DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50253394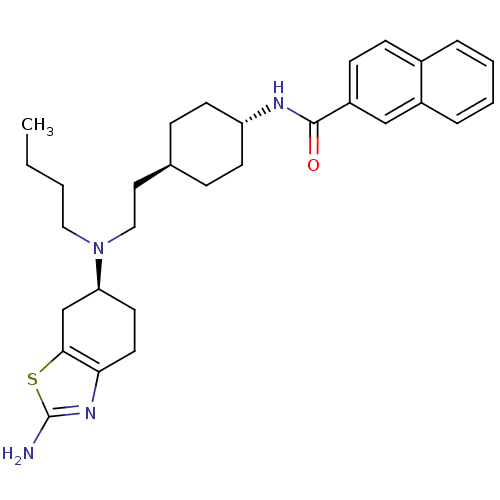 (CHEMBL494308 | Naphthalene-2-carboxylic acid (4-{2...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]PD128907 from dopamine D3 receptor in Sprague-Dawley rat ventral striatum | J Med Chem 51: 5905-8 (2008) Article DOI: 10.1021/jm800471h BindingDB Entry DOI: 10.7270/Q2FN161H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50116766 ((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]PD128907 from dopamine D3 receptor in Sprague-Dawley rat ventral striatum | J Med Chem 51: 5905-8 (2008) Article DOI: 10.1021/jm800471h BindingDB Entry DOI: 10.7270/Q2FN161H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50181841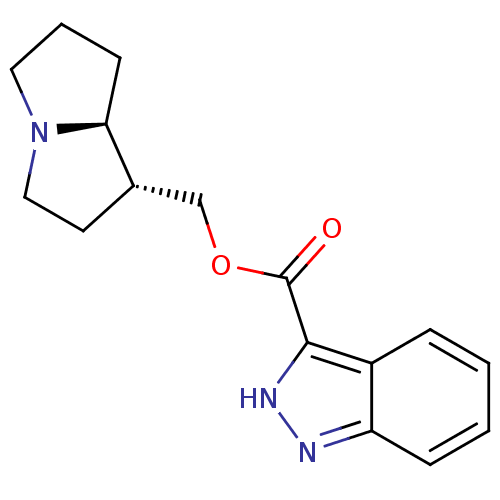 ((1S,7aR)-hexahydro-1H-pyrrolizin-1-ylmethyl 1H-ind...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50388994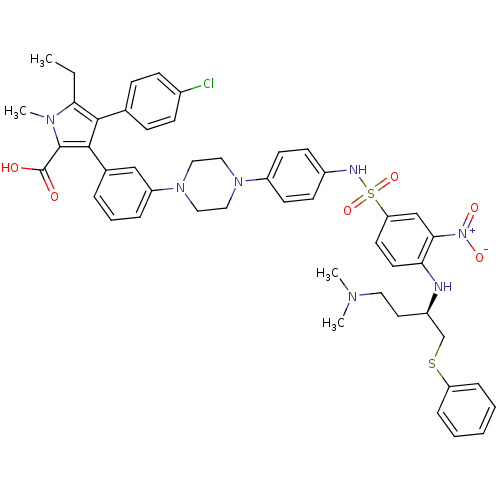 (CHEMBL2063897) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 6X His-tagged human Bcl2 expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization assay | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50397455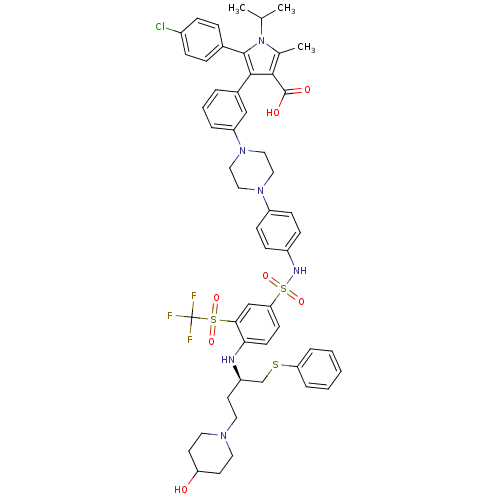 (CHEMBL2170838) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminal 6xHis-tagged human Bcl-2 expressed in Escherichia coli BL21 (DE3) after 2 hrs by fluorescence polarization assay | J Med Chem 55: 8502-14 (2012) Article DOI: 10.1021/jm3010306 BindingDB Entry DOI: 10.7270/Q2V69KQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50366724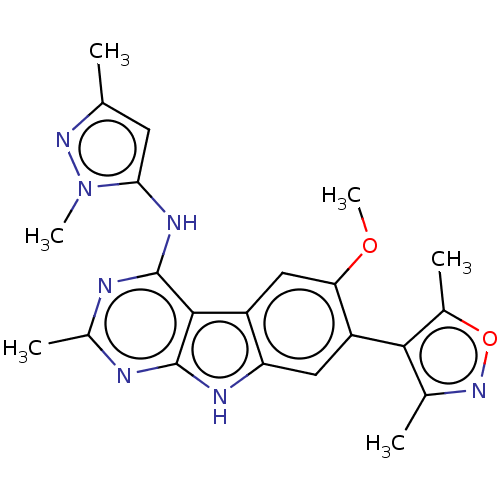 (CHEMBL4172277) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM26218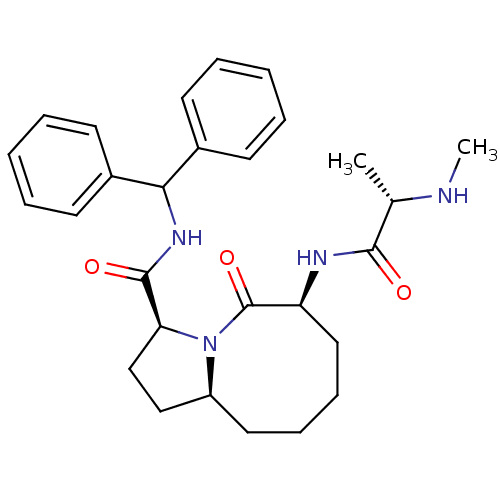 ((3S,6S,10aS)-N-(diphenylmethyl)-6-[(2S)-2-(methyla...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of fluorescent SM5F peptide from His-tagged human cIAP1 BIR3 domain expressed in Escherichia coli BL21(DE3) cells by fluorescence polari... | J Med Chem 52: 593-6 (2009) Article DOI: 10.1021/jm801101z BindingDB Entry DOI: 10.7270/Q2Z03816 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50315191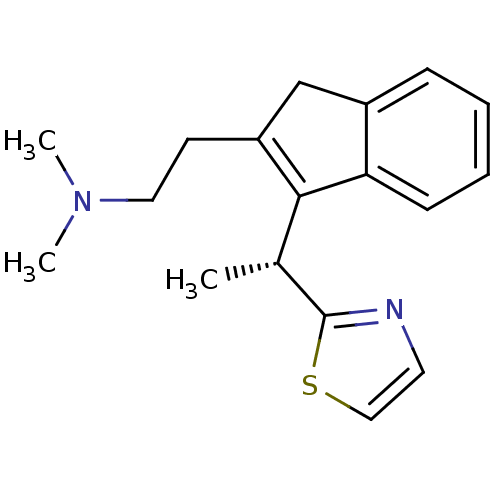 ((R)-N,N-dimethyl-2-(3-(1-(thiazol-2-yl)ethyl)-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Binding affinity at histamine H1 receptor | Bioorg Med Chem Lett 20: 2629-33 (2010) Article DOI: 10.1016/j.bmcl.2010.02.055 BindingDB Entry DOI: 10.7270/Q2NG4QSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 13086 total ) | Next | Last >> |