 Found 493 hits with Last Name = 'yao' and Initial = 'c'
Found 493 hits with Last Name = 'yao' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50595097

(CHEMBL5170337)Show SMILES COC[C@H]1CCCN1c1cc(Nc2ccccn2)nc(n1)-n1cccn1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50092173
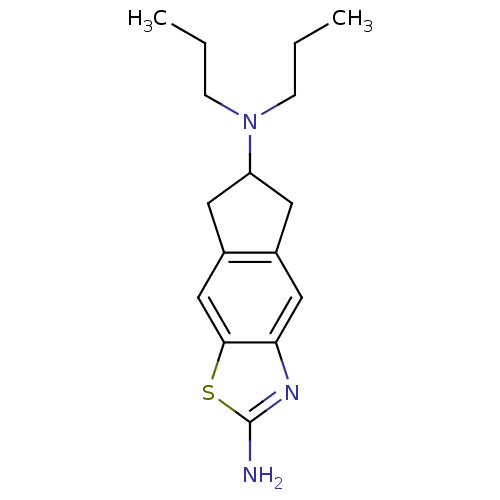
(CHEMBL325710 | N*6*,N*6*-Dipropyl-6,7-dihydro-5H-1...)Show InChI InChI=1S/C16H23N3S/c1-3-5-19(6-4-2)13-7-11-9-14-15(10-12(11)8-13)20-16(17)18-14/h9-10,13H,3-8H2,1-2H3,(H2,17,18) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50123627

((S)-6-Dipropylamino-5,6,7,8-tetrahydro-naphthalene...)Show InChI InChI=1S/C16H25NO2/c1-3-9-17(10-4-2)13-6-7-14-12(11-13)5-8-15(18)16(14)19/h5,8,13,18-19H,3-4,6-7,9-11H2,1-2H3/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50048466
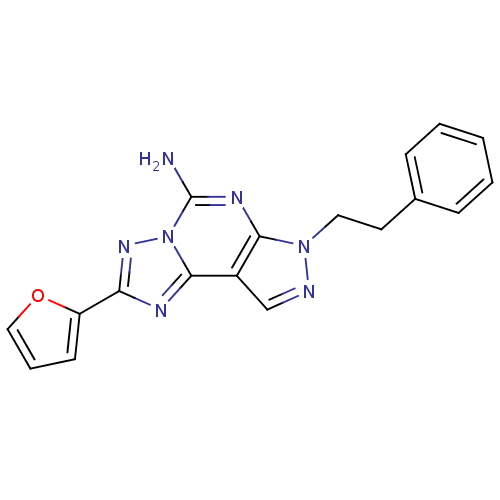
(2-(furan-2-yl)-7-phenethyl-7H-pyrazolo[4,3-e][1,2,...)Show InChI InChI=1S/C18H15N7O/c19-18-22-16-13(11-20-24(16)9-8-12-5-2-1-3-6-12)17-21-15(23-25(17)18)14-7-4-10-26-14/h1-7,10-11H,8-9H2,(H2,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50123627

((S)-6-Dipropylamino-5,6,7,8-tetrahydro-naphthalene...)Show InChI InChI=1S/C16H25NO2/c1-3-9-17(10-4-2)13-6-7-14-12(11-13)5-8-15(18)16(14)19/h5,8,13,18-19H,3-4,6-7,9-11H2,1-2H3/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50152240

(CHEMBL184309 | N*5*-{2-[4-(2,4-Difluoro-phenyl)-pi...)Show SMILES CN(CCN1CCN(CC1)c1ccc(F)cc1F)c1nc(N)n2nc(nc2n1)-c1ccco1 Show InChI InChI=1S/C21H23F2N9O/c1-29(6-7-30-8-10-31(11-9-30)16-5-4-14(22)13-15(16)23)20-26-19(24)32-21(27-20)25-18(28-32)17-3-2-12-33-17/h2-5,12-13H,6-11H2,1H3,(H2,24,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50499055
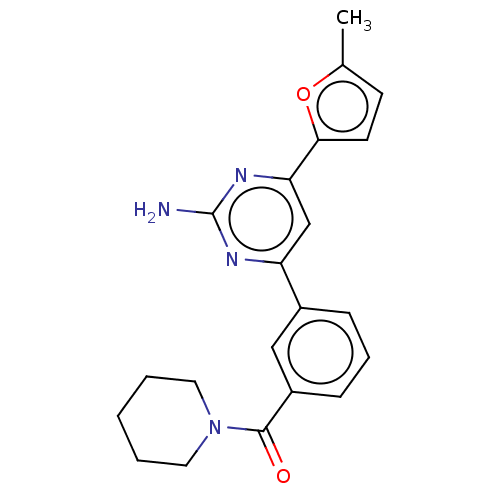
(CHEMBL3735985)Show SMILES Cc1ccc(o1)-c1cc(nc(N)n1)-c1cccc(c1)C(=O)N1CCCCC1 Show InChI InChI=1S/C21H22N4O2/c1-14-8-9-19(27-14)18-13-17(23-21(22)24-18)15-6-5-7-16(12-15)20(26)25-10-3-2-4-11-25/h5-9,12-13H,2-4,10-11H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50499055

(CHEMBL3735985)Show SMILES Cc1ccc(o1)-c1cc(nc(N)n1)-c1cccc(c1)C(=O)N1CCCCC1 Show InChI InChI=1S/C21H22N4O2/c1-14-8-9-19(27-14)18-13-17(23-21(22)24-18)15-6-5-7-16(12-15)20(26)25-10-3-2-4-11-25/h5-9,12-13H,2-4,10-11H2,1H3,(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50152215

(7N-[1-(2-chloro-4-pyridylmethyl)-(2R)-tetrahydro-1...)Show SMILES Nc1nc(NC[C@H]2CCCN2Cc2ccnc(Cl)c2)nc2nc(nn12)-c1ccco1 Show InChI InChI=1S/C19H20ClN9O/c20-15-9-12(5-6-22-15)11-28-7-1-3-13(28)10-23-18-25-17(21)29-19(26-18)24-16(27-29)14-4-2-8-30-14/h2,4-6,8-9,13H,1,3,7,10-11H2,(H3,21,23,24,25,26,27)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50011294
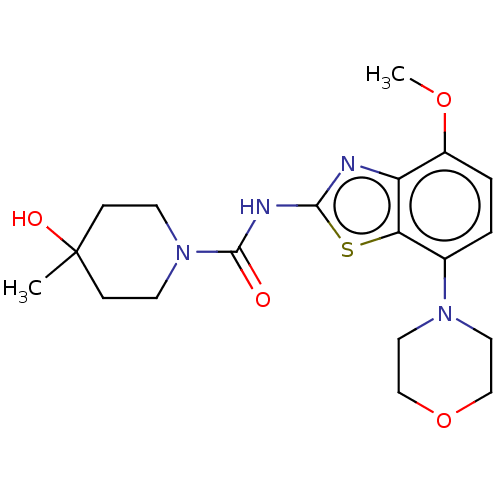
(A2A | Ro-4494351 | Ro-4494351-002 | Ro-4494351000 ...)Show SMILES COc1ccc(N2CCOCC2)c2sc(NC(=O)N3CCC(C)(O)CC3)nc12 Show InChI InChI=1S/C19H26N4O4S/c1-19(25)5-7-23(8-6-19)18(24)21-17-20-15-14(26-2)4-3-13(16(15)28-17)22-9-11-27-12-10-22/h3-4,25H,5-12H2,1-2H3,(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50092173

(CHEMBL325710 | N*6*,N*6*-Dipropyl-6,7-dihydro-5H-1...)Show InChI InChI=1S/C16H23N3S/c1-3-5-19(6-4-2)13-7-11-9-14-15(10-12(11)8-13)20-16(17)18-14/h9-10,13H,3-8H2,1-2H3,(H2,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50594647

(CHEMBL5182020)Show SMILES Cc1cccc(Nc2nc(NCCCO)nc3n(ncc23)-c2ccccc2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50377446

((S,R)-MEFLOQUINE)Show SMILES O[C@@H]([C@@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50156628

((R)-2-furan-2-yl-7-(hexahydropyrrolo[1,2-a]pyrazin...)Show SMILES Nc1nc(cc2nc(nn12)-c1ccco1)N1CCN2CCC[C@@H]2C1 |r| Show InChI InChI=1S/C16H19N7O/c17-16-19-13(22-7-6-21-5-1-3-11(21)10-22)9-14-18-15(20-23(14)16)12-4-2-8-24-12/h2,4,8-9,11H,1,3,5-7,10H2,(H2,17,19)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50151188
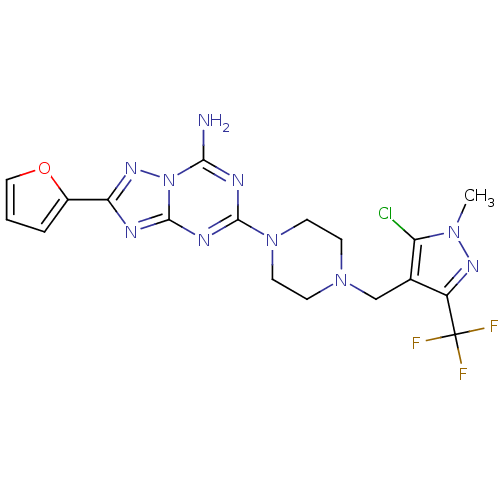
(5-(4-((5-chloro-1-methyl-3-(trifluoromethyl)-1H-py...)Show SMILES Cn1nc(c(CN2CCN(CC2)c2nc(N)n3nc(nc3n2)-c2ccco2)c1Cl)C(F)(F)F Show InChI InChI=1S/C18H18ClF3N10O/c1-29-13(19)10(12(27-29)18(20,21)22)9-30-4-6-31(7-5-30)16-25-15(23)32-17(26-16)24-14(28-32)11-3-2-8-33-11/h2-3,8H,4-7,9H2,1H3,(H2,23,24,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50048466

(2-(furan-2-yl)-7-phenethyl-7H-pyrazolo[4,3-e][1,2,...)Show InChI InChI=1S/C18H15N7O/c19-18-22-16-13(11-20-24(16)9-8-12-5-2-1-3-6-12)17-21-15(23-25(17)18)14-7-4-10-26-14/h1-7,10-11H,8-9H2,(H2,19,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50152215

(7N-[1-(2-chloro-4-pyridylmethyl)-(2R)-tetrahydro-1...)Show SMILES Nc1nc(NC[C@H]2CCCN2Cc2ccnc(Cl)c2)nc2nc(nn12)-c1ccco1 Show InChI InChI=1S/C19H20ClN9O/c20-15-9-12(5-6-22-15)11-28-7-1-3-13(28)10-23-18-25-17(21)29-19(26-18)24-16(27-29)14-4-2-8-30-14/h2,4-6,8-9,13H,1,3,7,10-11H2,(H3,21,23,24,25,26,27)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50377446

((S,R)-MEFLOQUINE)Show SMILES O[C@@H]([C@@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50594647

(CHEMBL5182020)Show SMILES Cc1cccc(Nc2nc(NCCCO)nc3n(ncc23)-c2ccccc2)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50152240

(CHEMBL184309 | N*5*-{2-[4-(2,4-Difluoro-phenyl)-pi...)Show SMILES CN(CCN1CCN(CC1)c1ccc(F)cc1F)c1nc(N)n2nc(nc2n1)-c1ccco1 Show InChI InChI=1S/C21H23F2N9O/c1-29(6-7-30-8-10-31(11-9-30)16-5-4-14(22)13-15(16)23)20-26-19(24)32-21(27-20)25-18(28-32)17-3-2-12-33-17/h2-5,12-13H,6-11H2,1H3,(H2,24,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50156628

((R)-2-furan-2-yl-7-(hexahydropyrrolo[1,2-a]pyrazin...)Show SMILES Nc1nc(cc2nc(nn12)-c1ccco1)N1CCN2CCC[C@@H]2C1 |r| Show InChI InChI=1S/C16H19N7O/c17-16-19-13(22-7-6-21-5-1-3-11(21)10-22)9-14-18-15(20-23(14)16)12-4-2-8-24-12/h2,4,8-9,11H,1,3,5-7,10H2,(H2,17,19)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50011294

(A2A | Ro-4494351 | Ro-4494351-002 | Ro-4494351000 ...)Show SMILES COc1ccc(N2CCOCC2)c2sc(NC(=O)N3CCC(C)(O)CC3)nc12 Show InChI InChI=1S/C19H26N4O4S/c1-19(25)5-7-23(8-6-19)18(24)21-17-20-15-14(26-2)4-3-13(16(15)28-17)22-9-11-27-12-10-22/h3-4,25H,5-12H2,1-2H3,(H,20,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50133184
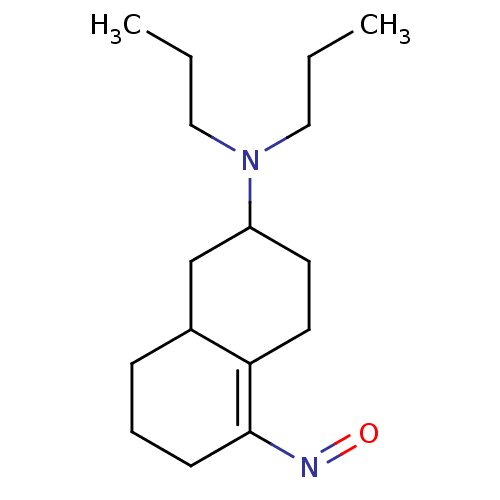
(6-Dipropylamino-3,4,5,6,7,8-hexahydro-2H-naphthale...)Show InChI InChI=1S/C16H28N2O/c1-3-10-18(11-4-2)14-8-9-15-13(12-14)6-5-7-16(15)17-19/h13-14H,3-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50151188

(5-(4-((5-chloro-1-methyl-3-(trifluoromethyl)-1H-py...)Show SMILES Cn1nc(c(CN2CCN(CC2)c2nc(N)n3nc(nc3n2)-c2ccco2)c1Cl)C(F)(F)F Show InChI InChI=1S/C18H18ClF3N10O/c1-29-13(19)10(12(27-29)18(20,21)22)9-30-4-6-31(7-5-30)16-25-15(23)32-17(26-16)24-14(28-32)11-3-2-8-33-11/h2-3,8H,4-7,9H2,1H3,(H2,23,24,25,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50270331
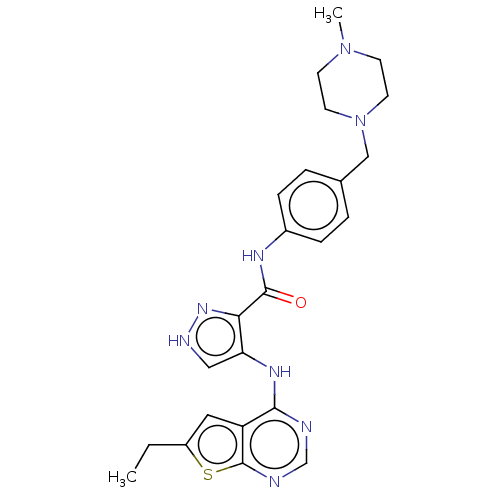
(CHEMBL4086149)Show SMILES CCc1cc2c(Nc3c[nH]nc3C(=O)Nc3ccc(CN4CCN(C)CC4)cc3)ncnc2s1 Show InChI InChI=1S/C24H28N8OS/c1-3-18-12-19-22(25-15-26-24(19)34-18)29-20-13-27-30-21(20)23(33)28-17-6-4-16(5-7-17)14-32-10-8-31(2)9-11-32/h4-7,12-13,15H,3,8-11,14H2,1-2H3,(H,27,30)(H,28,33)(H,25,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.101 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... |
J Med Chem 61: 1499-1518 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01261
BindingDB Entry DOI: 10.7270/Q2FR003K |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50270307
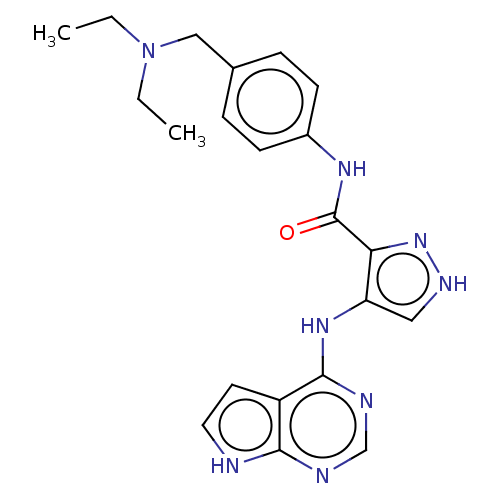
(CHEMBL4092761)Show SMILES CCN(CC)Cc1ccc(NC(=O)c2n[nH]cc2Nc2ncnc3[nH]ccc23)cc1 Show InChI InChI=1S/C21H24N8O/c1-3-29(4-2)12-14-5-7-15(8-6-14)26-21(30)18-17(11-25-28-18)27-20-16-9-10-22-19(16)23-13-24-20/h5-11,13H,3-4,12H2,1-2H3,(H,25,28)(H,26,30)(H2,22,23,24,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... |
J Med Chem 61: 1499-1518 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01261
BindingDB Entry DOI: 10.7270/Q2FR003K |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50270330
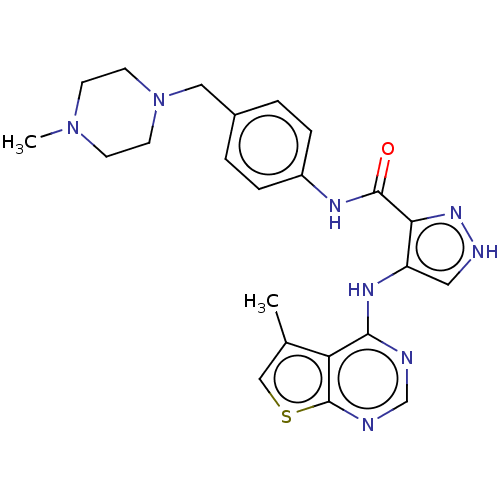
(CHEMBL4078261)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3n[nH]cc3Nc3ncnc4scc(C)c34)cc2)CC1 Show InChI InChI=1S/C23H26N8OS/c1-15-13-33-23-19(15)21(24-14-25-23)28-18-11-26-29-20(18)22(32)27-17-5-3-16(4-6-17)12-31-9-7-30(2)8-10-31/h3-6,11,13-14H,7-10,12H2,1-2H3,(H,26,29)(H,27,32)(H,24,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.167 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... |
J Med Chem 61: 1499-1518 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01261
BindingDB Entry DOI: 10.7270/Q2FR003K |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50449547
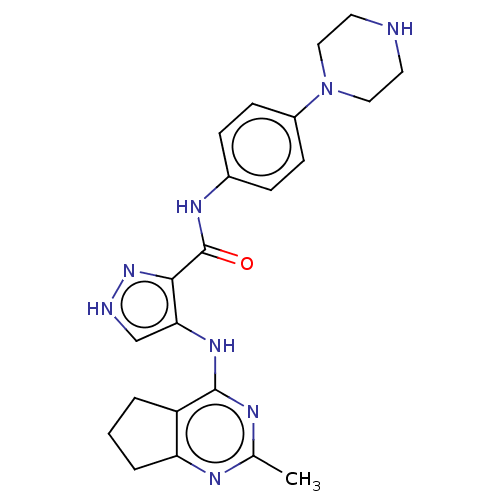
(CHEMBL4163870)Show SMILES Cc1nc2CCCc2c(Nc2c[nH]nc2C(=O)Nc2ccc(cc2)N2CCNCC2)n1 Show InChI InChI=1S/C22H26N8O/c1-14-25-18-4-2-3-17(18)21(26-14)28-19-13-24-29-20(19)22(31)27-15-5-7-16(8-6-15)30-11-9-23-10-12-30/h5-8,13,23H,2-4,9-12H2,1H3,(H,24,29)(H,27,31)(H,25,26,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 by Hotspot assay |
Eur J Med Chem 155: 303-315 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.010
BindingDB Entry DOI: 10.7270/Q2668GSP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50270304
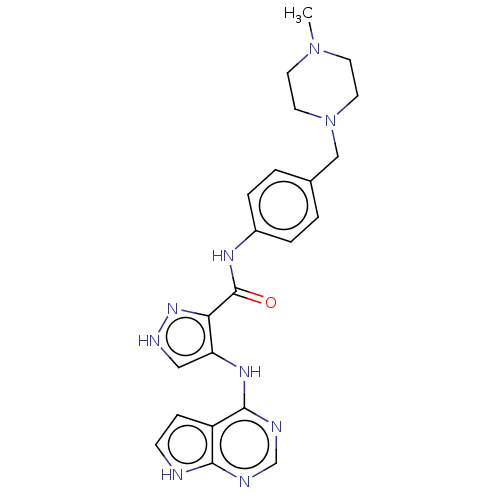
(CHEMBL4077071)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3n[nH]cc3Nc3ncnc4[nH]ccc34)cc2)CC1 Show InChI InChI=1S/C22H25N9O/c1-30-8-10-31(11-9-30)13-15-2-4-16(5-3-15)27-22(32)19-18(12-26-29-19)28-21-17-6-7-23-20(17)24-14-25-21/h2-7,12,14H,8-11,13H2,1H3,(H,26,29)(H,27,32)(H2,23,24,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... |
J Med Chem 61: 1499-1518 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01261
BindingDB Entry DOI: 10.7270/Q2FR003K |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50270309
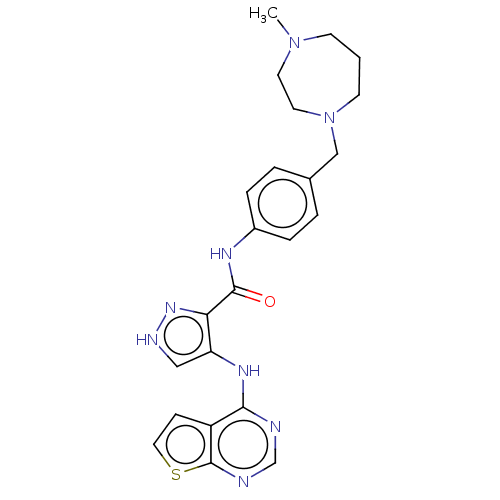
(CHEMBL4101635)Show SMILES CN1CCCN(Cc2ccc(NC(=O)c3n[nH]cc3Nc3ncnc4sccc34)cc2)CC1 Show InChI InChI=1S/C23H26N8OS/c1-30-8-2-9-31(11-10-30)14-16-3-5-17(6-4-16)27-22(32)20-19(13-26-29-20)28-21-18-7-12-33-23(18)25-15-24-21/h3-7,12-13,15H,2,8-11,14H2,1H3,(H,26,29)(H,27,32)(H,24,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... |
J Med Chem 61: 1499-1518 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01261
BindingDB Entry DOI: 10.7270/Q2FR003K |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50270316
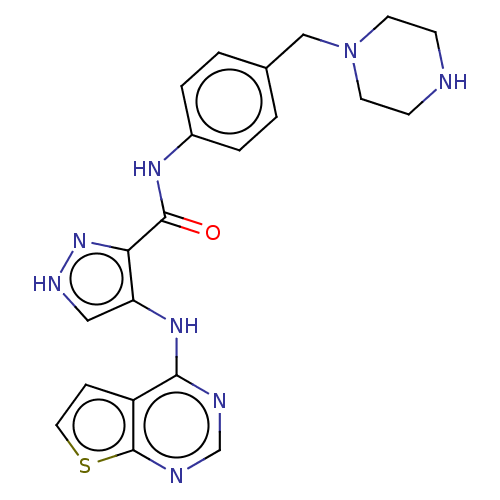
(CHEMBL4104057)Show SMILES O=C(Nc1ccc(CN2CCNCC2)cc1)c1n[nH]cc1Nc1ncnc2sccc12 Show InChI InChI=1S/C21H22N8OS/c30-20(26-15-3-1-14(2-4-15)12-29-8-6-22-7-9-29)18-17(11-25-28-18)27-19-16-5-10-31-21(16)24-13-23-19/h1-5,10-11,13,22H,6-9,12H2,(H,25,28)(H,26,30)(H,23,24,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... |
J Med Chem 61: 1499-1518 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01261
BindingDB Entry DOI: 10.7270/Q2FR003K |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50270329
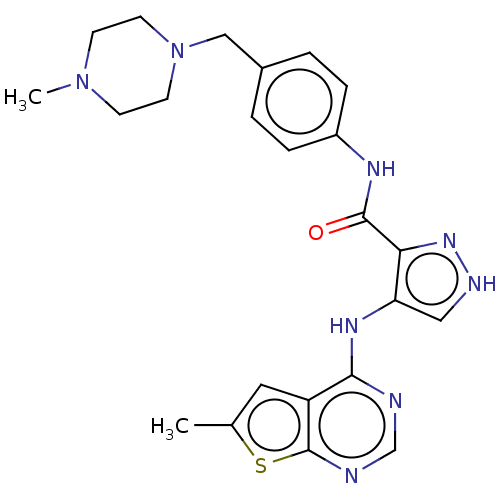
(CHEMBL4073053)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3n[nH]cc3Nc3ncnc4sc(C)cc34)cc2)CC1 Show InChI InChI=1S/C23H26N8OS/c1-15-11-18-21(24-14-25-23(18)33-15)28-19-12-26-29-20(19)22(32)27-17-5-3-16(4-6-17)13-31-9-7-30(2)8-10-31/h3-6,11-12,14H,7-10,13H2,1-2H3,(H,26,29)(H,27,32)(H,24,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.384 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... |
J Med Chem 61: 1499-1518 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01261
BindingDB Entry DOI: 10.7270/Q2FR003K |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50270298

(CHEMBL4075720)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3n[nH]cc3Nc3ncnc4sccc34)cc2)CC1 Show InChI InChI=1S/C22H24N8OS/c1-29-7-9-30(10-8-29)13-15-2-4-16(5-3-15)26-21(31)19-18(12-25-28-19)27-20-17-6-11-32-22(17)24-14-23-20/h2-6,11-12,14H,7-10,13H2,1H3,(H,25,28)(H,26,31)(H,23,24,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.438 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... |
J Med Chem 61: 1499-1518 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01261
BindingDB Entry DOI: 10.7270/Q2FR003K |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50449554
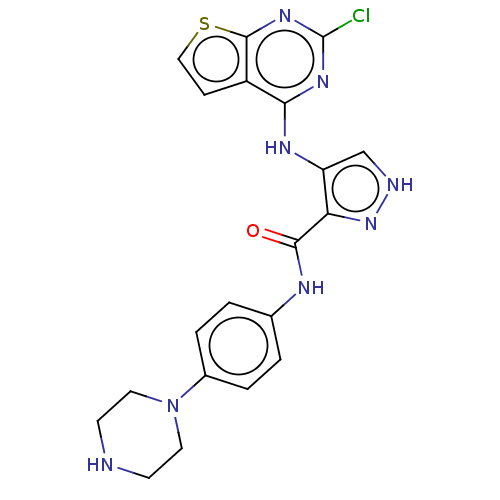
(CHEMBL4172223)Show SMILES Clc1nc(Nc2c[nH]nc2C(=O)Nc2ccc(cc2)N2CCNCC2)c2ccsc2n1 Show InChI InChI=1S/C20H19ClN8OS/c21-20-26-17(14-5-10-31-19(14)27-20)25-15-11-23-28-16(15)18(30)24-12-1-3-13(4-2-12)29-8-6-22-7-9-29/h1-5,10-11,22H,6-9H2,(H,23,28)(H,24,30)(H,25,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 by Hotspot assay |
Eur J Med Chem 155: 303-315 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.010
BindingDB Entry DOI: 10.7270/Q2668GSP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50270303
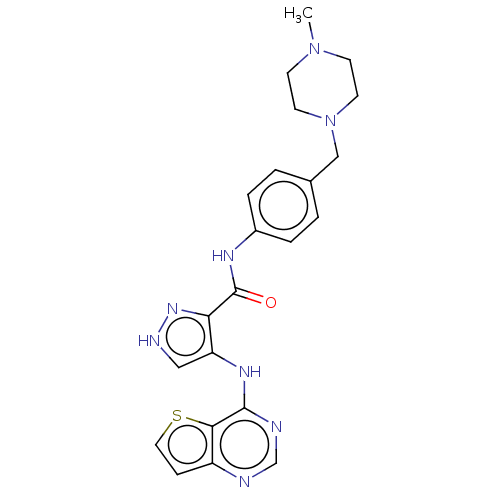
(CHEMBL4061686)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3n[nH]cc3Nc3ncnc4ccsc34)cc2)CC1 Show InChI InChI=1S/C22H24N8OS/c1-29-7-9-30(10-8-29)13-15-2-4-16(5-3-15)26-22(31)19-18(12-25-28-19)27-21-20-17(6-11-32-20)23-14-24-21/h2-6,11-12,14H,7-10,13H2,1H3,(H,25,28)(H,26,31)(H,23,24,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... |
J Med Chem 61: 1499-1518 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01261
BindingDB Entry DOI: 10.7270/Q2FR003K |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50270298

(CHEMBL4075720)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3n[nH]cc3Nc3ncnc4sccc34)cc2)CC1 Show InChI InChI=1S/C22H24N8OS/c1-29-7-9-30(10-8-29)13-15-2-4-16(5-3-15)26-21(31)19-18(12-25-28-19)27-20-17-6-11-32-22(17)24-14-23-20/h2-6,11-12,14H,7-10,13H2,1H3,(H,25,28)(H,26,31)(H,23,24,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 D835Y mutant by Hotspot assay |
Eur J Med Chem 155: 303-315 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.010
BindingDB Entry DOI: 10.7270/Q2668GSP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50270319
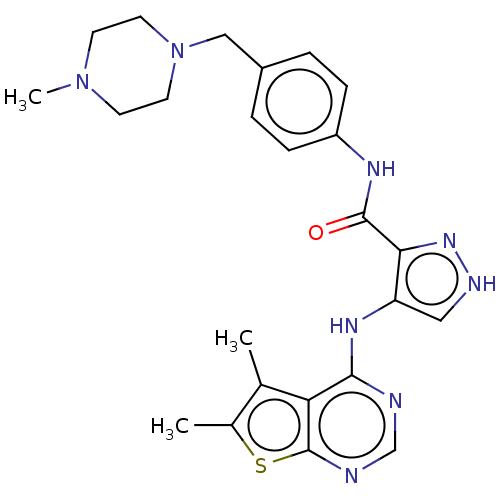
(CHEMBL4093755)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3n[nH]cc3Nc3ncnc4sc(C)c(C)c34)cc2)CC1 Show InChI InChI=1S/C24H28N8OS/c1-15-16(2)34-24-20(15)22(25-14-26-24)29-19-12-27-30-21(19)23(33)28-18-6-4-17(5-7-18)13-32-10-8-31(3)9-11-32/h4-7,12,14H,8-11,13H2,1-3H3,(H,27,30)(H,28,33)(H,25,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.576 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... |
J Med Chem 61: 1499-1518 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01261
BindingDB Entry DOI: 10.7270/Q2FR003K |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50270335
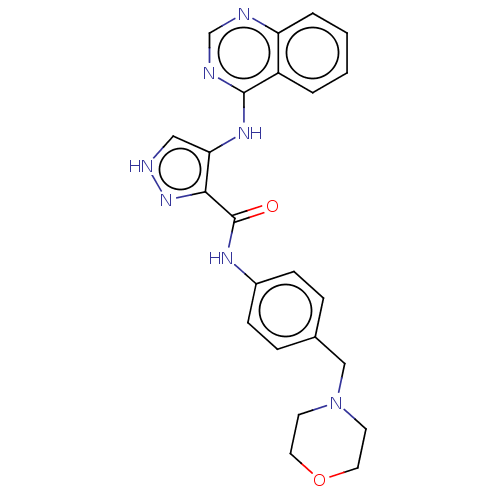
(CHEMBL4088753)Show SMILES O=C(Nc1ccc(CN2CCOCC2)cc1)c1n[nH]cc1Nc1ncnc2ccccc12 Show InChI InChI=1S/C23H23N7O2/c31-23(27-17-7-5-16(6-8-17)14-30-9-11-32-12-10-30)21-20(13-26-29-21)28-22-18-3-1-2-4-19(18)24-15-25-22/h1-8,13,15H,9-12,14H2,(H,26,29)(H,27,31)(H,24,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.588 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... |
J Med Chem 61: 1499-1518 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01261
BindingDB Entry DOI: 10.7270/Q2FR003K |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50270298

(CHEMBL4075720)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3n[nH]cc3Nc3ncnc4sccc34)cc2)CC1 Show InChI InChI=1S/C22H24N8OS/c1-29-7-9-30(10-8-29)13-15-2-4-16(5-3-15)26-21(31)19-18(12-25-28-19)27-20-17-6-11-32-22(17)24-14-23-20/h2-6,11-12,14H,7-10,13H2,1H3,(H,25,28)(H,26,31)(H,23,24,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 by Hotspot assay |
Eur J Med Chem 155: 303-315 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.010
BindingDB Entry DOI: 10.7270/Q2668GSP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50449553
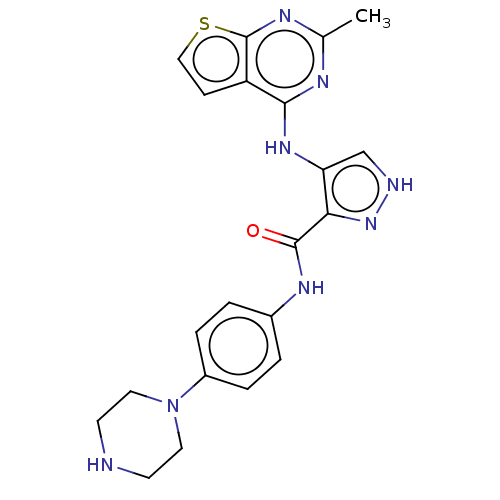
(CHEMBL4164331)Show SMILES Cc1nc(Nc2c[nH]nc2C(=O)Nc2ccc(cc2)N2CCNCC2)c2ccsc2n1 Show InChI InChI=1S/C21H22N8OS/c1-13-24-19(16-6-11-31-21(16)25-13)27-17-12-23-28-18(17)20(30)26-14-2-4-15(5-3-14)29-9-7-22-8-10-29/h2-6,11-12,22H,7-10H2,1H3,(H,23,28)(H,26,30)(H,24,25,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 by Hotspot assay |
Eur J Med Chem 155: 303-315 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.010
BindingDB Entry DOI: 10.7270/Q2668GSP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50270306
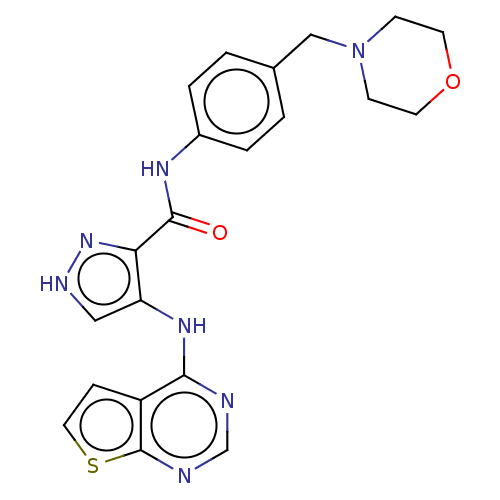
(CHEMBL4077074)Show SMILES O=C(Nc1ccc(CN2CCOCC2)cc1)c1n[nH]cc1Nc1ncnc2sccc12 Show InChI InChI=1S/C21H21N7O2S/c29-20(25-15-3-1-14(2-4-15)12-28-6-8-30-9-7-28)18-17(11-24-27-18)26-19-16-5-10-31-21(16)23-13-22-19/h1-5,10-11,13H,6-9,12H2,(H,24,27)(H,25,29)(H,22,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.623 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... |
J Med Chem 61: 1499-1518 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01261
BindingDB Entry DOI: 10.7270/Q2FR003K |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50449548
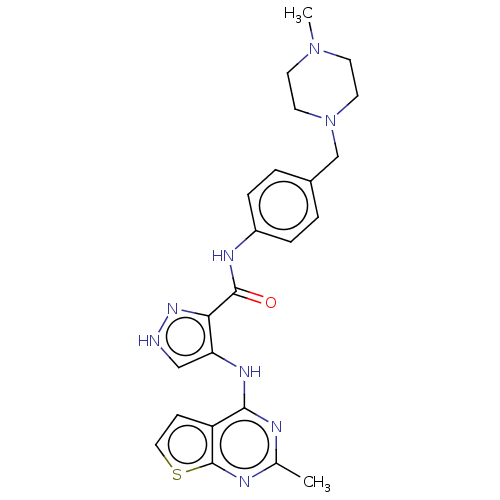
(CHEMBL4175865)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3n[nH]cc3Nc3nc(C)nc4sccc34)cc2)CC1 Show InChI InChI=1S/C23H26N8OS/c1-15-25-21(18-7-12-33-23(18)26-15)28-19-13-24-29-20(19)22(32)27-17-5-3-16(4-6-17)14-31-10-8-30(2)9-11-31/h3-7,12-13H,8-11,14H2,1-2H3,(H,24,29)(H,27,32)(H,25,26,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 by Hotspot assay |
Eur J Med Chem 155: 303-315 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.010
BindingDB Entry DOI: 10.7270/Q2668GSP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50449551

(CHEMBL4171138)Show SMILES CC(C)c1cc2c(Nc3c[nH]nc3C(=O)Nc3ccc(CN4CCOCC4)cc3)nc(C)nc2s1 Show InChI InChI=1S/C25H29N7O2S/c1-15(2)21-12-19-23(27-16(3)28-25(19)35-21)30-20-13-26-31-22(20)24(33)29-18-6-4-17(5-7-18)14-32-8-10-34-11-9-32/h4-7,12-13,15H,8-11,14H2,1-3H3,(H,26,31)(H,29,33)(H,27,28,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 by Hotspot assay |
Eur J Med Chem 155: 303-315 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.010
BindingDB Entry DOI: 10.7270/Q2668GSP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50449559
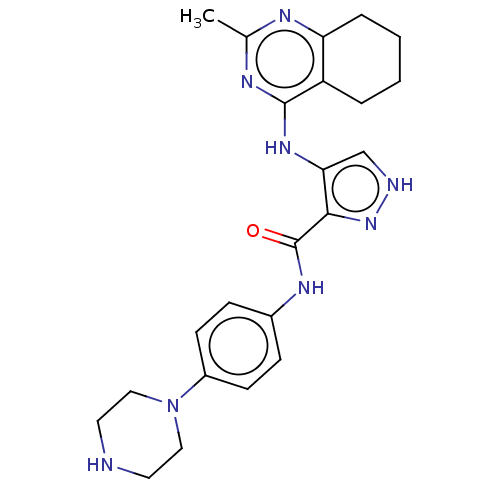
(CHEMBL4159478)Show SMILES Cc1nc2CCCCc2c(Nc2c[nH]nc2C(=O)Nc2ccc(cc2)N2CCNCC2)n1 Show InChI InChI=1S/C23H28N8O/c1-15-26-19-5-3-2-4-18(19)22(27-15)29-20-14-25-30-21(20)23(32)28-16-6-8-17(9-7-16)31-12-10-24-11-13-31/h6-9,14,24H,2-5,10-13H2,1H3,(H,25,30)(H,28,32)(H,26,27,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 by Hotspot assay |
Eur J Med Chem 155: 303-315 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.010
BindingDB Entry DOI: 10.7270/Q2668GSP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 D835Y mutant by Hotspot assay |
Eur J Med Chem 155: 303-315 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.010
BindingDB Entry DOI: 10.7270/Q2668GSP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50449544
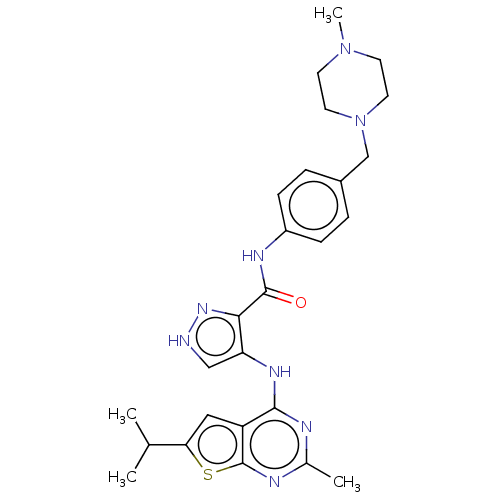
(CHEMBL4160544)Show SMILES CC(C)c1cc2c(Nc3c[nH]nc3C(=O)Nc3ccc(CN4CCN(C)CC4)cc3)nc(C)nc2s1 Show InChI InChI=1S/C26H32N8OS/c1-16(2)22-13-20-24(28-17(3)29-26(20)36-22)31-21-14-27-32-23(21)25(35)30-19-7-5-18(6-8-19)15-34-11-9-33(4)10-12-34/h5-8,13-14,16H,9-12,15H2,1-4H3,(H,27,32)(H,30,35)(H,28,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 by Hotspot assay |
Eur J Med Chem 155: 303-315 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.010
BindingDB Entry DOI: 10.7270/Q2668GSP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | CHEMBL5274847
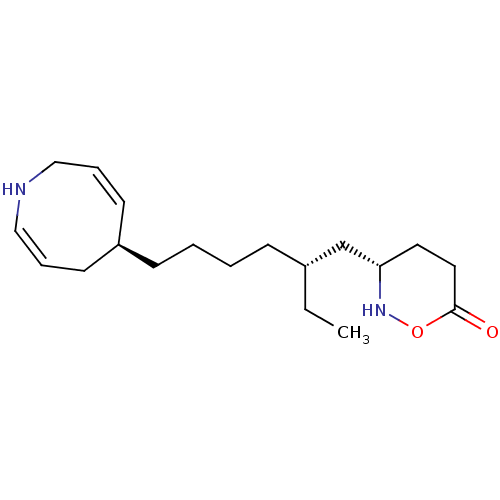
Show InChI InChI=1S/C20H24N4S/c1-2-3-4-5-7-14-10-12-15(13-11-14)25-17-9-6-8-16-18(17)19(21)24-20(22)23-16/h6,8-13H,2-5,7H2,1H3,(H4,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity for carbachol induced contractions in guinea pig ileum against Muscarinic acetylcholine receptor M3 in the presence of mepyrami... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 R595_E596 mutant by Hotspot assay |
Eur J Med Chem 155: 303-315 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.010
BindingDB Entry DOI: 10.7270/Q2668GSP |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50270304

(CHEMBL4077071)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3n[nH]cc3Nc3ncnc4[nH]ccc34)cc2)CC1 Show InChI InChI=1S/C22H25N9O/c1-30-8-10-31(11-9-30)13-15-2-4-16(5-3-15)27-22(32)19-18(12-26-29-19)28-21-17-6-7-23-20(17)24-14-25-21/h2-7,12,14H,8-11,13H2,1H3,(H,26,29)(H,27,32)(H2,23,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/Cyclin D1 using RB protein as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins... |
J Med Chem 61: 1499-1518 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01261
BindingDB Entry DOI: 10.7270/Q2FR003K |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50449546
(CHEMBL4160930)Show SMILES CN1CCN(CC1)c1ccc(NC(=O)c2n[nH]cc2Nc2nc(C)nc3sccc23)cc1 Show InChI InChI=1S/C22H24N8OS/c1-14-24-20(17-7-12-32-22(17)25-14)27-18-13-23-28-19(18)21(31)26-15-3-5-16(6-4-15)30-10-8-29(2)9-11-30/h3-7,12-13H,8-11H2,1-2H3,(H,23,28)(H,26,31)(H,24,25,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 by Hotspot assay |
Eur J Med Chem 155: 303-315 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.010
BindingDB Entry DOI: 10.7270/Q2668GSP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data