

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12493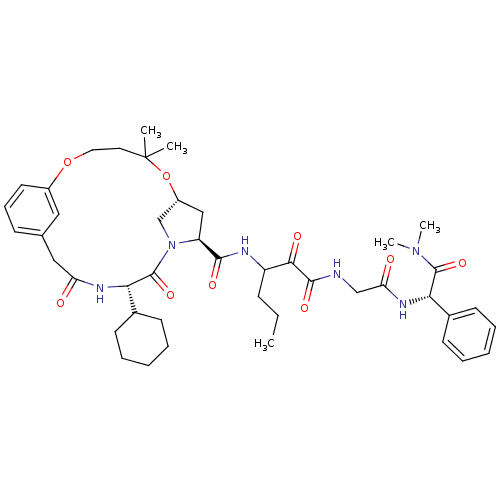 ((2S)-({N-[3-({[(7R,9S,12S)-12-cyclohexyl-5,5-dimet...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12483 ((2S)-({N-[3-({[(5S,8S,10R)-5-cyclohexyl-3,6-dioxo-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | -47.3 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12476 ((2S)-({N-[(3S)-3-({[(7R,9S,12S)-12-cyclohexyl-11,1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | -47.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12475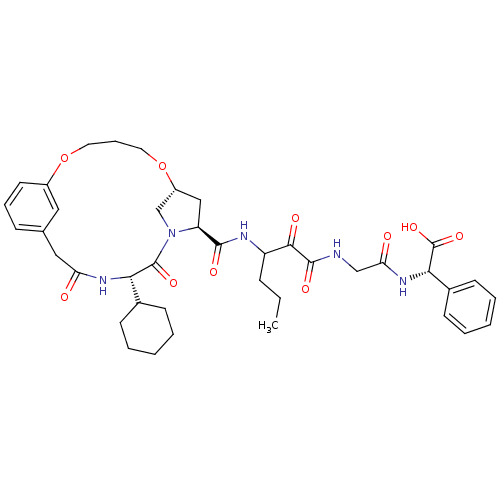 ((2S)-({N-[3-({[(7R,9S,12S)-12-cyclohexyl-11,14-dio...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | -46.4 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12484 ((2S)-({N-[3-({[(7R,9S,12S)-12-cyclohexyl-11,14-dio...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | -46.4 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12311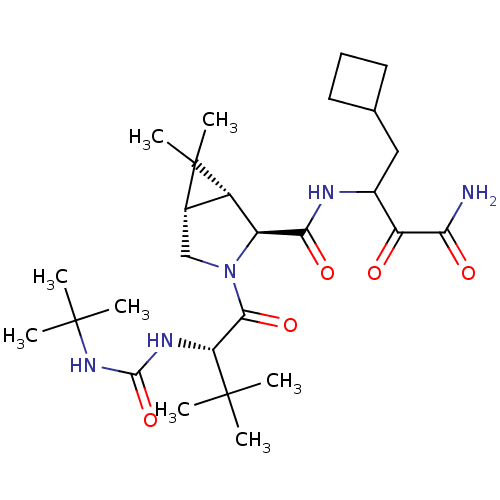 ((1R,5S)-N-[3-Amino-1-(cyclobutylmethyl)-2,3-dioxop...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 14 | -45.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12480 ((2S)-({N-[(3S)-3-({[(7R,9S,12S)-12-tert-butyl-11,1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | -45.4 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM9699 ((2S)-2-[2-(3-{[(3S,18S)-3-cyclohexyl-2,5-dioxo-12-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | -45.4 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 567-74 (2006) Article DOI: 10.1021/jm050520a BindingDB Entry DOI: 10.7270/Q2QJ7FJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12287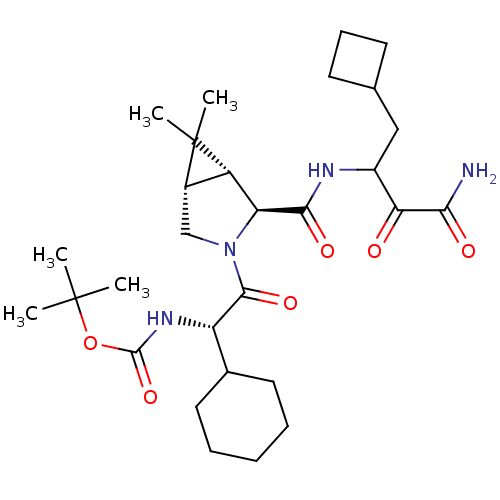 (SCH 491762 | tert-butyl N-[(1S)-2-[(1R,2S,5S)-2-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 15 | -45.4 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12492 (Proline-Based Macrocycle 44 | tert-butyl (2S)-({N-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12494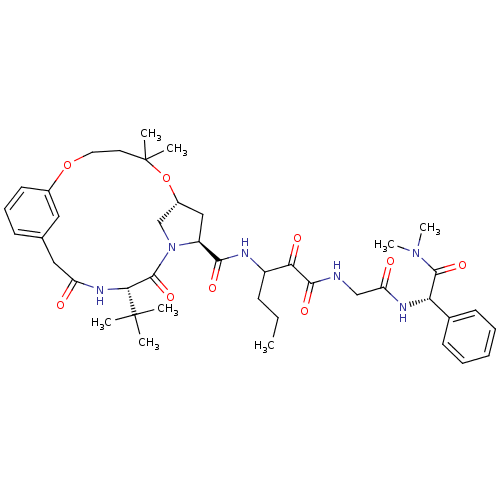 ((2S)-({N-[3-({[(7R,9S,12S)-12-tert-butyl-5,5-dimet...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12495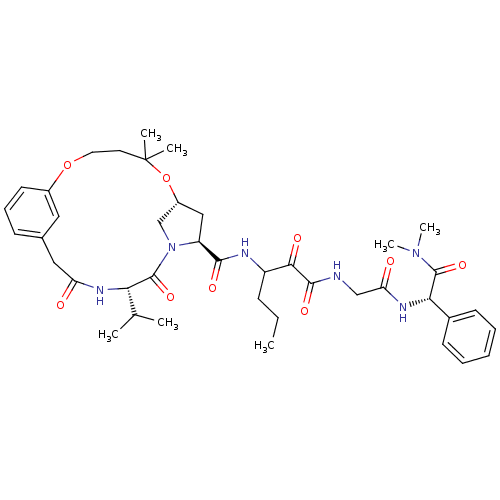 ((2S)-({N-[3-({[(7R,9S,12S)-12-isopropyl-5,5-dimeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17886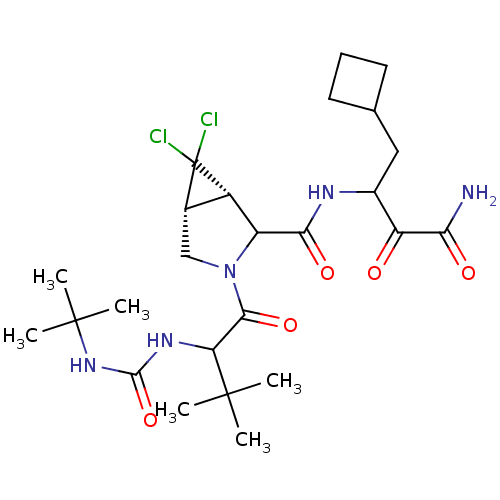 (3-{[(1S,5R)-3-{2-[(tert-butylcarbamoyl)amino]-3,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM9700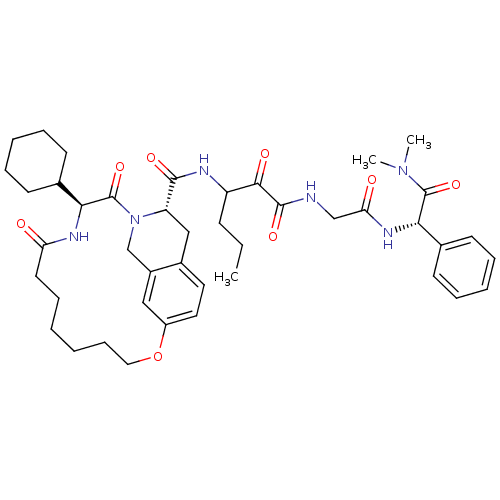 (3-{[(3S,18S)-3-cyclohexyl-2,5-dioxo-12-oxa-1,4-dia...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | -44.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 567-74 (2006) Article DOI: 10.1021/jm050520a BindingDB Entry DOI: 10.7270/Q2QJ7FJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12482 (3-{[(6R,8S,11S)-11-cyclohexyl-10,13-dioxo-2,5-diox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | -43.7 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12486 ((2S)-({N-[3-({[(7R,9S,12S)-12-cyclohexyl-11,14-dio...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17898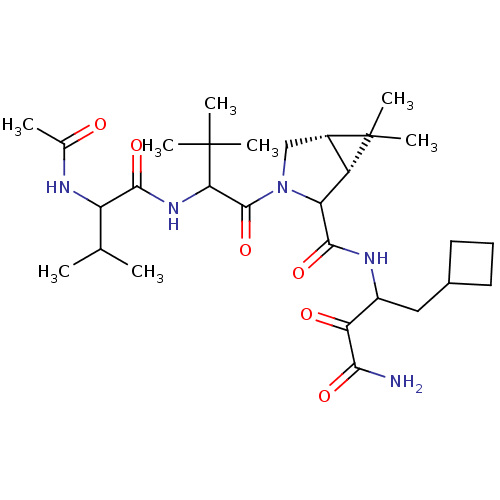 (Ketoamide inhibitor, 35 | N-{1-[(1R,5S)-2-[(1-carb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12498 ((2S)-({N-[3-({[(7R,9S,12S)-12-(2,3-dihydro-1H-inde...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12474 (Proline-Based Macrocycle 22 | tert-butyl (2S)-({N-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 44 | -42.7 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12497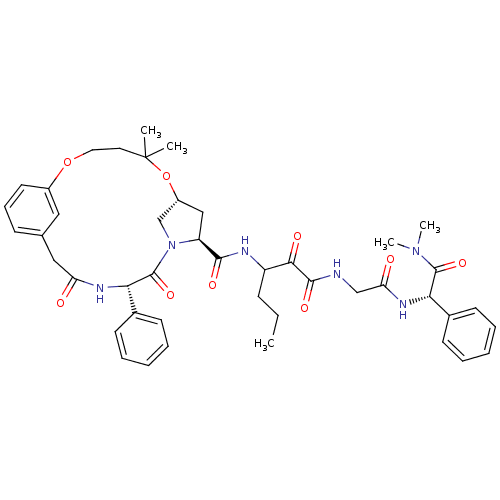 ((2S)-({N-[3-({[(7R,9S,12S)-5,5-dimethyl-11,14-diox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17884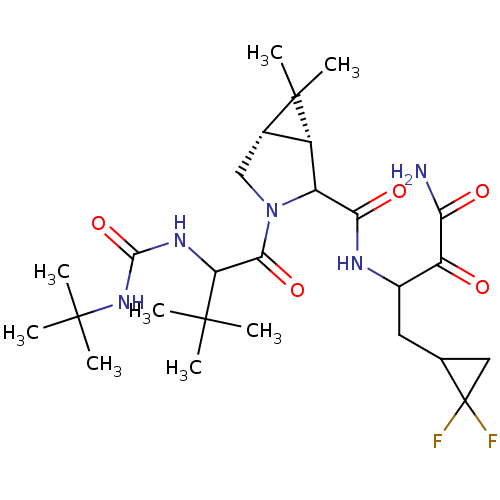 (3-{[(1R,5S)-3-{2-[(tert-butylcarbamoyl)amino]-3,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17875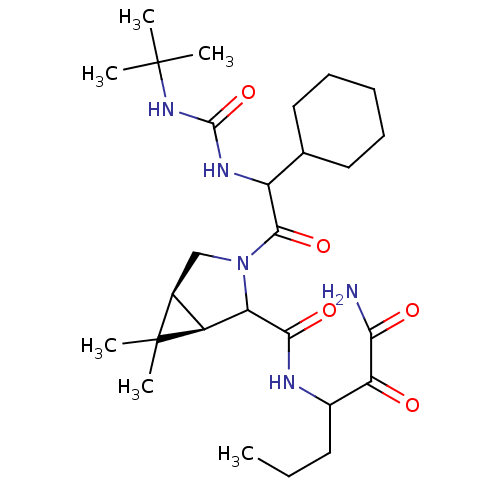 (3-{[(1R,5S)-3-{2-[(tert-butylcarbamoyl)amino]-2-cy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 54 | -42.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12297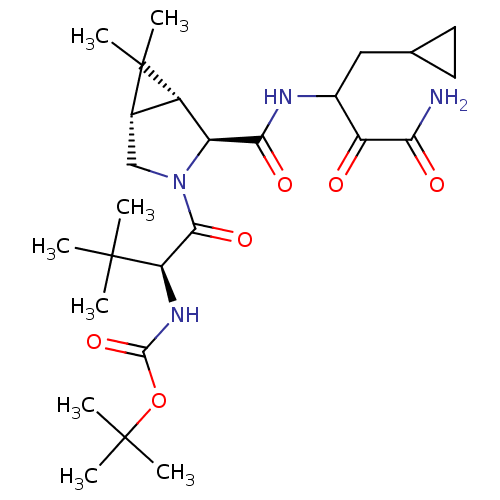 (1,1-Dimethylethyl-[1(S)-[[(1R,5S)-2(S)-[[[3-amino-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 57 | -42.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12479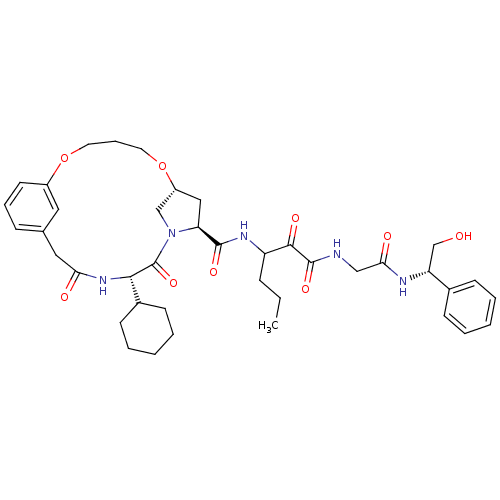 ((2S)-({N-[3-({[(7R,9S,12S)-12-cyclohexyl-11,14-dio...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 60 | -41.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17896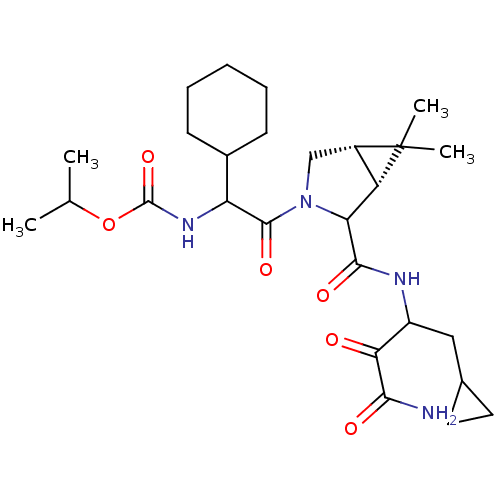 (Ketoamide inhibitor, 33 | propan-2-yl N-{2-[(1R,5S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM9727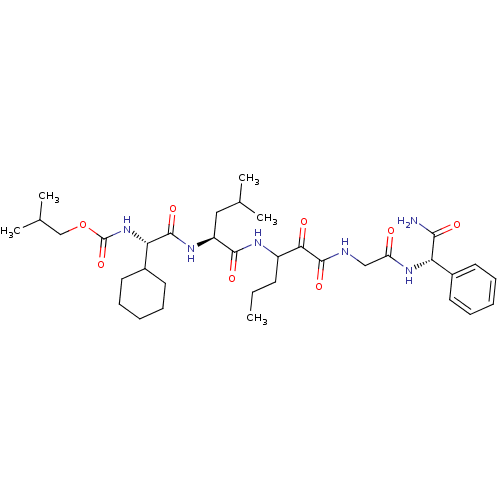 (2-methylpropyl N-[(S)-{[(1S)-1-({1-[({[(S)-carbamo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 15: 4180-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.091 BindingDB Entry DOI: 10.7270/Q2KS6PSP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17897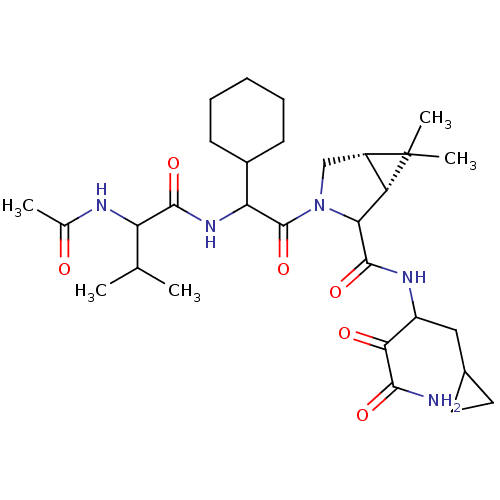 (Ketoamide inhibitor, 34 | N-{2-[(1R,5S)-2-[(1-carb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12496 ((2S)-({N-[3-({[(7R,9S,12S)-12-isobutyl-5,5-dimethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12490 (Proline-Based Macrocycle 42 | benzyl 2-(3-{[(7R,9S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM9731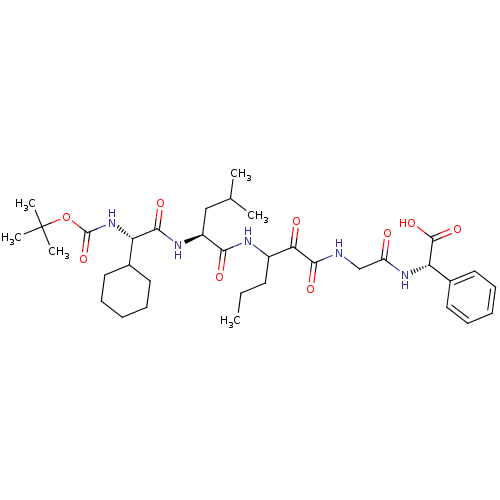 ((2S)-2-(2-{3-[(2S)-2-[(2S)-2-{[(tert-butoxy)carbon...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 15: 4180-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.091 BindingDB Entry DOI: 10.7270/Q2KS6PSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17893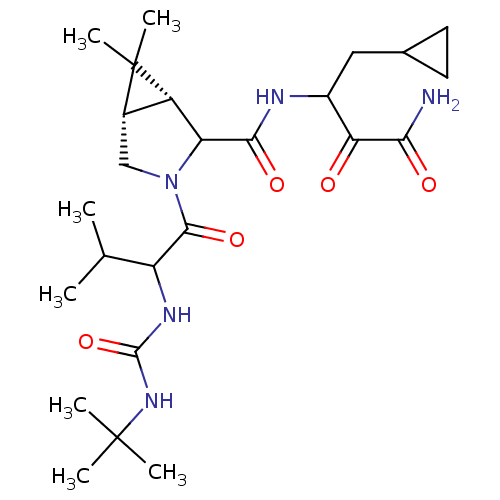 (3-{[(1R,5S)-3-{2-[(tert-butylcarbamoyl)amino]-3-me...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17882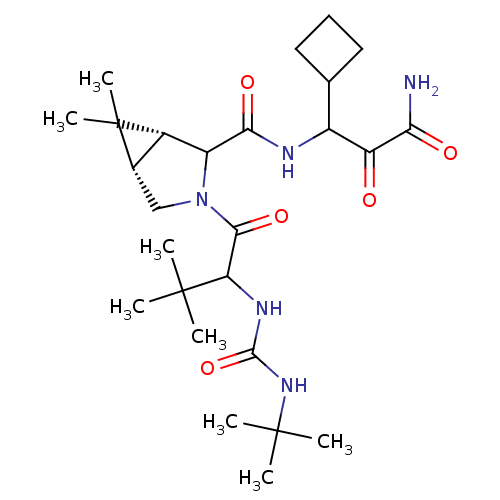 (3-{[(1R,5S)-3-{2-[(tert-butylcarbamoyl)amino]-3,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | -40.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50171301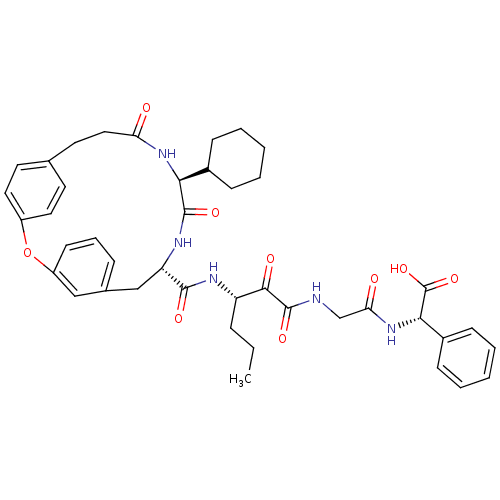 ((S)-(2-{3-[((9S,12S)-12-Cyclohexyl-11,14-dioxo-2-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit the hydrolysis of chromogenic 4-phenylazophenyl ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV protease continu... | J Med Chem 48: 5088-91 (2005) Article DOI: 10.1021/jm0489556 BindingDB Entry DOI: 10.7270/Q2DN45T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17885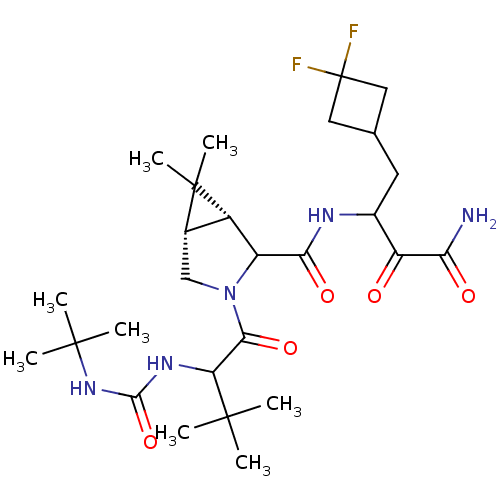 (3-{[(1R,5S)-3-{2-[(tert-butylcarbamoyl)amino]-3,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM9729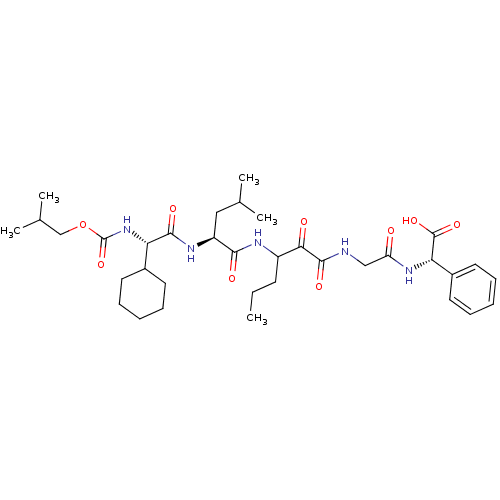 ((2S)-2-(2-{3-[(2S)-2-[(2S)-2-cyclohexyl-2-{[(2-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 15: 4180-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.091 BindingDB Entry DOI: 10.7270/Q2KS6PSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12481 ((2S)-({N-[(3S)-3-({[(7S,9S,12S)-12-tert-butyl-11,1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 130 | -40.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17887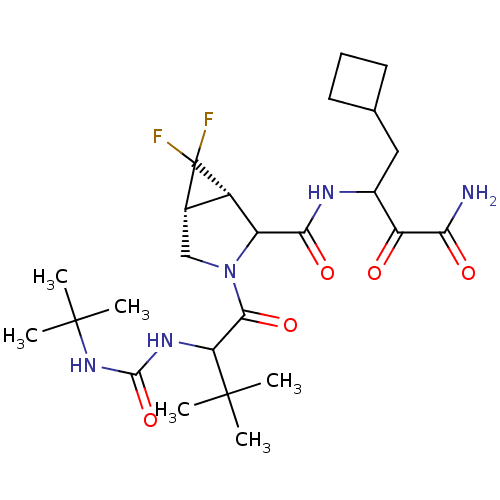 (3-{[(1S,5R)-3-{2-[(tert-butylcarbamoyl)amino]-3,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50174451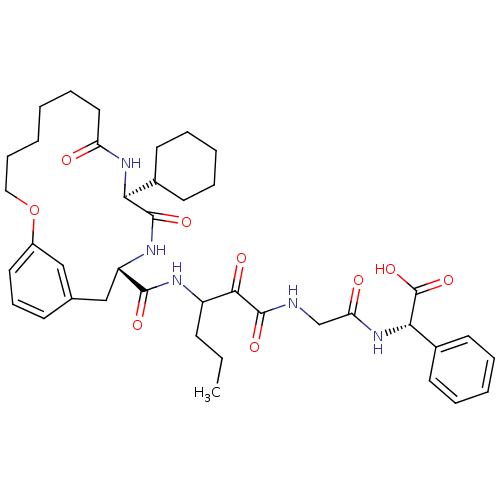 ((S)-(2-{3-[((11S,14S)-11-Cyclohexyl-9,12-dioxo-2-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity for HCV NS3 protease measured by HCV continuous assay | J Med Chem 48: 6229-35 (2005) Article DOI: 10.1021/jm050323b BindingDB Entry DOI: 10.7270/Q2XW4JC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50171302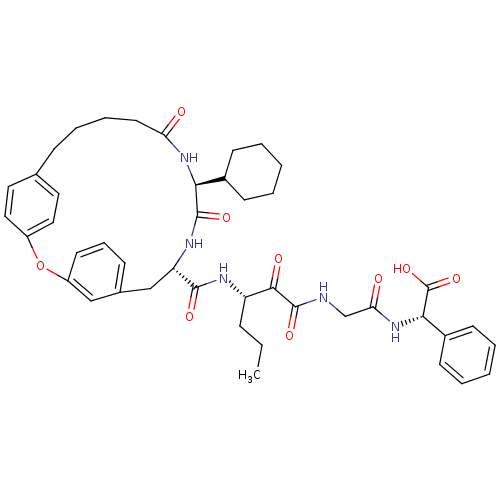 ((S)-(2-{3-[((9S,12S)-12-Cyclohexyl-11,14-dioxo-2-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit the hydrolysis of chromogenic 4-phenylazophenyl ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV protease continu... | J Med Chem 48: 5088-91 (2005) Article DOI: 10.1021/jm0489556 BindingDB Entry DOI: 10.7270/Q2DN45T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50174449 ((11S,14S)-11-Cyclohexyl-9,12-dioxo-2-oxa-10,13-dia...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity for HCV NS3 protease measured by HCV continuous assay | J Med Chem 48: 6229-35 (2005) Article DOI: 10.1021/jm050323b BindingDB Entry DOI: 10.7270/Q2XW4JC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12487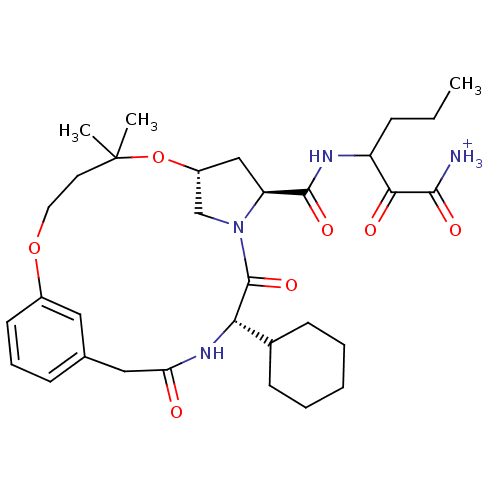 (3-({[(7R,9S,12S)-12-cyclohexyl-5,5-dimethyl-11,14-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17876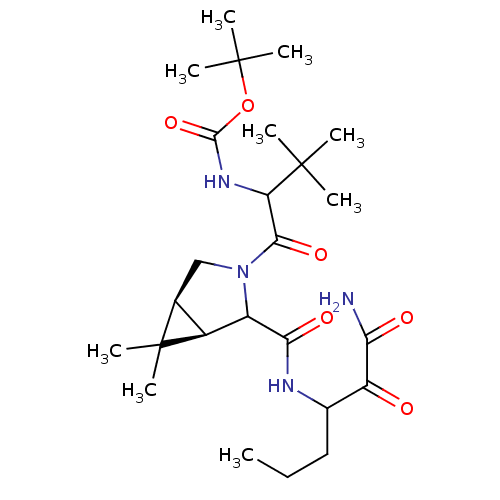 (Ketoamide inhibitor, 5 | tert-butyl N-{1-[(1R,5S)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 220 | -38.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17895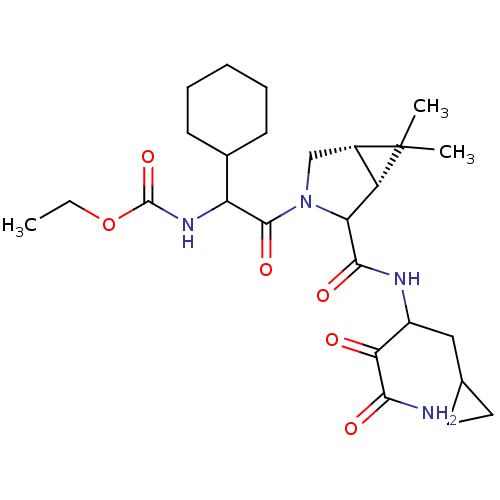 (Ketoamide inhibitor, 32 | ethyl N-{2-[(1R,5S)-2-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM9698 (tert-butyl (2S)-2-[2-(3-{[(3S,18S)-3-cyclohexyl-2,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 260 | -38.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 567-74 (2006) Article DOI: 10.1021/jm050520a BindingDB Entry DOI: 10.7270/Q2QJ7FJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12491 (N-[(benzylcarbamoyl)methyl]-3-{[(7R,9S,12S)-12-cyc...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 269 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM9725 (2-methylpropyl N-[(S)-{[(1S)-1-({1-[({[(1S)-1-carb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 15: 4180-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.091 BindingDB Entry DOI: 10.7270/Q2KS6PSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50171305 ((S)-(2-{3-[((9S,12S)-12-Cyclohexyl-11,14-dioxo-2-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit the hydrolysis of chromogenic 4-phenylazophenyl ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV protease continu... | J Med Chem 48: 5088-91 (2005) Article DOI: 10.1021/jm0489556 BindingDB Entry DOI: 10.7270/Q2DN45T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50171302 ((S)-(2-{3-[((9S,12S)-12-Cyclohexyl-11,14-dioxo-2-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit the hydrolysis of chromogenic 4-chlorophenylbutyric acid ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV proteas... | J Med Chem 48: 5088-91 (2005) Article DOI: 10.1021/jm0489556 BindingDB Entry DOI: 10.7270/Q2DN45T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50174448 ((11S,14S)-11-Cyclohexyl-9,12-dioxo-2-oxa-10,13-dia...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity for HCV NS3 protease measured by HCV continuous assay | J Med Chem 48: 6229-35 (2005) Article DOI: 10.1021/jm050323b BindingDB Entry DOI: 10.7270/Q2XW4JC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12478 ((2S)-({N-[3-({[(7R,9S,12S)-12-cyclohexyl-11,14-dio...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 310 | -37.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 995-1005 (2006) Article DOI: 10.1021/jm050820s BindingDB Entry DOI: 10.7270/Q2H70D13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1144 total ) | Next | Last >> |