

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433027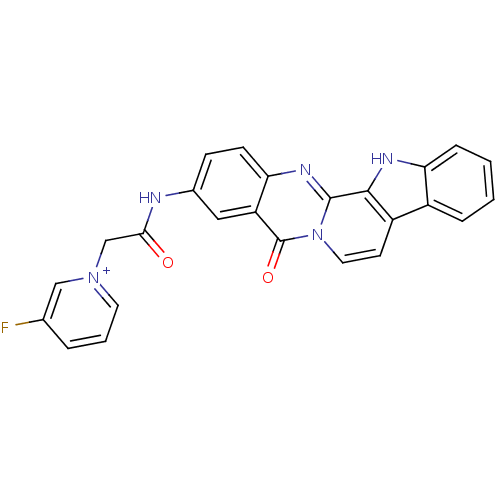 (CHEMBL2375941) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50283689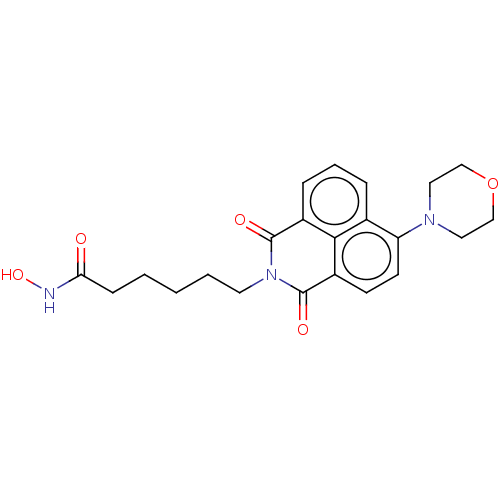 (CHEMBL4160632) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. D'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Inhibition of human recombinant full-length HDAC6 expressed in baculovirus infected Sf9 cells using Boc-Lys(acetyl)-AMC as substrate after 30 mins by... | Eur J Med Chem 143: 1543-1552 (2018) Article DOI: 10.1016/j.ejmech.2017.10.022 BindingDB Entry DOI: 10.7270/Q2028V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433028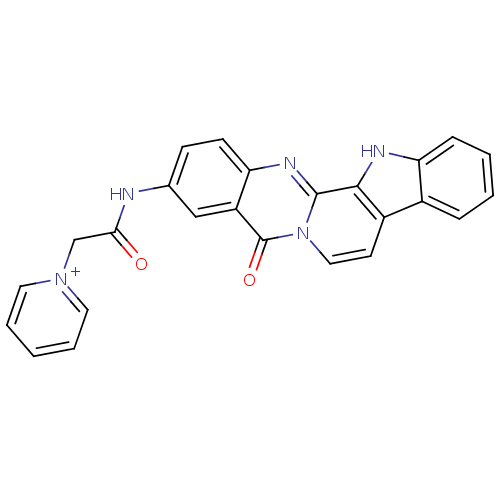 (CHEMBL2375940) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433018 (CHEMBL2375923) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433019 (CHEMBL2375922) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50283689 (CHEMBL4160632) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. D'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Inhibition of human recombinant full-length C-terminal GST-tagged HDAC3 expressed in baculovirus infected Sf9 cells using Boc-Lys(acetyl)-AMC as subs... | Eur J Med Chem 143: 1543-1552 (2018) Article DOI: 10.1016/j.ejmech.2017.10.022 BindingDB Entry DOI: 10.7270/Q2028V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50283688 (CHEMBL4176291) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. D'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Inhibition of human recombinant full-length HDAC6 expressed in baculovirus infected Sf9 cells using Boc-Lys(acetyl)-AMC as substrate after 30 mins by... | Eur J Med Chem 143: 1543-1552 (2018) Article DOI: 10.1016/j.ejmech.2017.10.022 BindingDB Entry DOI: 10.7270/Q2028V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433022 (CHEMBL2375919) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433005 (CHEMBL2375936) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50283689 (CHEMBL4160632) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. D'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Inhibition of human recombinant full-length HDAC1 (1 to 482 residues) expressed in baculovirus using Boc-Lys(acetyl)-AMC as substrate after 30 mins b... | Eur J Med Chem 143: 1543-1552 (2018) Article DOI: 10.1016/j.ejmech.2017.10.022 BindingDB Entry DOI: 10.7270/Q2028V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19443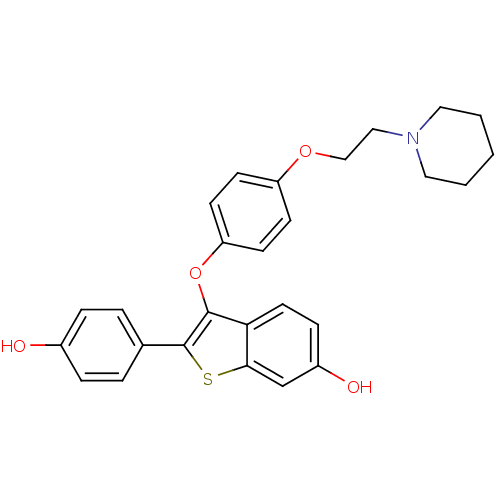 (2-(4-hydroxyphenyl)-3-{4-[2-(piperidin-1-yl)ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Illinois at Chicago | Assay Description Arzoxifene and its analogues were assayed in the standard ER competitive radioligand binding assay, using full length human recombinant ER-alpha and ... | J Med Chem 50: 2682-92 (2007) Article DOI: 10.1021/jm070079j BindingDB Entry DOI: 10.7270/Q2VQ30Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50492417 (CHEMBL2401843) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 after 30 mins by fluorescence assay | J Med Chem 56: 5782-96 (2013) Article DOI: 10.1021/jm400467w BindingDB Entry DOI: 10.7270/Q2XP77W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433026 (CHEMBL2375942) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433017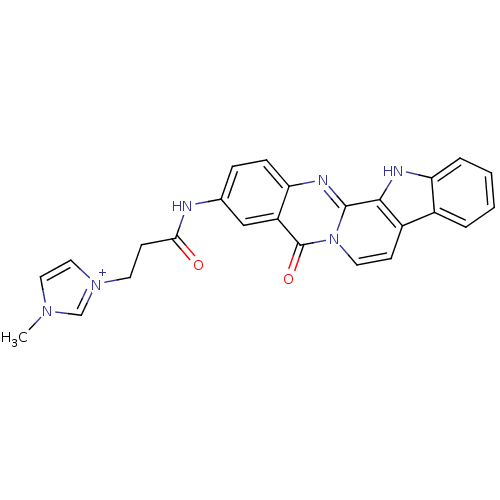 (CHEMBL2375924) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19443 (2-(4-hydroxyphenyl)-3-{4-[2-(piperidin-1-yl)ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Illinois at Chicago | Assay Description Arzoxifene and its analogues were assayed in the standard ER competitive radioligand binding assay, using full length human recombinant ER-alpha and ... | J Med Chem 50: 2682-92 (2007) Article DOI: 10.1021/jm070079j BindingDB Entry DOI: 10.7270/Q2VQ30Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19446 (2-phenyl-3-{4-[2-(piperidin-1-yl)ethoxy]phenoxy}-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10.9 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Illinois at Chicago | Assay Description Arzoxifene and its analogues were assayed in the standard ER competitive radioligand binding assay, using full length human recombinant ER-alpha and ... | J Med Chem 50: 2682-92 (2007) Article DOI: 10.1021/jm070079j BindingDB Entry DOI: 10.7270/Q2VQ30Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50283688 (CHEMBL4176291) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. D'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Inhibition of human recombinant full-length C-terminal GST-tagged HDAC3 expressed in baculovirus infected Sf9 cells using Boc-Lys(acetyl)-AMC as subs... | Eur J Med Chem 143: 1543-1552 (2018) Article DOI: 10.1016/j.ejmech.2017.10.022 BindingDB Entry DOI: 10.7270/Q2028V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433004 (CHEMBL2375937) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50492420 (CHEMBL2401846) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 after 30 mins by fluorescence assay | J Med Chem 56: 5782-96 (2013) Article DOI: 10.1021/jm400467w BindingDB Entry DOI: 10.7270/Q2XP77W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433010 (CHEMBL2375931) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433014 (CHEMBL2375927) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50492417 (CHEMBL2401843) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 after 30 mins by fluorescence assay | J Med Chem 56: 5782-96 (2013) Article DOI: 10.1021/jm400467w BindingDB Entry DOI: 10.7270/Q2XP77W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380399 (CHEMBL2018302 | Tubastatin A | US10227295, Compoun...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. D'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Inhibition of human recombinant full-length HDAC6 expressed in baculovirus infected Sf9 cells using Boc-Lys(acetyl)-AMC as substrate after 30 mins by... | Eur J Med Chem 143: 1543-1552 (2018) Article DOI: 10.1016/j.ejmech.2017.10.022 BindingDB Entry DOI: 10.7270/Q2028V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19446 (2-phenyl-3-{4-[2-(piperidin-1-yl)ethoxy]phenoxy}-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16.3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Illinois at Chicago | Assay Description Arzoxifene and its analogues were assayed in the standard ER competitive radioligand binding assay, using full length human recombinant ER-alpha and ... | J Med Chem 50: 2682-92 (2007) Article DOI: 10.1021/jm070079j BindingDB Entry DOI: 10.7270/Q2VQ30Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19444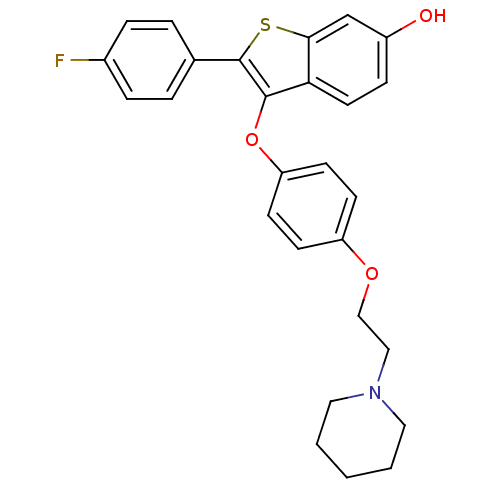 (2-(4-fluorophenyl)-3-{4-[2-(piperidin-1-yl)ethoxy]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17.2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Illinois at Chicago | Assay Description Arzoxifene and its analogues were assayed in the standard ER competitive radioligand binding assay, using full length human recombinant ER-alpha and ... | J Med Chem 50: 2682-92 (2007) Article DOI: 10.1021/jm070079j BindingDB Entry DOI: 10.7270/Q2VQ30Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19448 (2-(4-aminophenyl)-3-{4-[2-(piperidin-1-yl)ethoxy]p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Illinois at Chicago | Assay Description Arzoxifene and its analogues were assayed in the standard ER competitive radioligand binding assay, using full length human recombinant ER-alpha and ... | J Med Chem 50: 2682-92 (2007) Article DOI: 10.1021/jm070079j BindingDB Entry DOI: 10.7270/Q2VQ30Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20.6 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Illinois at Chicago | Assay Description Arzoxifene and its analogues were assayed in the standard ER competitive radioligand binding assay, using full length human recombinant ER-alpha and ... | J Med Chem 50: 2682-92 (2007) Article DOI: 10.1021/jm070079j BindingDB Entry DOI: 10.7270/Q2VQ30Z9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19442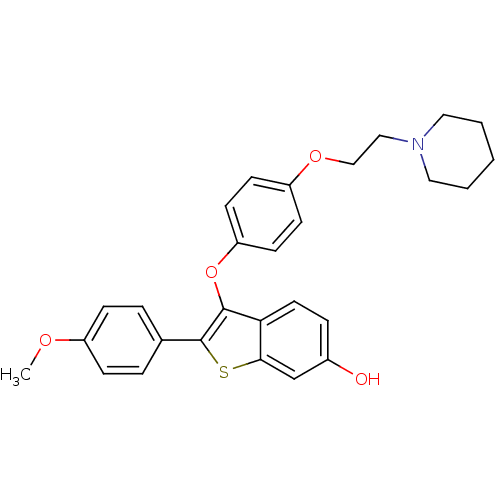 (2-(4-methoxyphenyl)-3-[4-(2-piperidin-1-ylethoxy)p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Illinois at Chicago | Assay Description Arzoxifene and its analogues were assayed in the standard ER competitive radioligand binding assay, using full length human recombinant ER-alpha and ... | J Med Chem 50: 2682-92 (2007) Article DOI: 10.1021/jm070079j BindingDB Entry DOI: 10.7270/Q2VQ30Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. D'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Inhibition of human recombinant full-length HDAC6 expressed in baculovirus infected Sf9 cells using Boc-Lys(acetyl)-AMC as substrate after 30 mins by... | Eur J Med Chem 143: 1543-1552 (2018) Article DOI: 10.1016/j.ejmech.2017.10.022 BindingDB Entry DOI: 10.7270/Q2028V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50283689 (CHEMBL4160632) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. D'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Inhibition of human recombinant full-length C-terminal GST-tagged HDAC2 expressed in baculovirus infected Sf9 cells using Boc-Lys(acetyl)-AMC as subs... | Eur J Med Chem 143: 1543-1552 (2018) Article DOI: 10.1016/j.ejmech.2017.10.022 BindingDB Entry DOI: 10.7270/Q2028V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19445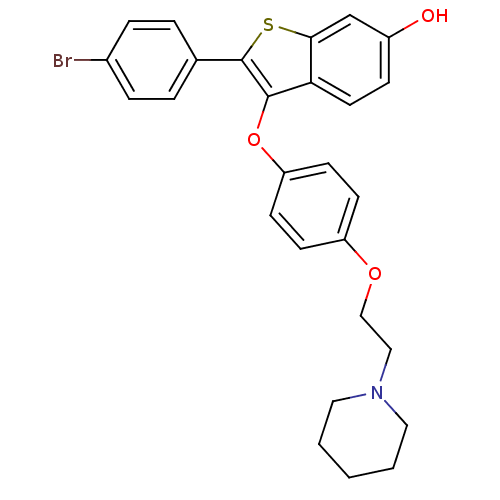 (2-(4-bromophenyl)-3-{4-[2-(piperidin-1-yl)ethoxy]p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Illinois at Chicago | Assay Description Arzoxifene and its analogues were assayed in the standard ER competitive radioligand binding assay, using full length human recombinant ER-alpha and ... | J Med Chem 50: 2682-92 (2007) Article DOI: 10.1021/jm070079j BindingDB Entry DOI: 10.7270/Q2VQ30Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19447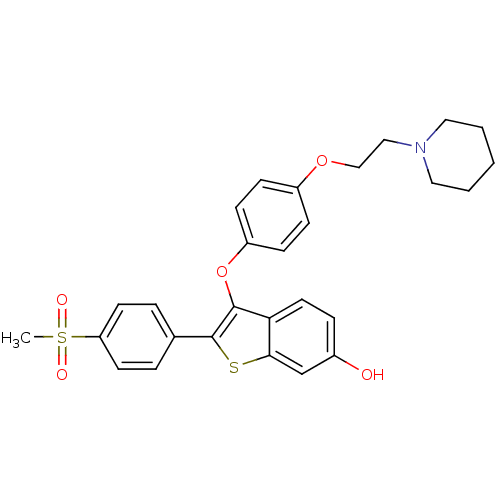 (2-(4-methanesulfonylphenyl)-3-{4-[2-(piperidin-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Illinois at Chicago | Assay Description Arzoxifene and its analogues were assayed in the standard ER competitive radioligand binding assay, using full length human recombinant ER-alpha and ... | J Med Chem 50: 2682-92 (2007) Article DOI: 10.1021/jm070079j BindingDB Entry DOI: 10.7270/Q2VQ30Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19444 (2-(4-fluorophenyl)-3-{4-[2-(piperidin-1-yl)ethoxy]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27.9 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Illinois at Chicago | Assay Description Arzoxifene and its analogues were assayed in the standard ER competitive radioligand binding assay, using full length human recombinant ER-alpha and ... | J Med Chem 50: 2682-92 (2007) Article DOI: 10.1021/jm070079j BindingDB Entry DOI: 10.7270/Q2VQ30Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50492420 (CHEMBL2401846) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 after 30 mins by fluorescence assay | J Med Chem 56: 5782-96 (2013) Article DOI: 10.1021/jm400467w BindingDB Entry DOI: 10.7270/Q2XP77W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. D'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Inhibition of human recombinant full-length C-terminal GST-tagged HDAC3 expressed in baculovirus infected Sf9 cells using Boc-Lys(acetyl)-AMC as subs... | Eur J Med Chem 143: 1543-1552 (2018) Article DOI: 10.1016/j.ejmech.2017.10.022 BindingDB Entry DOI: 10.7270/Q2028V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50492421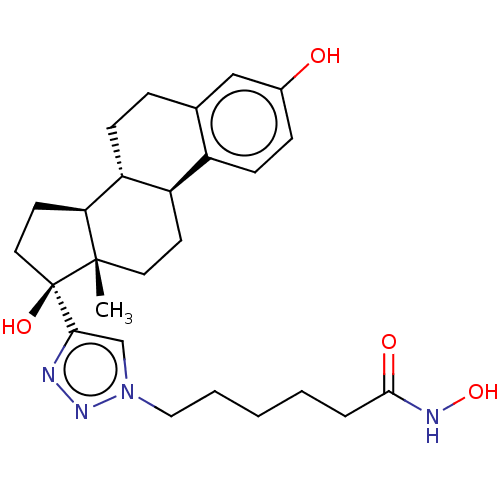 (CHEMBL2401845) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 after 30 mins by fluorescence assay | J Med Chem 56: 5782-96 (2013) Article DOI: 10.1021/jm400467w BindingDB Entry DOI: 10.7270/Q2XP77W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butylthiocholine as substrate incubated for 15 mins followed by substrate addition measured for... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 after 30 mins by fluorescence assay | J Med Chem 56: 5782-96 (2013) Article DOI: 10.1021/jm400467w BindingDB Entry DOI: 10.7270/Q2XP77W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19450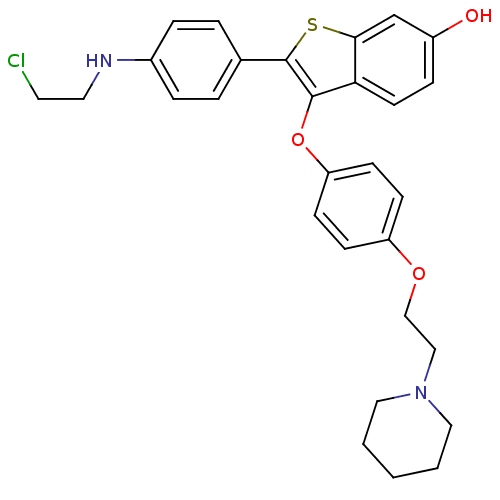 (2-{4-[(2-chloroethyl)amino]phenyl}-3-{4-[2-(piperi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35.6 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Illinois at Chicago | Assay Description Arzoxifene and its analogues were assayed in the standard ER competitive radioligand binding assay, using full length human recombinant ER-alpha and ... | J Med Chem 50: 2682-92 (2007) Article DOI: 10.1021/jm070079j BindingDB Entry DOI: 10.7270/Q2VQ30Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50283688 (CHEMBL4176291) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. D'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Inhibition of human recombinant full-length HDAC1 (1 to 482 residues) expressed in baculovirus using Boc-Lys(acetyl)-AMC as substrate after 30 mins b... | Eur J Med Chem 143: 1543-1552 (2018) Article DOI: 10.1016/j.ejmech.2017.10.022 BindingDB Entry DOI: 10.7270/Q2028V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50491819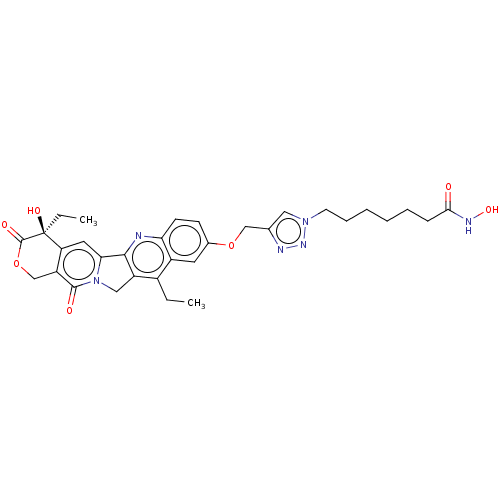 (CHEMBL2386908) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50491817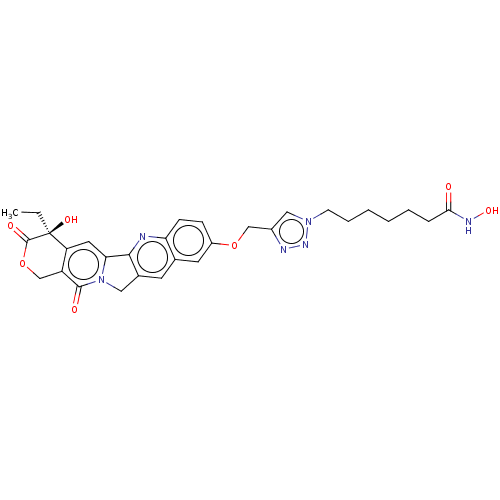 (CHEMBL2386912) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC1 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC1 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM20607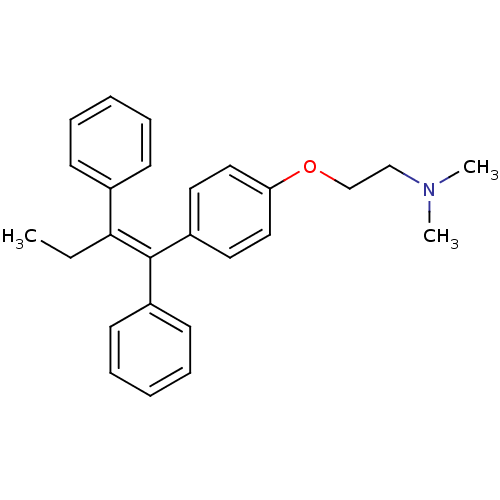 ((2-{4-[(1Z)-1,2-diphenylbut-1-en-1-yl]phenoxy}ethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Antagonist activity at Gal4 DBD-fused human ERalpha LBD expressed in HEK293T cells assessed as inhibition of estradiol-induced transcriptional activa... | J Med Chem 56: 5782-96 (2013) Article DOI: 10.1021/jm400467w BindingDB Entry DOI: 10.7270/Q2XP77W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50491820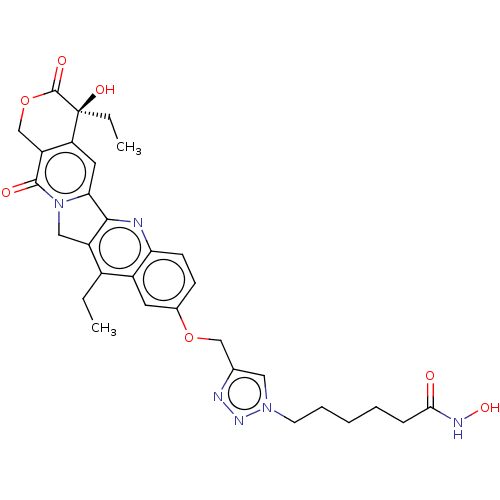 (CHEMBL2386907) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 after 30 mins by fluorescence assay | J Med Chem 56: 5782-96 (2013) Article DOI: 10.1021/jm400467w BindingDB Entry DOI: 10.7270/Q2XP77W3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19448 (2-(4-aminophenyl)-3-{4-[2-(piperidin-1-yl)ethoxy]p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48.6 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Illinois at Chicago | Assay Description Arzoxifene and its analogues were assayed in the standard ER competitive radioligand binding assay, using full length human recombinant ER-alpha and ... | J Med Chem 50: 2682-92 (2007) Article DOI: 10.1021/jm070079j BindingDB Entry DOI: 10.7270/Q2VQ30Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50491819 (CHEMBL2386908) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC1 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433013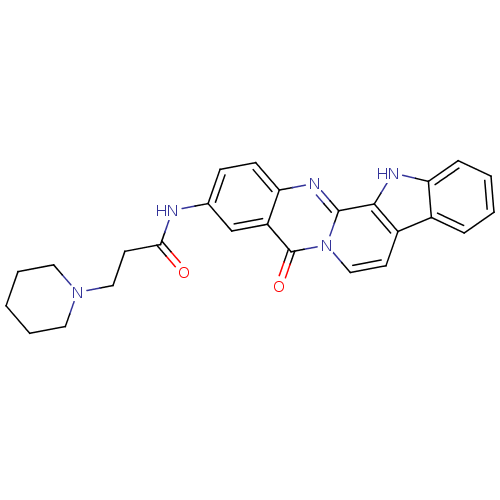 (CHEMBL2375928) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 175 total ) | Next | Last >> |