

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synaptic vesicular amine transporter (Rattus norvegicus (Rat)) | BDBM50301021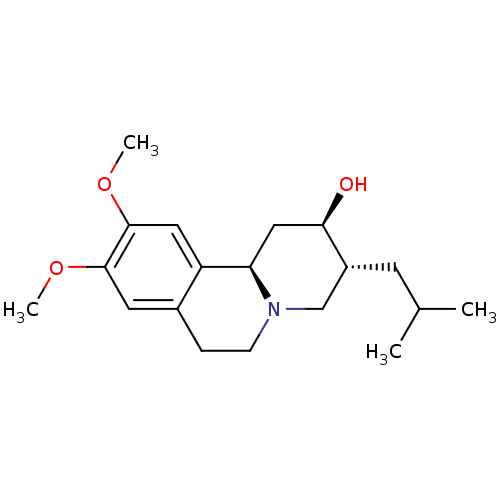 ((+)-dihydrotetrabenzaine | CHEMBL576222 | US110532...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation counting | Eur J Med Chem 46: 1841-8 (2011) Article DOI: 10.1016/j.ejmech.2011.02.046 BindingDB Entry DOI: 10.7270/Q2251JHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicular amine transporter (Rattus norvegicus (Rat)) | BDBM50048891 ((3R,11bR)-3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation counting | Eur J Med Chem 46: 1841-8 (2011) Article DOI: 10.1016/j.ejmech.2011.02.046 BindingDB Entry DOI: 10.7270/Q2251JHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Synaptic vesicular amine transporter (Rattus norvegicus (Rat)) | BDBM50017701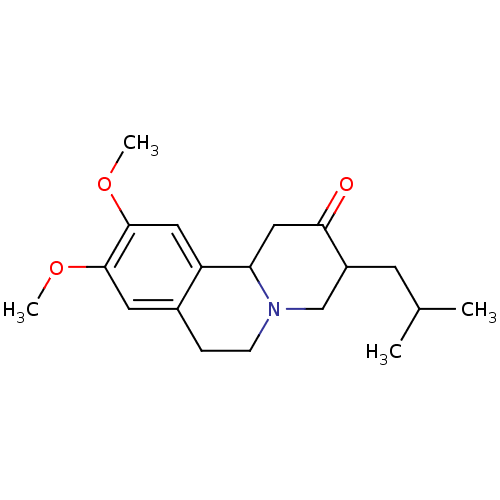 (3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 7.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation counting | Eur J Med Chem 46: 1841-8 (2011) Article DOI: 10.1016/j.ejmech.2011.02.046 BindingDB Entry DOI: 10.7270/Q2251JHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Synaptic vesicular amine transporter (Rattus norvegicus (Rat)) | BDBM50342820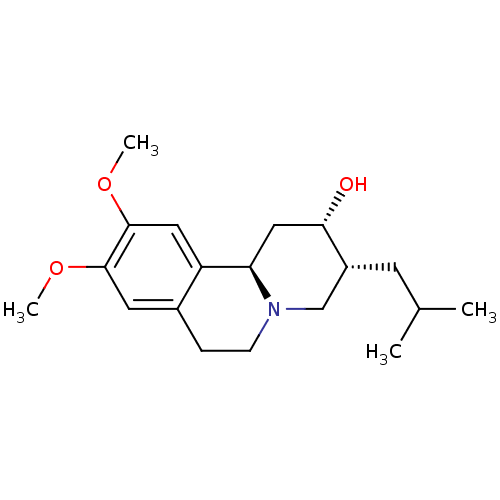 ((2S,3R,11bR)-3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 13.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation counting | Eur J Med Chem 46: 1841-8 (2011) Article DOI: 10.1016/j.ejmech.2011.02.046 BindingDB Entry DOI: 10.7270/Q2251JHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicular amine transporter (Rattus norvegicus (Rat)) | BDBM50342822 ((2R,3S,11bR)-3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 71.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation counting | Eur J Med Chem 46: 1841-8 (2011) Article DOI: 10.1016/j.ejmech.2011.02.046 BindingDB Entry DOI: 10.7270/Q2251JHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicular amine transporter (Rattus norvegicus (Rat)) | BDBM50342824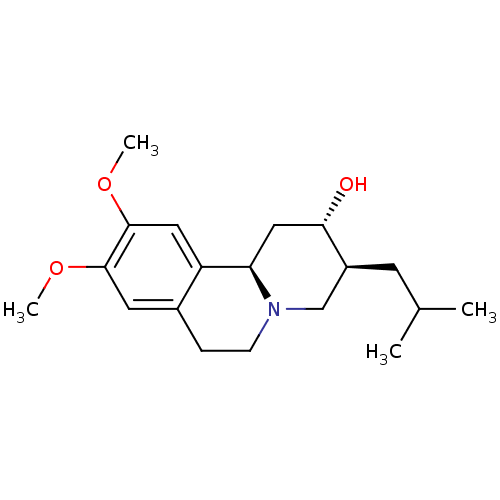 ((2S,3S,11bR)-3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 593 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation counting | Eur J Med Chem 46: 1841-8 (2011) Article DOI: 10.1016/j.ejmech.2011.02.046 BindingDB Entry DOI: 10.7270/Q2251JHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicular amine transporter (Rattus norvegicus (Rat)) | BDBM50342825 ((2R,3R,11bS)-3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation counting | Eur J Med Chem 46: 1841-8 (2011) Article DOI: 10.1016/j.ejmech.2011.02.046 BindingDB Entry DOI: 10.7270/Q2251JHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicular amine transporter (Rattus norvegicus (Rat)) | BDBM50342821 ((2R,3S,11bS)-3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation counting | Eur J Med Chem 46: 1841-8 (2011) Article DOI: 10.1016/j.ejmech.2011.02.046 BindingDB Entry DOI: 10.7270/Q2251JHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicular amine transporter (Rattus norvegicus (Rat)) | BDBM50342823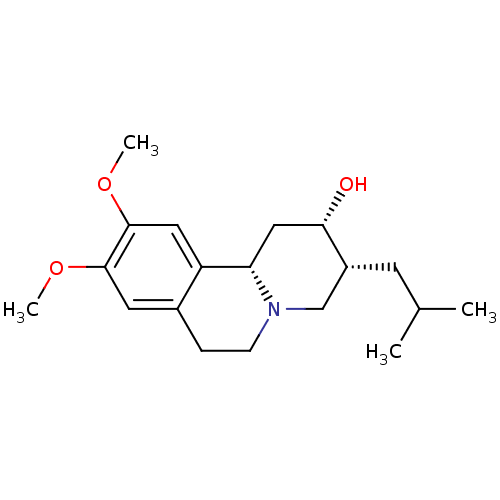 ((2S,3R,11bS)-3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation counting | Eur J Med Chem 46: 1841-8 (2011) Article DOI: 10.1016/j.ejmech.2011.02.046 BindingDB Entry DOI: 10.7270/Q2251JHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicular amine transporter (Rattus norvegicus (Rat)) | BDBM50342819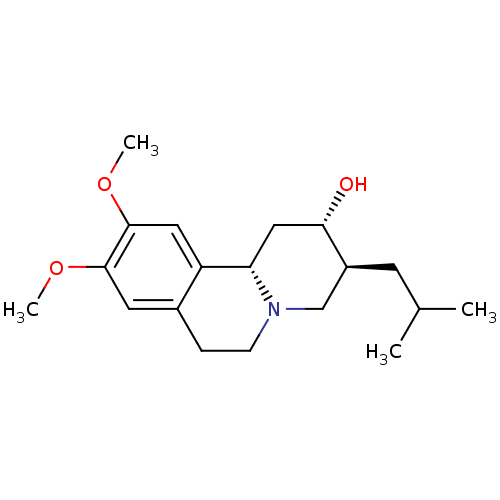 ((2S,3S,11bS)-3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation counting | Eur J Med Chem 46: 1841-8 (2011) Article DOI: 10.1016/j.ejmech.2011.02.046 BindingDB Entry DOI: 10.7270/Q2251JHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50131098 (2-(4-{2-((S)-1-(S)-Carbamoyl-3-methyl-butylcarbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory potency against human Protein-tyrosine phosphatase 1B expressed in E. coli BL21 (DE3) cells | Bioorg Med Chem Lett 13: 2577-81 (2003) BindingDB Entry DOI: 10.7270/Q2QC02VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicular amine transporter (Rattus norvegicus (Rat)) | BDBM50342818 ((3S,11BS)-TETRABENAZINE | CHEMBL519344) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation counting | Eur J Med Chem 46: 1841-8 (2011) Article DOI: 10.1016/j.ejmech.2011.02.046 BindingDB Entry DOI: 10.7270/Q2251JHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50578549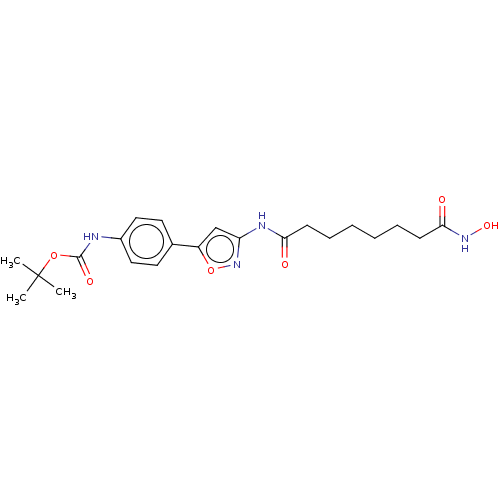 (CHEMBL4876454) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC6 (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113526 BindingDB Entry DOI: 10.7270/Q2D79G8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228403 ((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 preincubated for 30 mins followed by Gly-Pro-AMC addition measured for 50 mins by continuous fluorescence assay | ACS Med Chem Lett 7: 498-501 (2016) Article DOI: 10.1021/acsmedchemlett.6b00027 BindingDB Entry DOI: 10.7270/Q2CN75SM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046798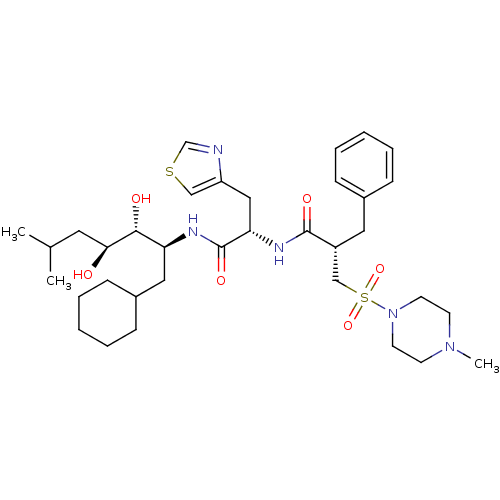 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory concentration against monkey plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM50464633 (CHEMBL4285830 | US11091476, Example 13) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University Curated by ChEMBL | Assay Description Inhibition of CDK6/Cyclin-D3 (unknown origin) using histoneH1 as substrate after 90 mins by ADP-Glo assay | Eur J Med Chem 144: 1-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.003 BindingDB Entry DOI: 10.7270/Q28C9ZX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50578549 (CHEMBL4876454) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC3 (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113526 BindingDB Entry DOI: 10.7270/Q2D79G8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119782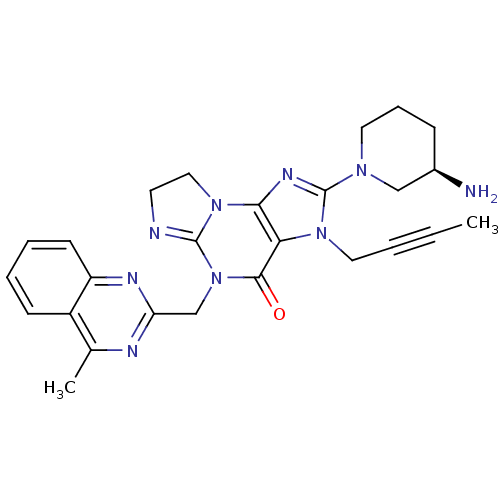 (US8691832, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM50464632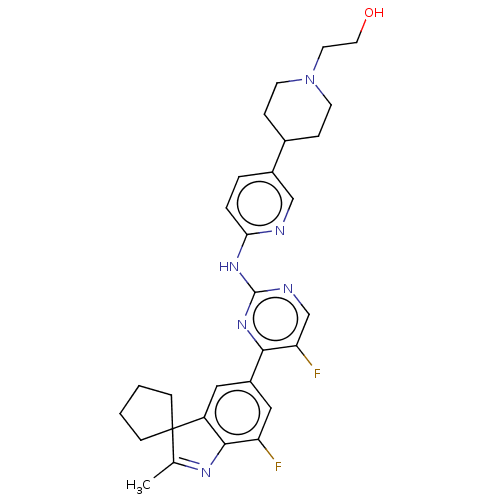 (CHEMBL4279832 | US11091476, Example 30) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University Curated by ChEMBL | Assay Description Inhibition of CDK6/Cyclin-D3 (unknown origin) using histoneH1 as substrate after 90 mins by ADP-Glo assay | Eur J Med Chem 144: 1-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.003 BindingDB Entry DOI: 10.7270/Q28C9ZX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046799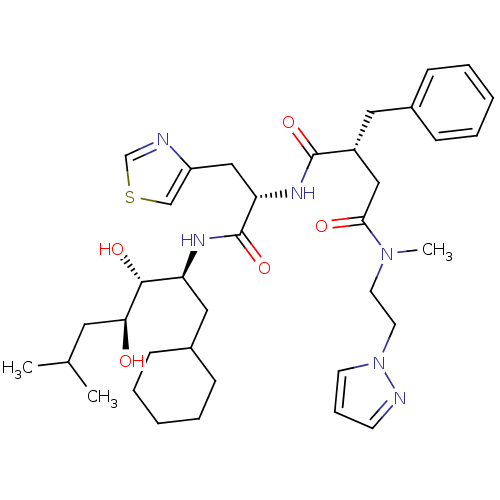 (2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046794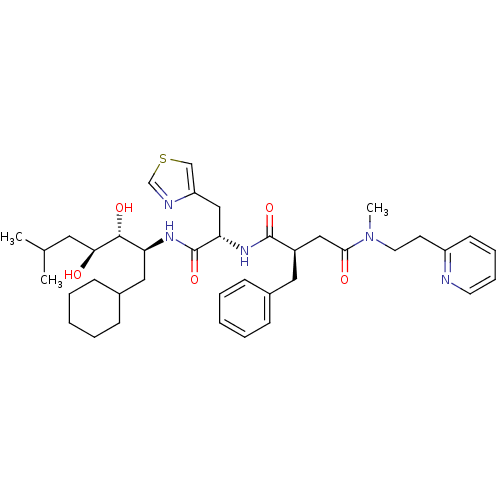 (2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory concentration against monkey plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046802 (2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046796 ((S)-N-[(S)-1-((1S,2R,3S)-1-Cyclohexylmethyl-2,3-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM50464606 (CHEMBL4277900 | US11091476, Example 17) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University Curated by ChEMBL | Assay Description Inhibition of CDK6/Cyclin-D3 (unknown origin) using histoneH1 as substrate after 90 mins by ADP-Glo assay | Eur J Med Chem 144: 1-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.003 BindingDB Entry DOI: 10.7270/Q28C9ZX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM50464630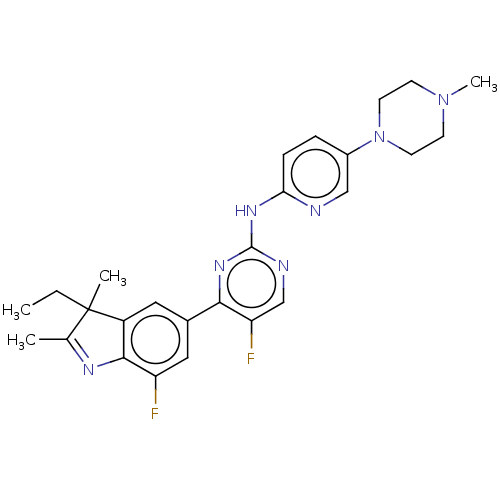 (CHEMBL4281514 | US11091476, Example 18) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University Curated by ChEMBL | Assay Description Inhibition of CDK6/Cyclin-D3 (unknown origin) using histoneH1 as substrate after 90 mins by ADP-Glo assay | Eur J Med Chem 144: 1-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.003 BindingDB Entry DOI: 10.7270/Q28C9ZX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM50243294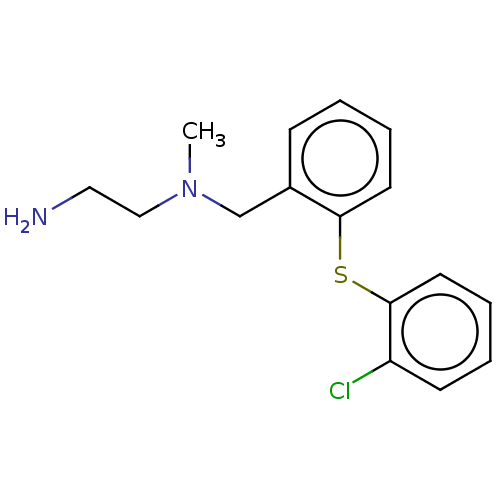 (CHEMBL4094513) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University Curated by ChEMBL | Assay Description Inhibition of PRMT8 (unknown origin) incubated for 15 mins followed by substrate addition measured after 60 mins by AlphaLisa method | J Med Chem 60: 8888-8905 (2017) Article DOI: 10.1021/acs.jmedchem.7b01134 BindingDB Entry DOI: 10.7270/Q27D2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 (Rattus norvegicus) | BDBM50243294 (CHEMBL4094513) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University Curated by ChEMBL | Assay Description Inhibition of rat His-tagged PRMT1 (11 to 353 residues) expressed in Escherichia coli BL21(DE3) incubated for 15 mins followed by substrate addition ... | J Med Chem 60: 8888-8905 (2017) Article DOI: 10.1021/acs.jmedchem.7b01134 BindingDB Entry DOI: 10.7270/Q27D2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046798 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory concentration against human plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046798 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory concentration against human plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM50243294 (CHEMBL4094513) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University Curated by ChEMBL | Assay Description Inhibition of PRMT6 (unknown origin) incubated for 15 mins followed by substrate addition measured after 60 mins by AlphaLisa method | J Med Chem 60: 8888-8905 (2017) Article DOI: 10.1021/acs.jmedchem.7b01134 BindingDB Entry DOI: 10.7270/Q27D2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119786 (US8691832, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011228 ((R)-2-Benzyl-N-[(S)-1-[(1S,2R)-1-cyclohexylmethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human plasma renin at pH 7.4 | J Med Chem 36: 449-59 (1993) BindingDB Entry DOI: 10.7270/Q20K27M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046801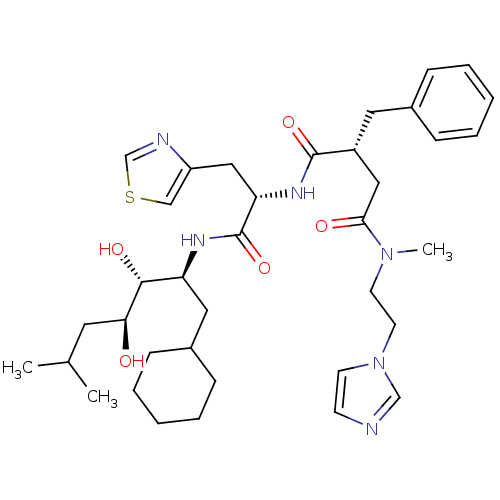 (2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046808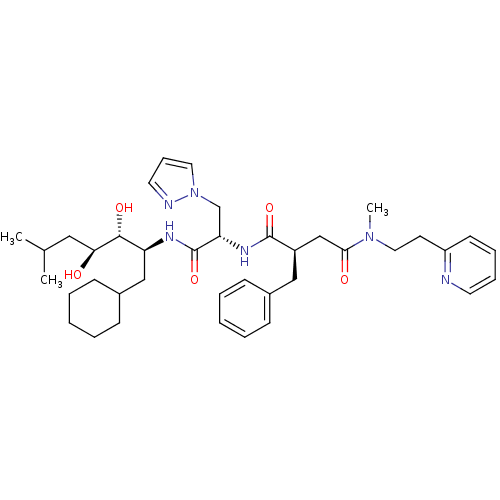 (2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046806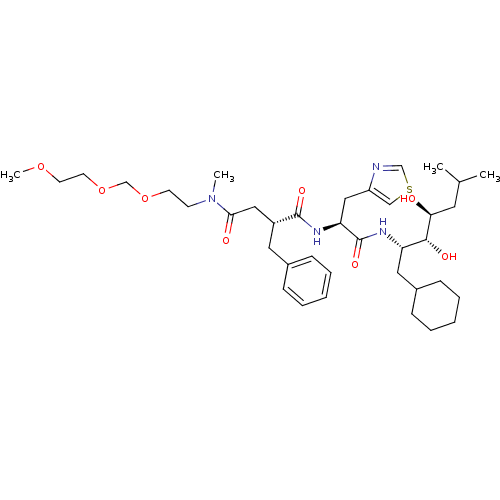 (2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022647 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human plasma renin at pH 7.4 | J Med Chem 36: 449-59 (1993) BindingDB Entry DOI: 10.7270/Q20K27M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119788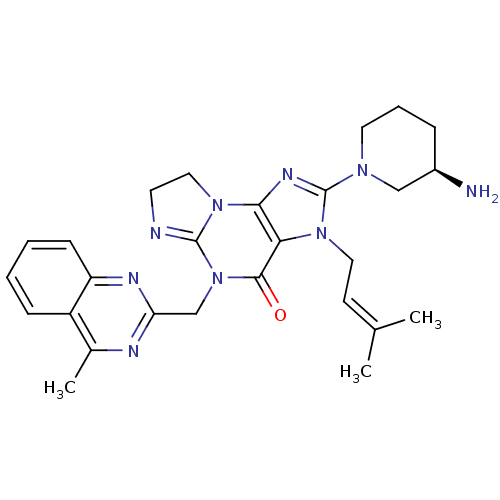 (US8691832, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM60417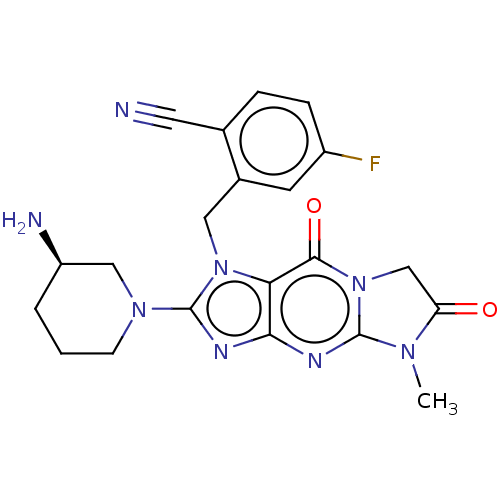 (US9051329, Example 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 preincubated for 30 mins followed by Gly-Pro-AMC addition measured for 50 mins by continuous fluorescence assay | ACS Med Chem Lett 7: 498-501 (2016) Article DOI: 10.1021/acsmedchemlett.6b00027 BindingDB Entry DOI: 10.7270/Q2CN75SM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50580530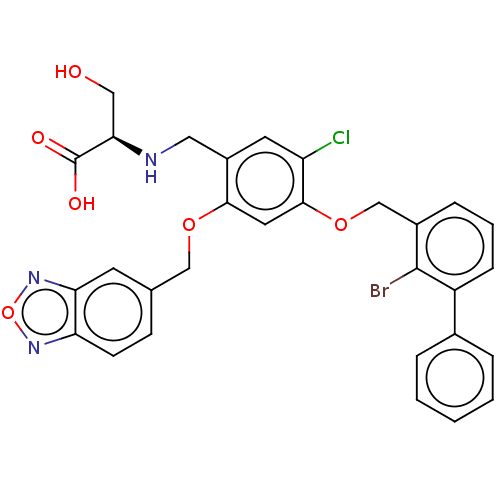 (CHEMBL5092744) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as blockade activity incubated for 15 mins by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00392 BindingDB Entry DOI: 10.7270/Q2XP78S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046805 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM50464615 (CHEMBL4289350 | US11091476, Example 3) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University Curated by ChEMBL | Assay Description Inhibition of CDK6/Cyclin-D3 (unknown origin) using histoneH1 as substrate after 90 mins by ADP-Glo assay | Eur J Med Chem 144: 1-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.003 BindingDB Entry DOI: 10.7270/Q28C9ZX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50085869 (CHEMBL367037 | N-[1-(1-{2-Carbamoyl-1-[3-(5-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of Growth factor receptor bound protein 2 binding by ELISA assay method | J Med Chem 43: 911-20 (2000) BindingDB Entry DOI: 10.7270/Q2WD3ZRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50578544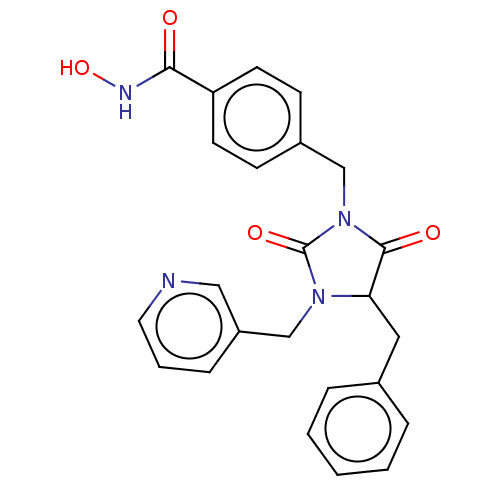 (CHEMBL4866683) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC6 (unknown origin) using Ac-LeuGlyLy-s(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition and further inc... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113526 BindingDB Entry DOI: 10.7270/Q2D79G8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50580548 (CHEMBL5081629) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as blockade activity incubated for 15 mins by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00392 BindingDB Entry DOI: 10.7270/Q2XP78S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046794 (2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory concentration against human plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046785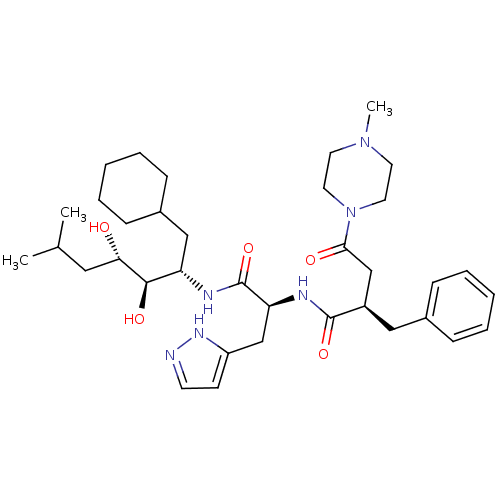 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human plasma renin at pH 7.4 | J Med Chem 36: 449-59 (1993) BindingDB Entry DOI: 10.7270/Q20K27M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM50464606 (CHEMBL4277900 | US11091476, Example 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University Curated by ChEMBL | Assay Description Inhibition of CDK4/Cyclin-D1 (unknown origin) using histone-H1 as substrate after 90 mins by ADP-Glo assay | Eur J Med Chem 144: 1-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.003 BindingDB Entry DOI: 10.7270/Q28C9ZX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated proteins 1A/1B light chain 3B (Human) | BDBM500022 (US11021457, Compound 26) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
WIGEN BIOMEDICINE TECHNOLOGY (SHANGHAI) CO., LTD. US Patent | Assay Description By constructing a prokaryotic expression system, the LC3B protein was successfully expressed and purified, and a preliminary screening and verificati... | US Patent US11021457 (2021) BindingDB Entry DOI: 10.7270/Q25H7KCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated proteins 1A/1B light chain 3B (Human) | BDBM500145 (US11021457, Compound 157) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
WIGEN BIOMEDICINE TECHNOLOGY (SHANGHAI) CO., LTD. US Patent | Assay Description By constructing a prokaryotic expression system, the LC3B protein was successfully expressed and purified, and a preliminary screening and verificati... | US Patent US11021457 (2021) BindingDB Entry DOI: 10.7270/Q25H7KCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated proteins 1A/1B light chain 3B (Human) | BDBM500144 (US11021457, Compound 156) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
WIGEN BIOMEDICINE TECHNOLOGY (SHANGHAI) CO., LTD. US Patent | Assay Description By constructing a prokaryotic expression system, the LC3B protein was successfully expressed and purified, and a preliminary screening and verificati... | US Patent US11021457 (2021) BindingDB Entry DOI: 10.7270/Q25H7KCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 810 total ) | Next | Last >> |