 Found 209 hits with Last Name = 'yates' and Initial = 'km'
Found 209 hits with Last Name = 'yates' and Initial = 'km' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190788
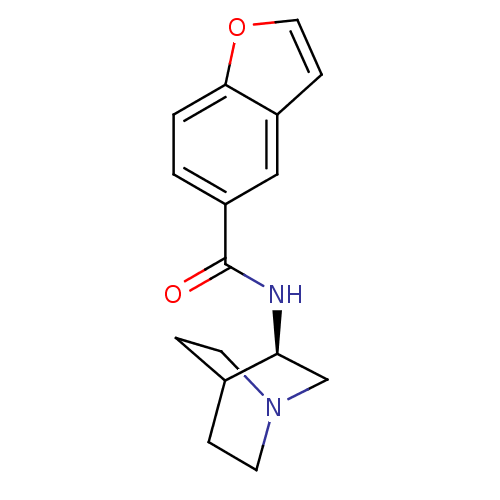
(CHEMBL378471 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2occc2c1 |wU:3.2,(-3.91,-15.15,;-3.91,-16.69,;-2.57,-17.45,;-1.24,-16.67,;-1.25,-15.14,;.09,-14.37,;1.43,-15.13,;1.43,-16.67,;.1,-17.45,;-.78,-16.3,;.04,-15.5,;-5.24,-17.46,;-5.23,-19.02,;-6.57,-19.79,;-7.91,-19.02,;-9.37,-19.49,;-10.28,-18.24,;-9.37,-17,;-7.9,-17.48,;-6.58,-16.7,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190785
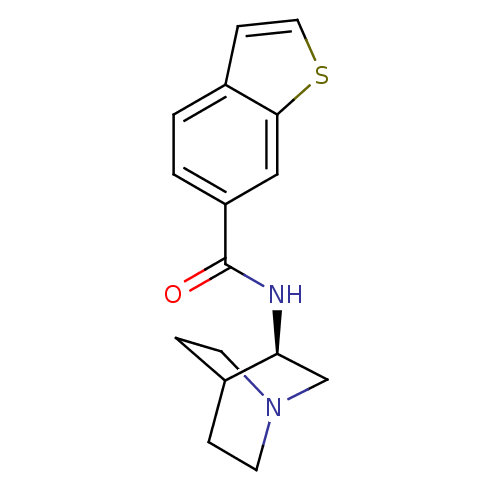
(CHEMBL378349 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccsc2c1 |wU:3.2,(28.02,-3.67,;28.02,-5.21,;29.36,-5.97,;30.69,-5.19,;30.68,-3.66,;32.02,-2.89,;33.36,-3.65,;33.36,-5.2,;32.03,-5.97,;31.15,-4.82,;31.97,-4.02,;26.69,-5.98,;26.7,-7.54,;25.35,-8.31,;24.02,-7.54,;22.56,-8.01,;21.65,-6.76,;22.56,-5.52,;24.03,-6,;25.35,-5.22,)| Show InChI InChI=1S/C16H18N2OS/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190793
(CHEMBL268939 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2sccc2cn1 |wU:3.2,(26.77,-30.5,;26.77,-32.04,;28.11,-32.81,;29.44,-32.03,;29.43,-30.5,;30.77,-29.72,;32.11,-30.49,;32.11,-32.03,;30.78,-32.8,;29.9,-31.66,;30.72,-30.86,;25.44,-32.82,;24.1,-32.06,;22.78,-32.83,;21.31,-32.36,;20.4,-33.6,;21.31,-34.85,;22.77,-34.37,;24.1,-35.14,;25.45,-34.37,)| Show InChI InChI=1S/C15H17N3OS/c19-15(12-7-14-11(8-16-12)3-6-20-14)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190791
(CHEMBL210865 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccsc2cn1 |wU:3.2,(11.37,-32.25,;11.38,-33.79,;12.72,-34.55,;14.05,-33.78,;14.04,-32.24,;15.38,-31.47,;16.72,-32.24,;16.71,-33.78,;15.39,-34.55,;14.51,-33.4,;15.33,-32.61,;10.05,-34.57,;8.71,-33.8,;7.38,-34.58,;5.92,-34.1,;5.01,-35.35,;5.92,-36.59,;7.38,-36.12,;8.71,-36.89,;10.06,-36.12,)| Show InChI InChI=1S/C15H17N3OS/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190789
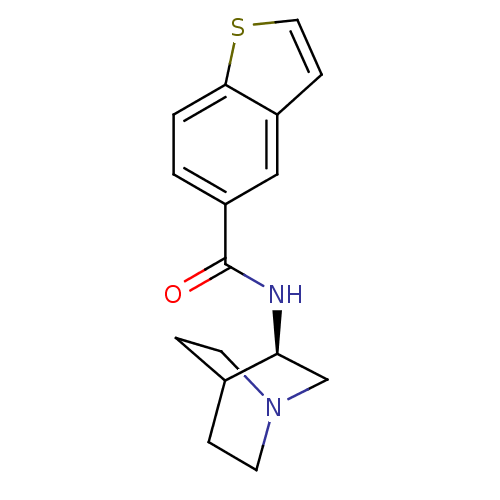
(CHEMBL208565 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2sccc2c1 |wU:3.2,(11.98,-5.28,;11.99,-6.82,;13.33,-7.58,;14.66,-6.8,;14.65,-5.27,;15.99,-4.5,;17.32,-5.26,;17.32,-6.8,;16,-7.58,;15.12,-6.43,;15.94,-5.63,;10.66,-7.59,;10.66,-9.15,;9.32,-9.92,;7.99,-9.15,;6.52,-9.62,;5.62,-8.37,;6.53,-7.13,;7.99,-7.61,;9.32,-6.83,)| Show InChI InChI=1S/C16H18N2OS/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190786
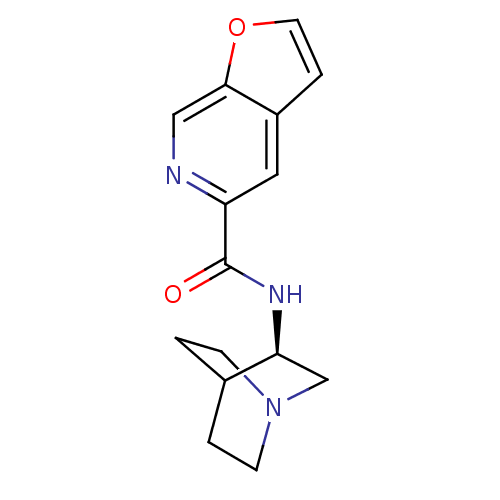
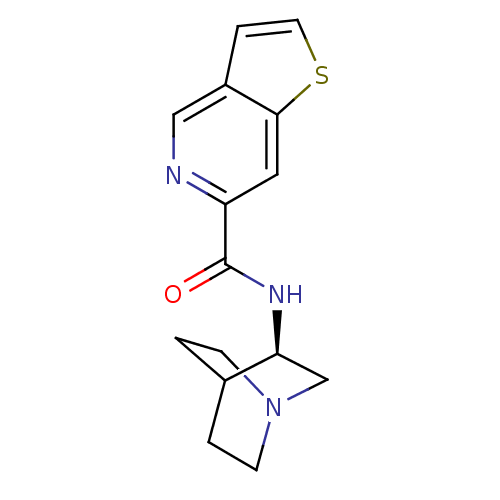
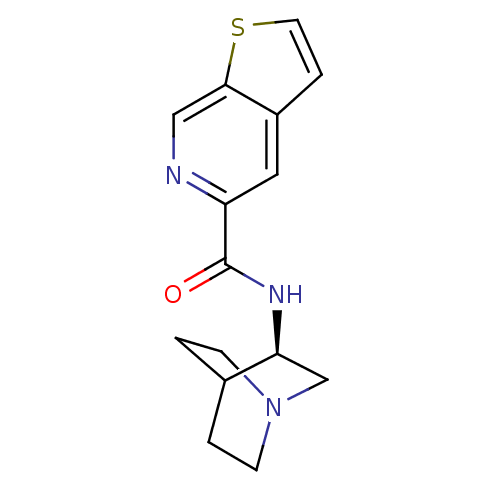
((R)-N-(quinuclidin-3-yl)furo[2,3-c]pyridine-5-carb...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccoc2cn1 |wU:3.2,(-5.16,-23.46,;-5.16,-25,;-3.82,-25.76,;-2.49,-24.99,;-2.5,-23.45,;-1.16,-22.68,;.18,-23.45,;.18,-24.99,;-1.15,-25.76,;-2.03,-24.61,;-1.21,-23.81,;-6.49,-25.78,;-7.83,-25.01,;-9.15,-25.79,;-10.62,-25.31,;-11.52,-26.55,;-10.62,-27.8,;-9.16,-27.33,;-7.82,-28.1,;-6.48,-27.33,)| Show InChI InChI=1S/C15H17N3O2/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190783
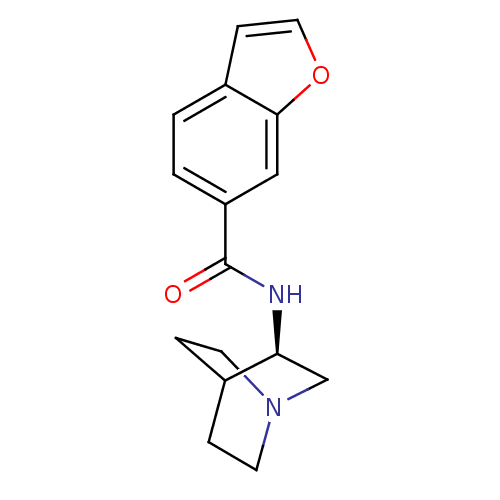
(CHEMBL379302 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccoc2c1 |wU:3.2,(27.6,-12.86,;27.61,-14.4,;28.95,-15.16,;30.28,-14.39,;30.27,-12.85,;31.61,-12.08,;32.95,-12.85,;32.94,-14.39,;31.62,-15.16,;30.74,-14.01,;31.56,-13.22,;26.28,-15.18,;26.29,-16.73,;24.94,-17.5,;23.61,-16.73,;22.15,-17.2,;21.24,-15.96,;22.15,-14.71,;23.61,-15.19,;24.94,-14.41,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190784
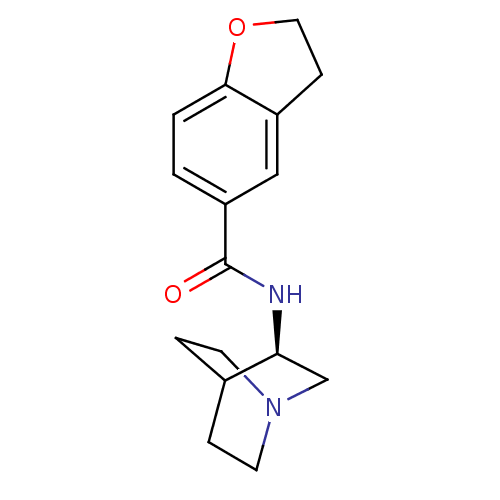
(CHEMBL378496 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2OCCc2c1 |wU:3.2,(11.67,-14.82,;11.67,-16.36,;13.01,-17.12,;14.34,-16.35,;14.33,-14.81,;15.67,-14.04,;17.01,-14.81,;17.01,-16.35,;15.68,-17.12,;14.8,-15.97,;15.62,-15.18,;10.34,-17.14,;10.35,-18.69,;9,-19.46,;7.67,-18.69,;6.21,-19.16,;5.3,-17.92,;6.21,-16.67,;7.68,-17.15,;9,-16.37,)| Show InChI InChI=1S/C16H20N2O2/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,9,11,14H,3-8,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50161764
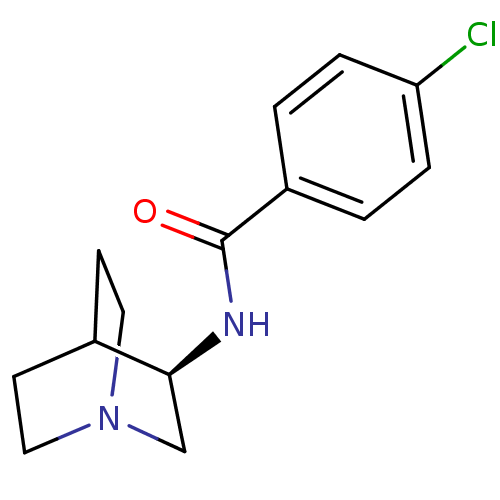
((R)-4-chloro-N-(quinuclidin-3-yl)benzamide | (R)-4...)Show SMILES Clc1ccc(cc1)C(=O)N[C@H]1CN2CCC1CC2 |r,wU:10.10,TLB:9:10:14.13:16.17,(9.34,-31.61,;10.67,-32.38,;10.67,-33.92,;12,-34.69,;13.35,-33.92,;13.34,-32.37,;12,-31.6,;14.68,-34.69,;16.01,-33.92,;14.68,-36.23,;16.02,-37,;16.46,-35.89,;16.53,-37.53,;15.18,-38.13,;14.9,-39.53,;16.27,-38.89,;17.81,-39.55,;18,-38.17,)| Show InChI InChI=1S/C14H17ClN2O/c15-12-3-1-11(2-4-12)14(18)16-13-9-17-7-5-10(13)6-8-17/h1-4,10,13H,5-9H2,(H,16,18)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190790
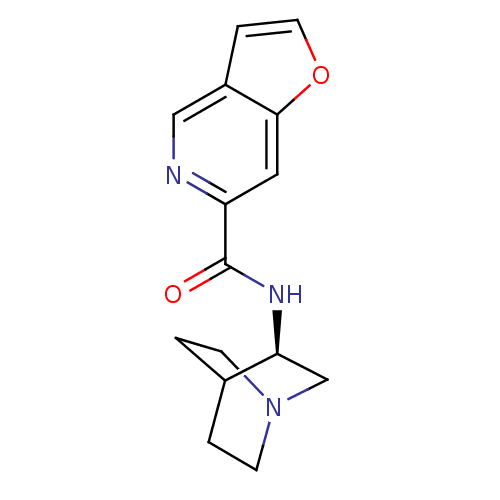
(CHEMBL214195 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2occc2cn1 |wU:3.2,(27.35,-21.97,;27.36,-23.51,;28.69,-24.27,;30.02,-23.5,;30.01,-21.96,;31.35,-21.19,;32.69,-21.96,;32.69,-23.5,;31.36,-24.27,;30.48,-23.12,;31.3,-22.32,;26.02,-24.29,;24.68,-23.52,;23.36,-24.3,;21.89,-23.82,;20.99,-25.07,;21.89,-26.31,;23.36,-25.84,;24.69,-26.61,;26.03,-25.84,)| Show InChI InChI=1S/C15H17N3O2/c19-15(12-7-14-11(8-16-12)3-6-20-14)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190794
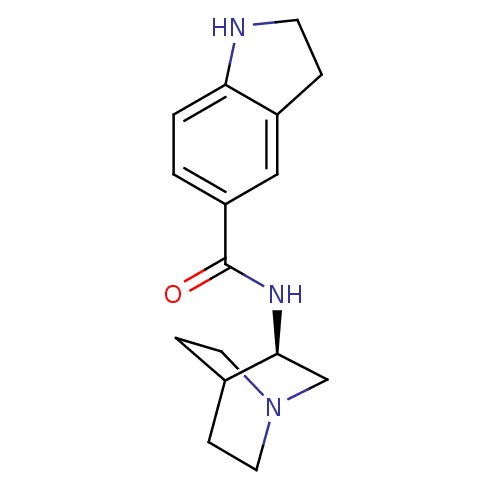
(CHEMBL211572 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2NCCc2c1 |wU:3.2,(-3.98,-5.4,;-3.98,-6.95,;-2.64,-7.71,;-1.31,-6.93,;-1.32,-5.4,;.02,-4.62,;1.36,-5.39,;1.36,-6.93,;.03,-7.7,;-.85,-6.56,;-.03,-5.76,;-5.31,-7.72,;-5.3,-9.27,;-6.64,-10.05,;-7.97,-9.28,;-9.44,-9.75,;-10.34,-8.5,;-9.44,-7.26,;-7.97,-7.74,;-6.65,-6.96,)| Show InChI InChI=1S/C16H21N3O/c20-16(13-1-2-14-12(9-13)3-6-17-14)18-15-10-19-7-4-11(15)5-8-19/h1-2,9,11,15,17H,3-8,10H2,(H,18,20)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190785

(CHEMBL378349 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccsc2c1 |wU:3.2,(28.02,-3.67,;28.02,-5.21,;29.36,-5.97,;30.69,-5.19,;30.68,-3.66,;32.02,-2.89,;33.36,-3.65,;33.36,-5.2,;32.03,-5.97,;31.15,-4.82,;31.97,-4.02,;26.69,-5.98,;26.7,-7.54,;25.35,-8.31,;24.02,-7.54,;22.56,-8.01,;21.65,-6.76,;22.56,-5.52,;24.03,-6,;25.35,-5.22,)| Show InChI InChI=1S/C16H18N2OS/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT3 receptor |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190785

(CHEMBL378349 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccsc2c1 |wU:3.2,(28.02,-3.67,;28.02,-5.21,;29.36,-5.97,;30.69,-5.19,;30.68,-3.66,;32.02,-2.89,;33.36,-3.65,;33.36,-5.2,;32.03,-5.97,;31.15,-4.82,;31.97,-4.02,;26.69,-5.98,;26.7,-7.54,;25.35,-8.31,;24.02,-7.54,;22.56,-8.01,;21.65,-6.76,;22.56,-5.52,;24.03,-6,;25.35,-5.22,)| Show InChI InChI=1S/C16H18N2OS/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Activity at 5HT3 receptor expressed in SHEP1 cells by FLIPR assay |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190783

(CHEMBL379302 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccoc2c1 |wU:3.2,(27.6,-12.86,;27.61,-14.4,;28.95,-15.16,;30.28,-14.39,;30.27,-12.85,;31.61,-12.08,;32.95,-12.85,;32.94,-14.39,;31.62,-15.16,;30.74,-14.01,;31.56,-13.22,;26.28,-15.18,;26.29,-16.73,;24.94,-17.5,;23.61,-16.73,;22.15,-17.2,;21.24,-15.96,;22.15,-14.71,;23.61,-15.19,;24.94,-14.41,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT3 receptor |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190786

((R)-N-(quinuclidin-3-yl)furo[2,3-c]pyridine-5-carb...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccoc2cn1 |wU:3.2,(-5.16,-23.46,;-5.16,-25,;-3.82,-25.76,;-2.49,-24.99,;-2.5,-23.45,;-1.16,-22.68,;.18,-23.45,;.18,-24.99,;-1.15,-25.76,;-2.03,-24.61,;-1.21,-23.81,;-6.49,-25.78,;-7.83,-25.01,;-9.15,-25.79,;-10.62,-25.31,;-11.52,-26.55,;-10.62,-27.8,;-9.16,-27.33,;-7.82,-28.1,;-6.48,-27.33,)| Show InChI InChI=1S/C15H17N3O2/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 511 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT3 receptor |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190788

(CHEMBL378471 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2occc2c1 |wU:3.2,(-3.91,-15.15,;-3.91,-16.69,;-2.57,-17.45,;-1.24,-16.67,;-1.25,-15.14,;.09,-14.37,;1.43,-15.13,;1.43,-16.67,;.1,-17.45,;-.78,-16.3,;.04,-15.5,;-5.24,-17.46,;-5.23,-19.02,;-6.57,-19.79,;-7.91,-19.02,;-9.37,-19.49,;-10.28,-18.24,;-9.37,-17,;-7.9,-17.48,;-6.58,-16.7,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Activity at 5HT3 receptor expressed in SHEP1 cells by FLIPR assay |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190786

((R)-N-(quinuclidin-3-yl)furo[2,3-c]pyridine-5-carb...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccoc2cn1 |wU:3.2,(-5.16,-23.46,;-5.16,-25,;-3.82,-25.76,;-2.49,-24.99,;-2.5,-23.45,;-1.16,-22.68,;.18,-23.45,;.18,-24.99,;-1.15,-25.76,;-2.03,-24.61,;-1.21,-23.81,;-6.49,-25.78,;-7.83,-25.01,;-9.15,-25.79,;-10.62,-25.31,;-11.52,-26.55,;-10.62,-27.8,;-9.16,-27.33,;-7.82,-28.1,;-6.48,-27.33,)| Show InChI InChI=1S/C15H17N3O2/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 628 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Activity at 5HT3 receptor expressed in SHEP1 cells by FLIPR assay |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190788

(CHEMBL378471 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2occc2c1 |wU:3.2,(-3.91,-15.15,;-3.91,-16.69,;-2.57,-17.45,;-1.24,-16.67,;-1.25,-15.14,;.09,-14.37,;1.43,-15.13,;1.43,-16.67,;.1,-17.45,;-.78,-16.3,;.04,-15.5,;-5.24,-17.46,;-5.23,-19.02,;-6.57,-19.79,;-7.91,-19.02,;-9.37,-19.49,;-10.28,-18.24,;-9.37,-17,;-7.9,-17.48,;-6.58,-16.7,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 663 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT3 receptor |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190783

(CHEMBL379302 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccoc2c1 |wU:3.2,(27.6,-12.86,;27.61,-14.4,;28.95,-15.16,;30.28,-14.39,;30.27,-12.85,;31.61,-12.08,;32.95,-12.85,;32.94,-14.39,;31.62,-15.16,;30.74,-14.01,;31.56,-13.22,;26.28,-15.18,;26.29,-16.73,;24.94,-17.5,;23.61,-16.73,;22.15,-17.2,;21.24,-15.96,;22.15,-14.71,;23.61,-15.19,;24.94,-14.41,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 962 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Activity at 5HT3 receptor expressed in SHEP1 cells by FLIPR assay |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50161764

((R)-4-chloro-N-(quinuclidin-3-yl)benzamide | (R)-4...)Show SMILES Clc1ccc(cc1)C(=O)N[C@H]1CN2CCC1CC2 |r,wU:10.10,TLB:9:10:14.13:16.17,(9.34,-31.61,;10.67,-32.38,;10.67,-33.92,;12,-34.69,;13.35,-33.92,;13.34,-32.37,;12,-31.6,;14.68,-34.69,;16.01,-33.92,;14.68,-36.23,;16.02,-37,;16.46,-35.89,;16.53,-37.53,;15.18,-38.13,;14.9,-39.53,;16.27,-38.89,;17.81,-39.55,;18,-38.17,)| Show InChI InChI=1S/C14H17ClN2O/c15-12-3-1-11(2-4-12)14(18)16-13-9-17-7-5-10(13)6-8-17/h1-4,10,13H,5-9H2,(H,16,18)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT3 receptor |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50161764

((R)-4-chloro-N-(quinuclidin-3-yl)benzamide | (R)-4...)Show SMILES Clc1ccc(cc1)C(=O)N[C@H]1CN2CCC1CC2 |r,wU:10.10,TLB:9:10:14.13:16.17,(9.34,-31.61,;10.67,-32.38,;10.67,-33.92,;12,-34.69,;13.35,-33.92,;13.34,-32.37,;12,-31.6,;14.68,-34.69,;16.01,-33.92,;14.68,-36.23,;16.02,-37,;16.46,-35.89,;16.53,-37.53,;15.18,-38.13,;14.9,-39.53,;16.27,-38.89,;17.81,-39.55,;18,-38.17,)| Show InChI InChI=1S/C14H17ClN2O/c15-12-3-1-11(2-4-12)14(18)16-13-9-17-7-5-10(13)6-8-17/h1-4,10,13H,5-9H2,(H,16,18)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Activity at 5HT3 receptor expressed in SHEP1 cells by FLIPR assay |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50177716
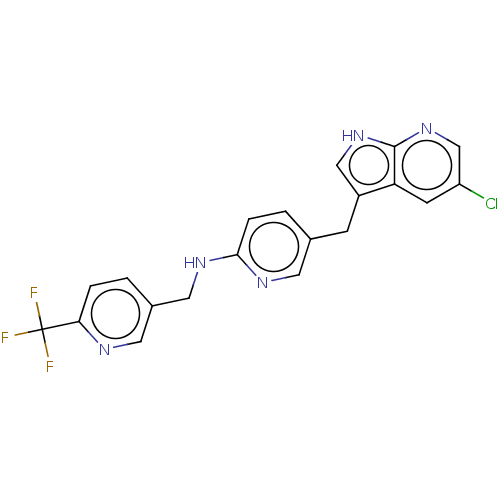
(CHEMBL3813873 | US11679110, Compound Pexidartinib ...)Show SMILES FC(F)(F)c1ccc(CNc2ccc(Cc3c[nH]c4ncc(Cl)cc34)cn2)cn1 Show InChI InChI=1S/C20H15ClF3N5/c21-15-6-16-14(10-28-19(16)29-11-15)5-12-2-4-18(26-7-12)27-9-13-1-3-17(25-8-13)20(22,23)24/h1-4,6-8,10-11H,5,9H2,(H,26,27)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Macrophage colony-stimulating factor 1 receptor [538-972]
(Homo sapiens (Human)) | BDBM181020
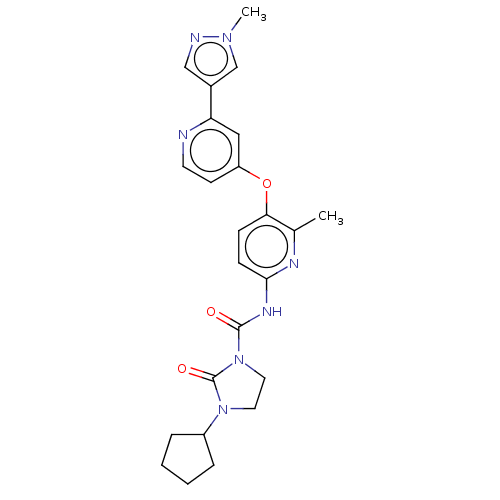
(US9133183, Example 29)Show SMILES Cc1nc(NC(=O)N2CCN(C3CCCC3)C2=O)ccc1Oc1ccnc(c1)-c1cnn(C)c1 Show InChI InChI=1S/C24H27N7O3/c1-16-21(34-19-9-10-25-20(13-19)17-14-26-29(2)15-17)7-8-22(27-16)28-23(32)31-12-11-30(24(31)33)18-5-3-4-6-18/h7-10,13-15,18H,3-6,11-12H2,1-2H3,(H,27,28,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Deciphera Pharmaceuticals, LLC
US Patent
| Assay Description
Activity of unphosphorylated c-FMS kinase (uFMS, Seq. ID no. 1) was determined by following the production of ADP from the FMS kinase reaction with A... |
US Patent US9133183 (2015)
BindingDB Entry DOI: 10.7270/Q2DN43TD |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50589694
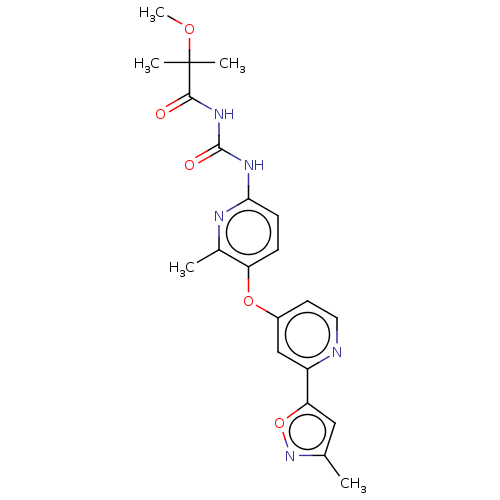
(CHEMBL5170451)Show SMILES COC(C)(C)C(=O)NC(=O)Nc1ccc(Oc2ccnc(c2)-c2cc(C)no2)c(C)n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50589686

(CHEMBL5197168)Show SMILES Cc1nc(NC(=O)NC(=O)C2(C)CCOCC2)ccc1Oc1ccnc(c1)-c1cnn(C)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50589672

(CHEMBL5176985)Show SMILES Cn1cc(cn1)-c1cc(Oc2ccc(NC(=O)NC(=O)Cc3ccc(F)cc3)nc2)ccn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor [538-972]
(Homo sapiens (Human)) | BDBM181019
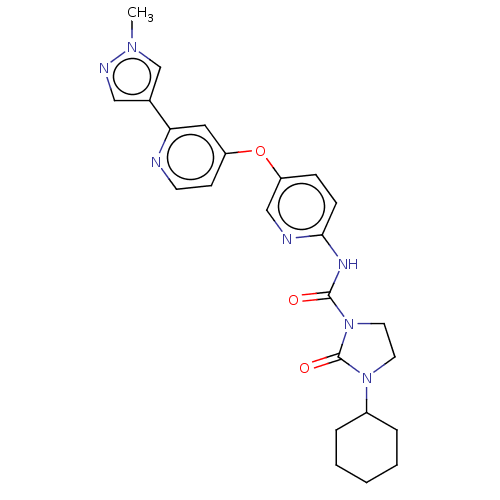
(US9133183, Example 15)Show SMILES Cn1cc(cn1)-c1cc(Oc2ccc(NC(=O)N3CCN(C4CCCCC4)C3=O)nc2)ccn1 Show InChI InChI=1S/C24H27N7O3/c1-29-16-17(14-27-29)21-13-19(9-10-25-21)34-20-7-8-22(26-15-20)28-23(32)31-12-11-30(24(31)33)18-5-3-2-4-6-18/h7-10,13-16,18H,2-6,11-12H2,1H3,(H,26,28,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Deciphera Pharmaceuticals, LLC
US Patent
| Assay Description
Activity of unphosphorylated c-FMS kinase (uFMS, Seq. ID no. 1) was determined by following the production of ADP from the FMS kinase reaction with A... |
US Patent US9133183 (2015)
BindingDB Entry DOI: 10.7270/Q2DN43TD |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50589678

(CHEMBL5181597)Show SMILES Cc1nc(NC(=O)NC(=O)C2CCC2)ccc1Oc1ccnc(c1)-c1cnn(C)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50589675
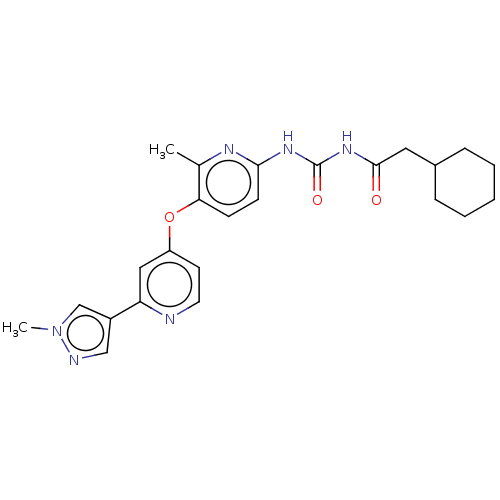
(CHEMBL5176270)Show SMILES Cc1nc(NC(=O)NC(=O)CC2CCCCC2)ccc1Oc1ccnc(c1)-c1cnn(C)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50589679
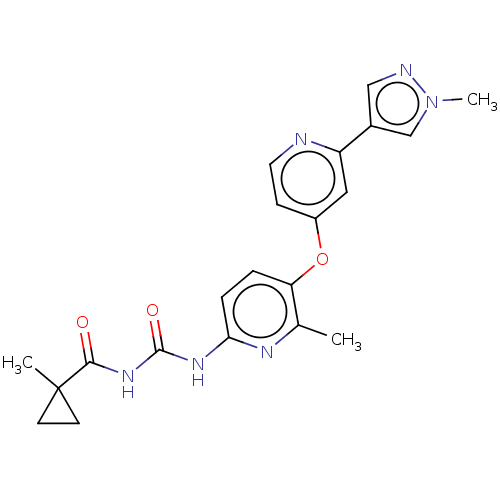
(CHEMBL5170039)Show SMILES Cc1nc(NC(=O)NC(=O)C2(C)CC2)ccc1Oc1ccnc(c1)-c1cnn(C)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50589674
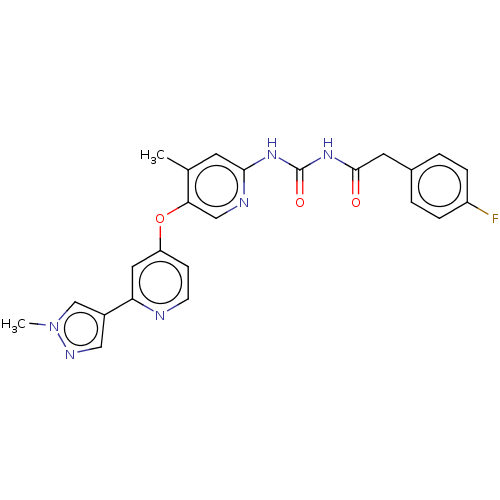
(CHEMBL5186979)Show SMILES Cc1cc(NC(=O)NC(=O)Cc2ccc(F)cc2)ncc1Oc1ccnc(c1)-c1cnn(C)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50589677
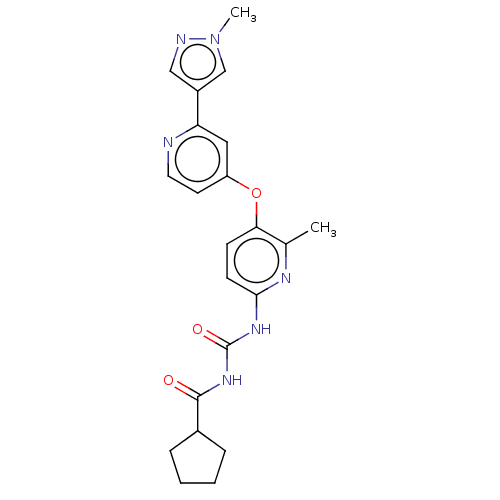
(CHEMBL5174778)Show SMILES Cc1nc(NC(=O)NC(=O)C2CCCC2)ccc1Oc1ccnc(c1)-c1cnn(C)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50589676
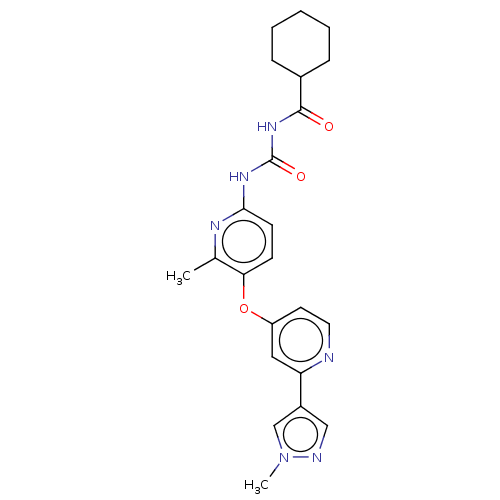
(CHEMBL5177368)Show SMILES Cc1nc(NC(=O)NC(=O)C2CCCCC2)ccc1Oc1ccnc(c1)-c1cnn(C)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50589680

(CHEMBL5195204)Show SMILES Cc1nc(NC(=O)NC(=O)C(C)(C)C)ccc1Oc1ccnc(c1)-c1cnn(C)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor [538-972]
(Homo sapiens (Human)) | BDBM181027

(US9133183, Example 90)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)N3CCN(C3=O)C(C)(C)C)nc2)ccn1 Show InChI InChI=1S/C20H24N6O4/c1-20(2,3)26-10-9-25(19(26)29)18(28)24-16-6-5-14(12-23-16)30-13-7-8-22-15(11-13)17(27)21-4/h5-8,11-12H,9-10H2,1-4H3,(H,21,27)(H,23,24,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Deciphera Pharmaceuticals, LLC
US Patent
| Assay Description
Activity of unphosphorylated c-FMS kinase (uFMS, Seq. ID no. 1) was determined by following the production of ADP from the FMS kinase reaction with A... |
US Patent US9133183 (2015)
BindingDB Entry DOI: 10.7270/Q2DN43TD |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50589676

(CHEMBL5177368)Show SMILES Cc1nc(NC(=O)NC(=O)C2CCCCC2)ccc1Oc1ccnc(c1)-c1cnn(C)c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50589675

(CHEMBL5176270)Show SMILES Cc1nc(NC(=O)NC(=O)CC2CCCCC2)ccc1Oc1ccnc(c1)-c1cnn(C)c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50589681
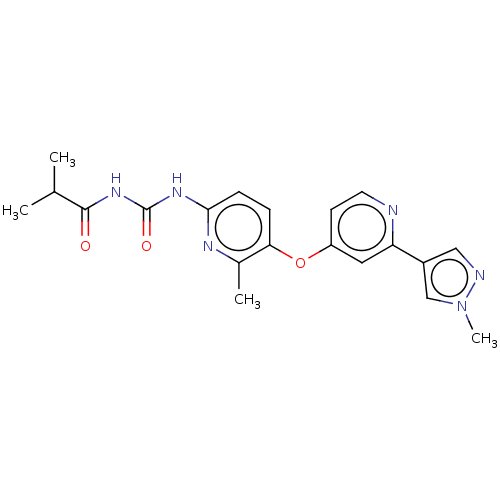
(CHEMBL5194023)Show SMILES CC(C)C(=O)NC(=O)Nc1ccc(Oc2ccnc(c2)-c2cnn(C)c2)c(C)n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor [538-972]
(Homo sapiens (Human)) | BDBM181018
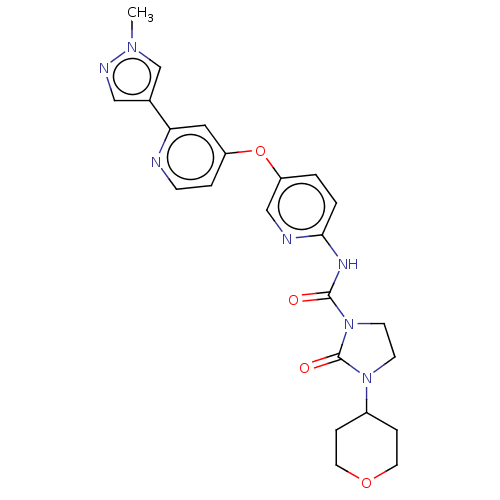
(US9133183, Example 1)Show SMILES Cn1cc(cn1)-c1cc(Oc2ccc(NC(=O)N3CCN(C4CCOCC4)C3=O)nc2)ccn1 Show InChI InChI=1S/C23H25N7O4/c1-28-15-16(13-26-28)20-12-18(4-7-24-20)34-19-2-3-21(25-14-19)27-22(31)30-9-8-29(23(30)32)17-5-10-33-11-6-17/h2-4,7,12-15,17H,5-6,8-11H2,1H3,(H,25,27,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Deciphera Pharmaceuticals, LLC
US Patent
| Assay Description
Activity of unphosphorylated c-FMS kinase (uFMS, Seq. ID no. 1) was determined by following the production of ADP from the FMS kinase reaction with A... |
US Patent US9133183 (2015)
BindingDB Entry DOI: 10.7270/Q2DN43TD |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50589689
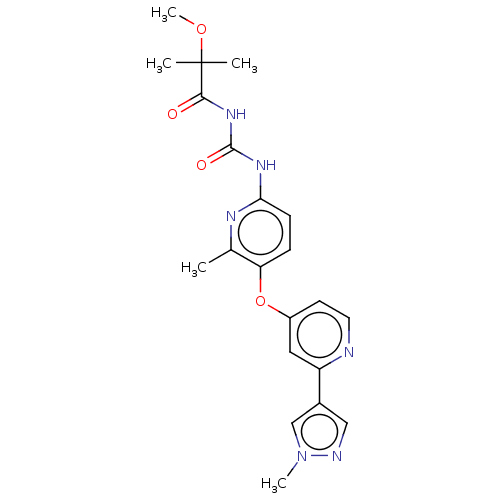
(CHEMBL5170609)Show SMILES COC(C)(C)C(=O)NC(=O)Nc1ccc(Oc2ccnc(c2)-c2cnn(C)c2)c(C)n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50589692
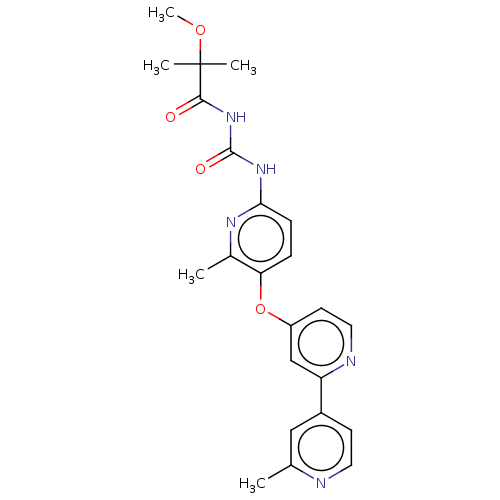
(CHEMBL5175832)Show SMILES COC(C)(C)C(=O)NC(=O)Nc1ccc(Oc2ccnc(c2)-c2ccnc(C)c2)c(C)n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor [538-972]
(Homo sapiens (Human)) | BDBM181034

(US9133183, Example 91)Show SMILES Cn1cc(cn1)-c1cc(Oc2cc(F)c(NC(=O)N3CCN(C4CCOCC4)C3=O)cc2F)ccn1 Show InChI InChI=1S/C24H24F2N6O4/c1-30-14-15(13-28-30)20-10-17(2-5-27-20)36-22-12-18(25)21(11-19(22)26)29-23(33)32-7-6-31(24(32)34)16-3-8-35-9-4-16/h2,5,10-14,16H,3-4,6-9H2,1H3,(H,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Deciphera Pharmaceuticals, LLC
US Patent
| Assay Description
Activity of unphosphorylated c-FMS kinase (uFMS, Seq. ID no. 1) was determined by following the production of ADP from the FMS kinase reaction with A... |
US Patent US9133183 (2015)
BindingDB Entry DOI: 10.7270/Q2DN43TD |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50177716

(CHEMBL3813873 | US11679110, Compound Pexidartinib ...)Show SMILES FC(F)(F)c1ccc(CNc2ccc(Cc3c[nH]c4ncc(Cl)cc34)cn2)cn1 Show InChI InChI=1S/C20H15ClF3N5/c21-15-6-16-14(10-28-19(16)29-11-15)5-12-2-4-18(26-7-12)27-9-13-1-3-17(25-8-13)20(22,23)24/h1-4,6-8,10-11H,5,9H2,(H,26,27)(H,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50589687
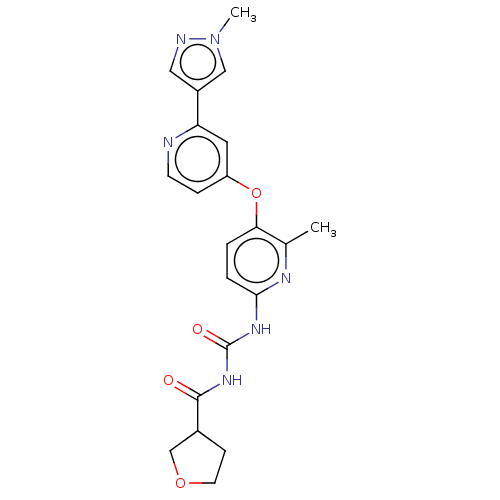
(CHEMBL5191635)Show SMILES Cc1nc(NC(=O)NC(=O)C2CCOC2)ccc1Oc1ccnc(c1)-c1cnn(C)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50589688

(CHEMBL5176187)Show SMILES COC1(CC1)C(=O)NC(=O)Nc1ccc(Oc2ccnc(c2)-c2cnn(C)c2)c(C)n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor [538-972]
(Homo sapiens (Human)) | BDBM181037
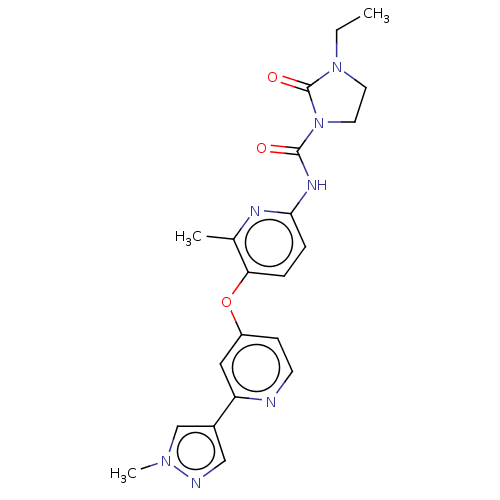
(US9133183, Example 42)Show SMILES CCN1CCN(C(=O)Nc2ccc(Oc3ccnc(c3)-c3cnn(C)c3)c(C)n2)C1=O Show InChI InChI=1S/C21H23N7O3/c1-4-27-9-10-28(21(27)30)20(29)25-19-6-5-18(14(2)24-19)31-16-7-8-22-17(11-16)15-12-23-26(3)13-15/h5-8,11-13H,4,9-10H2,1-3H3,(H,24,25,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Deciphera Pharmaceuticals, LLC
US Patent
| Assay Description
Activity of unphosphorylated c-FMS kinase (uFMS, Seq. ID no. 1) was determined by following the production of ADP from the FMS kinase reaction with A... |
US Patent US9133183 (2015)
BindingDB Entry DOI: 10.7270/Q2DN43TD |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor [538-972]
(Homo sapiens (Human)) | BDBM181035

(US9133183, Example 101)Show SMILES CN(C)C(=O)Nc1cc(Oc2cc(F)c(NC(=O)N3CCN(C4CCOCC4)C3=O)cc2Cl)ccn1 Show InChI InChI=1S/C23H26ClFN6O5/c1-29(2)21(32)28-20-11-15(3-6-26-20)36-19-13-17(25)18(12-16(19)24)27-22(33)31-8-7-30(23(31)34)14-4-9-35-10-5-14/h3,6,11-14H,4-5,7-10H2,1-2H3,(H,27,33)(H,26,28,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Deciphera Pharmaceuticals, LLC
US Patent
| Assay Description
Activity of unphosphorylated c-FMS kinase (uFMS, Seq. ID no. 1) was determined by following the production of ADP from the FMS kinase reaction with A... |
US Patent US9133183 (2015)
BindingDB Entry DOI: 10.7270/Q2DN43TD |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50589693

(CHEMBL5177523)Show SMILES COC(C)(C)C(=O)NC(=O)Nc1ccc(Oc2ccnc(c2)-c2cnc(C)s2)c(C)n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50589673
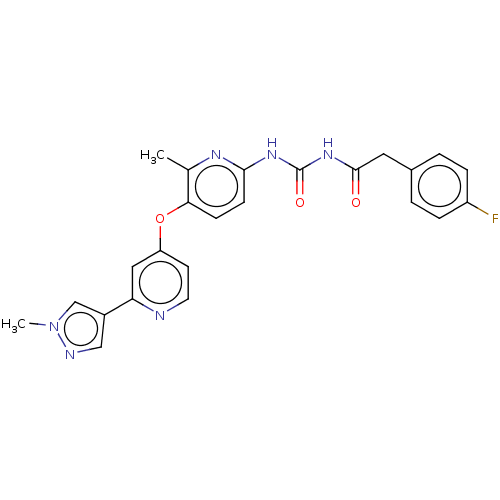
(CHEMBL5197530)Show SMILES Cc1nc(NC(=O)NC(=O)Cc2ccc(F)cc2)ccc1Oc1ccnc(c1)-c1cnn(C)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50177716

(CHEMBL3813873 | US11679110, Compound Pexidartinib ...)Show SMILES FC(F)(F)c1ccc(CNc2ccc(Cc3c[nH]c4ncc(Cl)cc34)cn2)cn1 Show InChI InChI=1S/C20H15ClF3N5/c21-15-6-16-14(10-28-19(16)29-11-15)5-12-2-4-18(26-7-12)27-9-13-1-3-17(25-8-13)20(22,23)24/h1-4,6-8,10-11H,5,9H2,(H,26,27)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data