

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM29525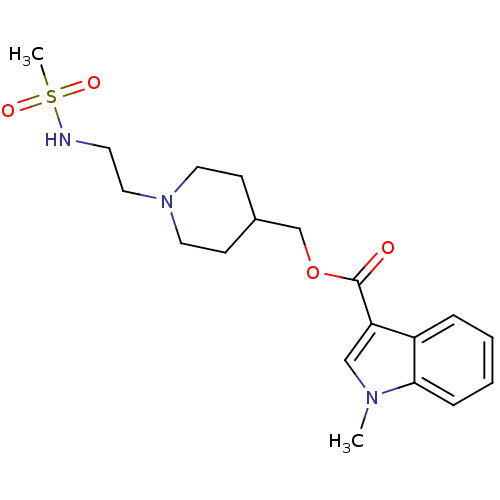 (3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5-HT4B receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 64: 629-37 (2013) Article DOI: 10.1016/j.ejmech.2013.03.060 BindingDB Entry DOI: 10.7270/Q27947NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM31883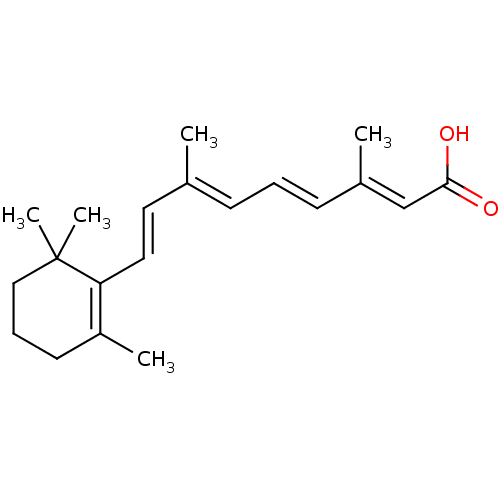 (9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description Agonistic activity of the compound towards retinoic acid receptor-gamma | J Med Chem 40: 4222-34 (1998) Article DOI: 10.1021/jm9704309 BindingDB Entry DOI: 10.7270/Q21J98VC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075807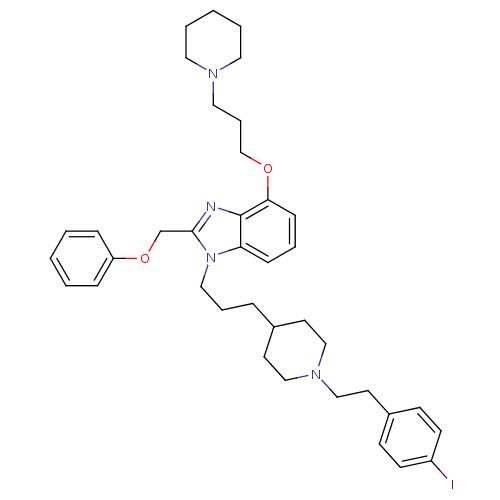 (1-(3-{1-[2-(4-Iodo-phenyl)-ethyl]-piperidin-4-yl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256265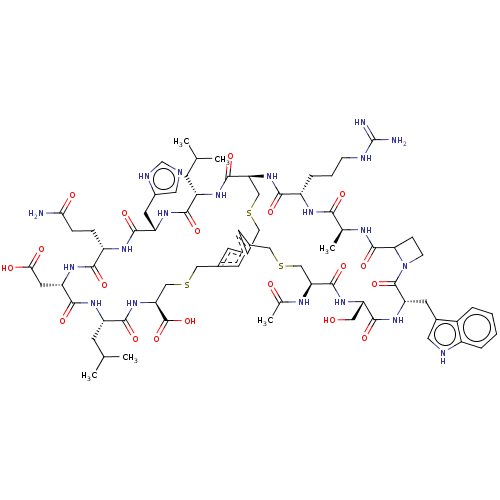 (CHEMBL4089486) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230846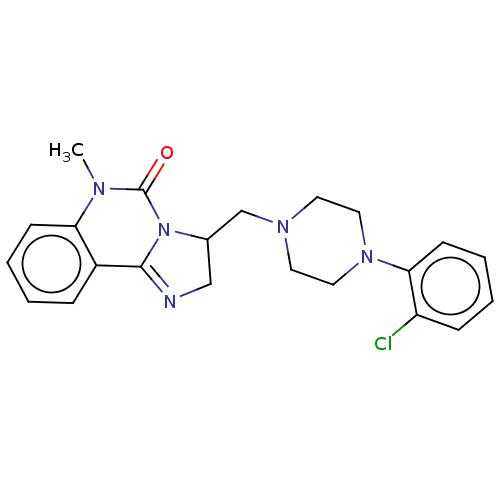 (CHEMBL2114071) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230859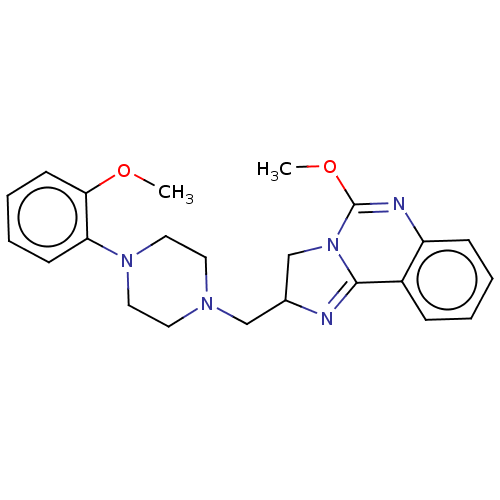 (CHEMBL309150) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256283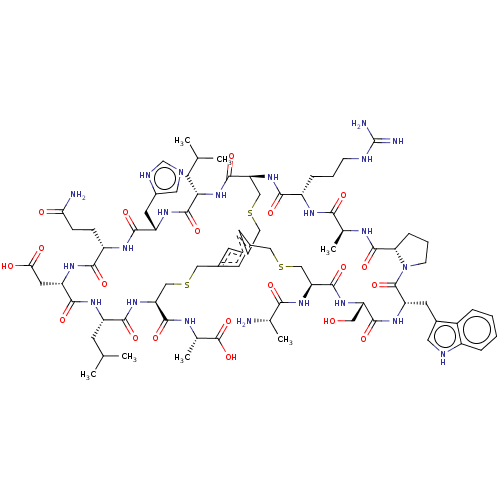 (CHEMBL4079711) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM466723 (US10800761, Example 42 | US10800761, Example 55 | ...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro inhibition of human carbonic anhydrase II (0.1 nM). | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM466723 (US10800761, Example 42 | US10800761, Example 55 | ...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro inhibition of human carbonic anhydrase II (0.1 nM). | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM85026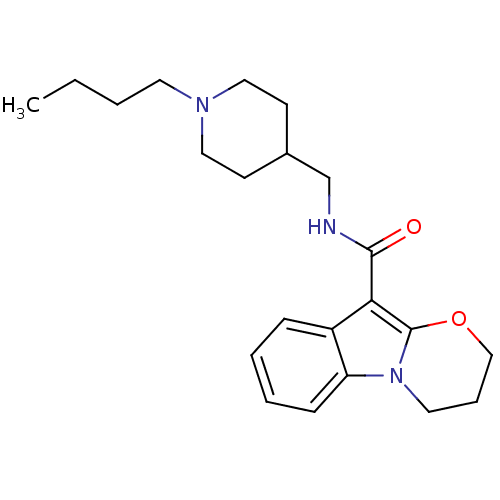 (N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5-HT4B receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 64: 629-37 (2013) Article DOI: 10.1016/j.ejmech.2013.03.060 BindingDB Entry DOI: 10.7270/Q27947NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075796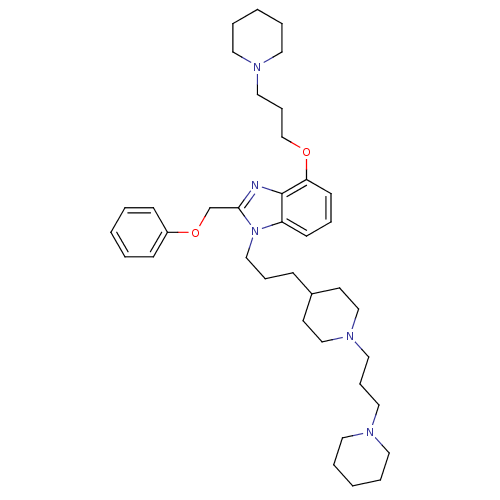 (2-Phenoxymethyl-4-(3-piperidin-1-yl-propoxy)-1-{3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230848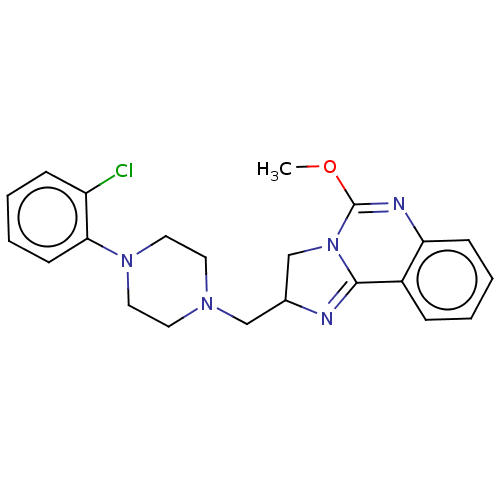 (CHEMBL70872) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075811 (3-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-propo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50492064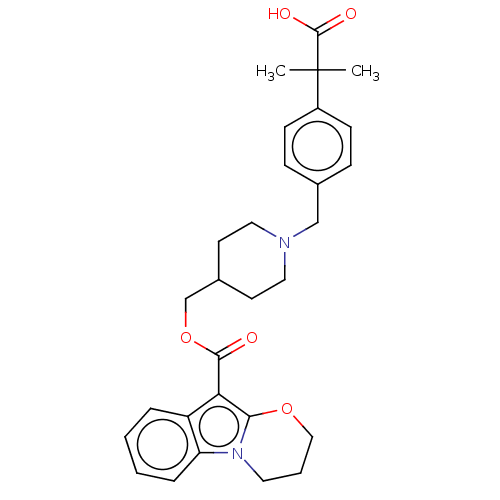 (CHEMBL2391994) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5-HT4B receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 64: 629-37 (2013) Article DOI: 10.1016/j.ejmech.2013.03.060 BindingDB Entry DOI: 10.7270/Q27947NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075803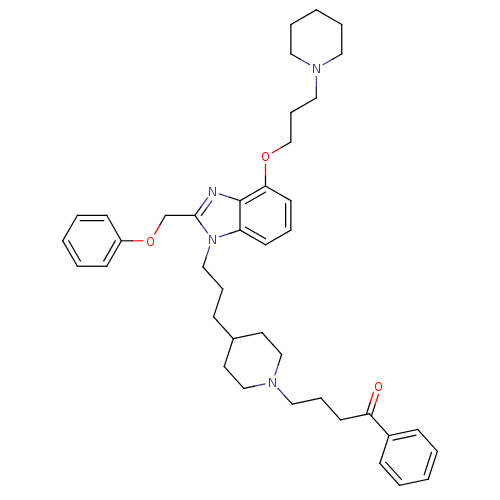 (4-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-propo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256264 (CHEMBL4099333) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256277 (CHEMBL4093698) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075812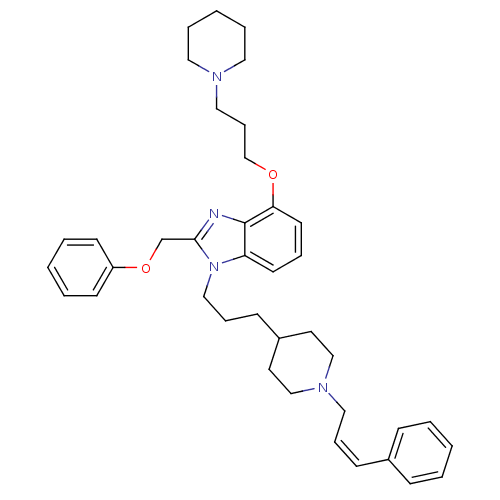 (2-Phenoxymethyl-1-{3-[1-((Z)-3-phenyl-allyl)-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075809 (1-[3-(1-Phenethyl-piperidin-4-yl)-propyl]-2-phenox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50066109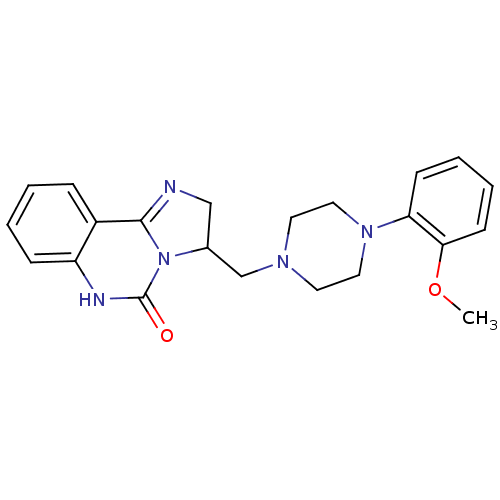 (3-[4-(2-Methoxy-phenyl)-piperazin-1-ylmethyl]-2,6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256286 (CHEMBL4076199) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University College of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma1 in human MDA-MB-468 cell membranes | Bioorg Med Chem Lett 27: 2216-2220 (2017) Article DOI: 10.1016/j.bmcl.2017.03.030 BindingDB Entry DOI: 10.7270/Q2P84F1X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18860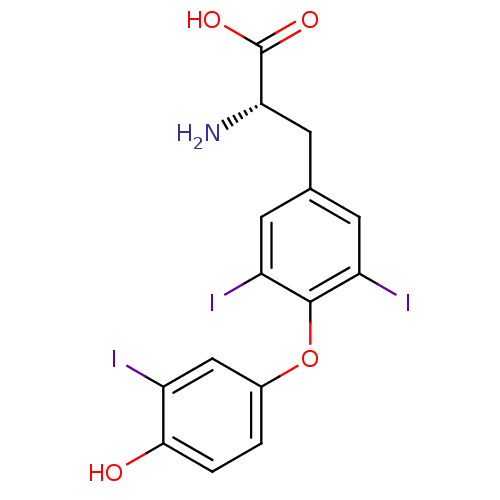 ((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]T3 from recombinant thyroid hormone receptor alpha (unknown origin) expressed in sf9 cells by scintillation proximity assay | Proc Natl Acad Sci U S A 104: 15490-5 (2007) Article DOI: 10.1073/pnas.0702759104 BindingDB Entry DOI: 10.7270/Q2JW8DPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50369383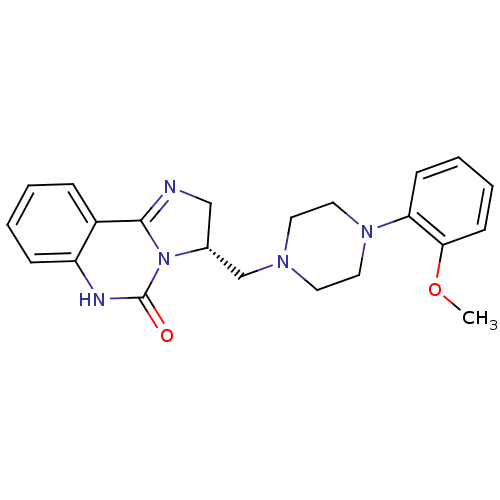 (CHEMBL1788222) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from Alpha-1A adrenergic receptorof rat submaxillary gland membranes | J Med Chem 41: 3128-41 (1998) Article DOI: 10.1021/jm970159v BindingDB Entry DOI: 10.7270/Q2GM880H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18869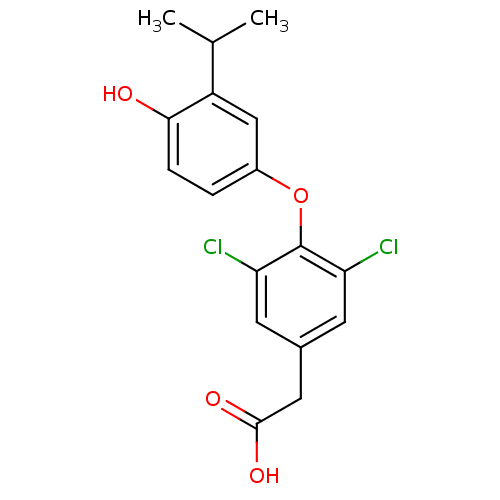 (2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]3,5,3'-triiodo-L-thyronine His-tagged human recombinant TRbeta1 by scintillation proximity assay | J Med Chem 51: 7075-93 (2009) Article DOI: 10.1021/jm800824d BindingDB Entry DOI: 10.7270/Q21V5DT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50040253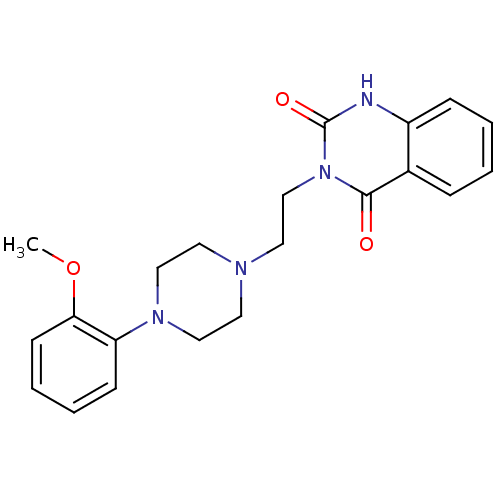 (3-{2-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-ethyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256262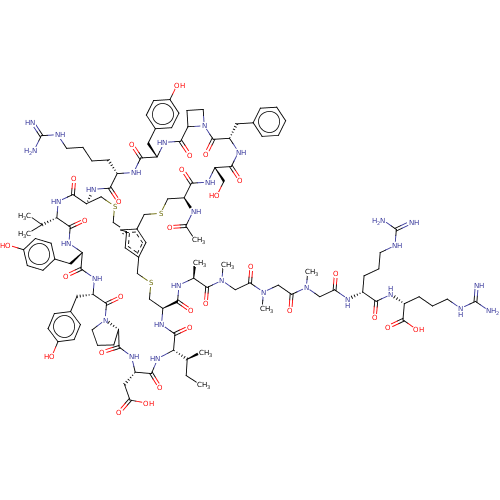 (CHEMBL4070056) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50066109 (3-[4-(2-Methoxy-phenyl)-piperazin-1-ylmethyl]-2,6-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from Alpha-1A adrenergic receptorof rat submaxillary gland membranes | J Med Chem 41: 3128-41 (1998) Article DOI: 10.1021/jm970159v BindingDB Entry DOI: 10.7270/Q2GM880H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50091096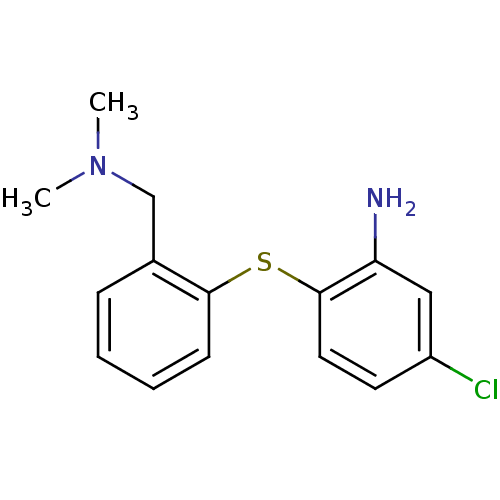 (5-Chloro-2-(2-dimethylaminomethyl-phenylsulfanyl)-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre for Addiction and Mental Health Curated by ChEMBL | Assay Description In vitro binding affinity on cloned Serotonin transporter | J Med Chem 43: 3103-10 (2000) BindingDB Entry DOI: 10.7270/Q2J67G4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075797 (1-{3-[1-(2-Cyclohexyl-ethyl)-piperidin-4-yl]-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.285 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075806 (1-{3-[1-(3-Methyl-butyl)-piperidin-4-yl]-propyl}-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50261255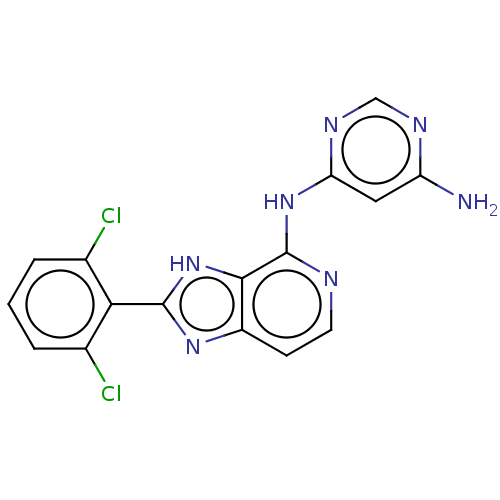 (CHEMBL4084436) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of TYK2 (unknown origin) by biochemical assay | Bioorg Med Chem Lett 27: 4370-4376 (2017) Article DOI: 10.1016/j.bmcl.2017.08.022 BindingDB Entry DOI: 10.7270/Q2RF5XG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor | J Med Chem 51: 5019-34 (2008) Article DOI: 10.1021/jm800463f BindingDB Entry DOI: 10.7270/Q2W096VS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM263346 (US9546162, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | 11 | n/a | n/a | 7.4 | n/a |
ALLERGAN, INC. US Patent | Assay Description Cells were seeded at a density of 5×104 cells per well in Biocoat® Poly-D-lysine-coated black-wall, clear-bottom 96-well plates (Becton-Dickinson) an... | US Patent US9546162 (2017) BindingDB Entry DOI: 10.7270/Q2P55QHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075802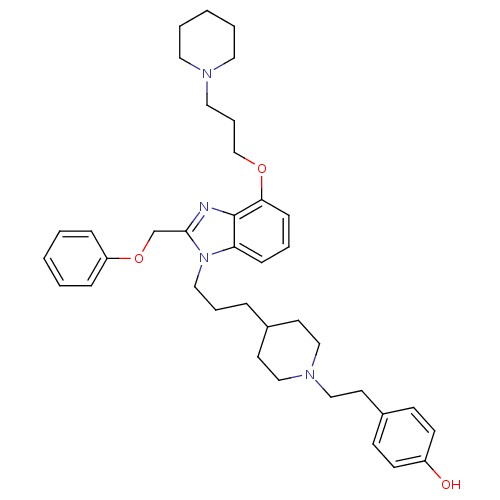 (4-[2-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50115668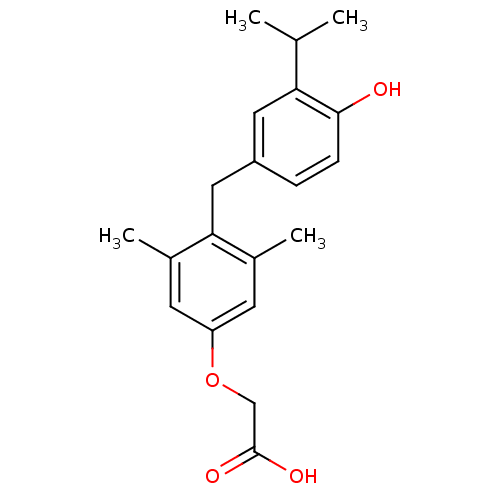 (3,5-dimethyl-4-(4'-hydroxy-3'-isopropylbenzyl)phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]3,5,3'-triiodo-L-thyronine His-tagged human recombinant TRbeta1 by scintillation proximity assay | J Med Chem 51: 7075-93 (2009) Article DOI: 10.1021/jm800824d BindingDB Entry DOI: 10.7270/Q21V5DT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18860 ((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]3,5,3'-triiodo-L-thyronine from His-tagged human recombinant TRalpha1 by scintillation proximity assay | J Med Chem 51: 7075-93 (2009) Article DOI: 10.1021/jm800824d BindingDB Entry DOI: 10.7270/Q21V5DT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50091098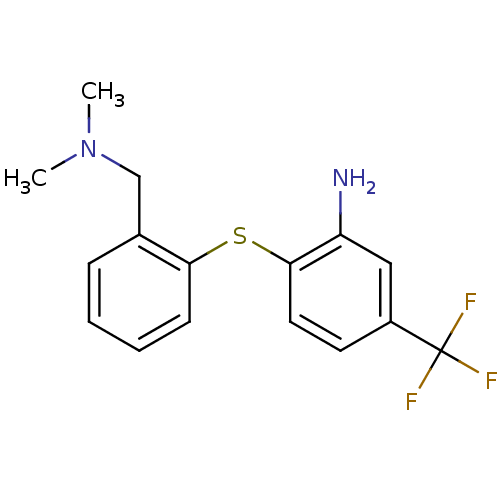 (2-(2-Dimethylaminomethyl-phenylsulfanyl)-5-trifluo...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre for Addiction and Mental Health Curated by ChEMBL | Assay Description In vitro binding affinity on cloned Serotonin transporter | J Med Chem 43: 3103-10 (2000) BindingDB Entry DOI: 10.7270/Q2J67G4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230844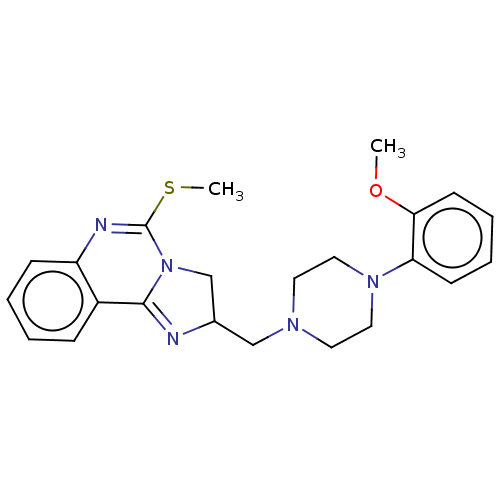 (CHEMBL71470) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230870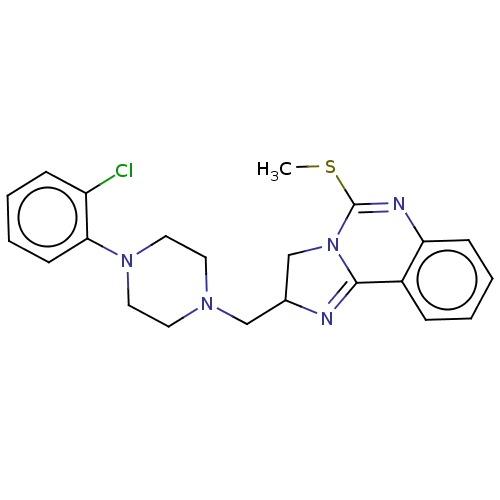 (CHEMBL70498) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230861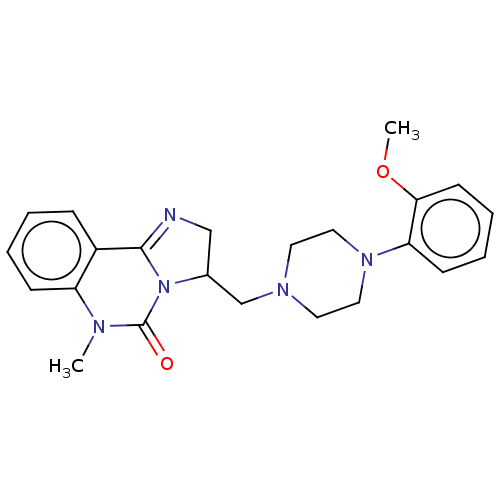 (CHEMBL2114072) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256260 (CHEMBL4094403) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075799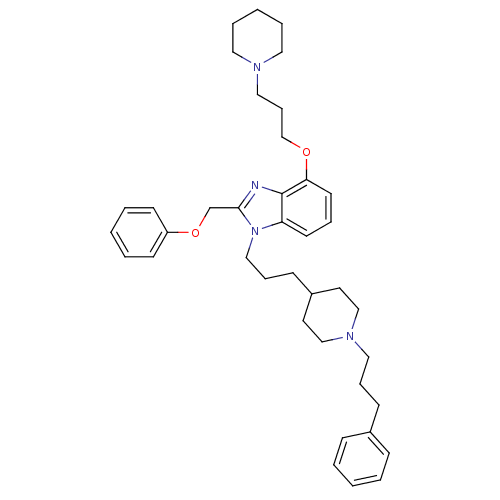 (2-Phenoxymethyl-1-{3-[1-(3-phenyl-propyl)-piperidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.361 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18869 (2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]T3 from recombinant thyroid hormone receptor beta (unknown origin) expressed in sf9 cells by scintillation proximity assay | Proc Natl Acad Sci U S A 104: 15490-5 (2007) Article DOI: 10.1073/pnas.0702759104 BindingDB Entry DOI: 10.7270/Q2JW8DPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230853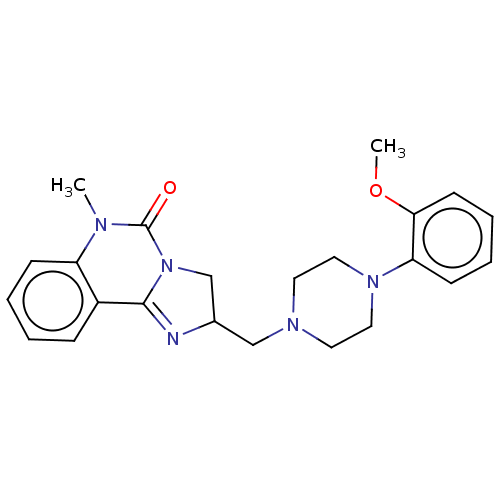 (CHEMBL304165) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256276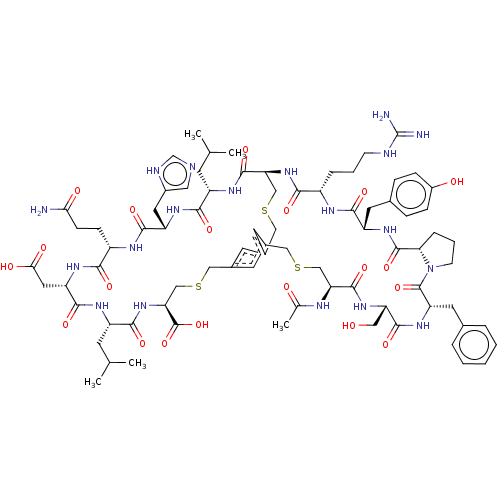 (CHEMBL4079260) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075798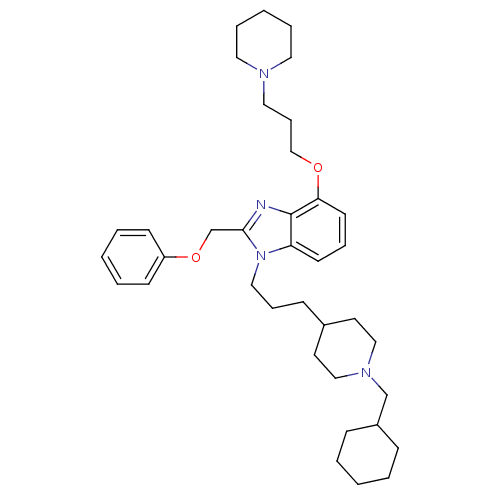 (1-[3-(1-Cyclohexylmethyl-piperidin-4-yl)-propyl]-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.393 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM50256284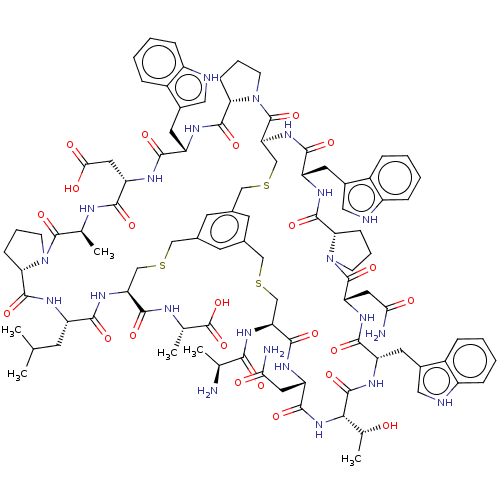 (CHEMBL4075544) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of recombinant Sprague-Dawley rat plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins foll... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50261268 (CHEMBL4062758) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of TYK2 (unknown origin) by biochemical assay | Bioorg Med Chem Lett 27: 4370-4376 (2017) Article DOI: 10.1016/j.bmcl.2017.08.022 BindingDB Entry DOI: 10.7270/Q2RF5XG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 16350 total ) | Next | Last >> |