 Found 6163 hits with Last Name = 'ye' and Initial = 'q'
Found 6163 hits with Last Name = 'ye' and Initial = 'q' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM466723

(US10800761, Example 42 | US10800761, Example 55 | ...)Show SMILES COc1cccc(F)c1-c1nccc(n1)C(=O)Nc1ccc(cc1N1C[C@@H](N)C[C@H]1CO)-c1cnccc1C#N |r| Show InChI InChI=1S/C29H26FN7O3/c1-40-26-4-2-3-22(30)27(26)28-34-10-8-24(35-28)29(39)36-23-6-5-17(21-14-33-9-7-18(21)13-31)11-25(23)37-15-19(32)12-20(37)16-38/h2-11,14,19-20,38H,12,15-16,32H2,1H3,(H,36,39)/t19-,20-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human carbonic anhydrase II (0.1 nM). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499195

(CHEMBL3735504)Show SMILES CC(C)(C)C(=O)Nc1ccc2n([C@@H]3CC[C@H](CO)CC3)c(NC(=O)c3cccc(c3)C(F)(F)F)nc2c1 |r,wD:12.11,15.15,(-7.45,.88,;-6.39,1.5,;-7.46,2.11,;-6.4,2.74,;-5.05,.74,;-5.04,-.49,;-3.72,1.53,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.24,-2.7,;3.72,-3.12,;4.09,-4.62,;2.98,-5.68,;3.36,-7.18,;4.54,-7.52,;1.5,-5.26,;1.13,-3.76,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;9,-2.3,;10.23,-2.29,;1.76,1.24,;.3,.77,;-1.03,1.55,)| Show InChI InChI=1S/C27H31F3N4O3/c1-26(2,3)24(37)31-19-9-12-22-21(14-19)32-25(34(22)20-10-7-16(15-35)8-11-20)33-23(36)17-5-4-6-18(13-17)27(28,29)30/h4-6,9,12-14,16,20,35H,7-8,10-11,15H2,1-3H3,(H,31,37)(H,32,33,36)/t16-,20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499203
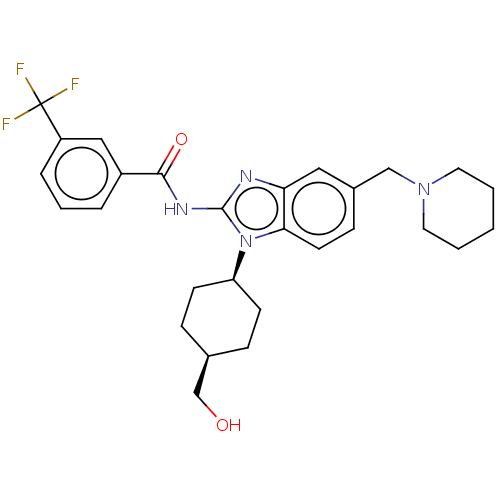
(CHEMBL3736036)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cc(CN3CCCCC3)ccc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;9,-2.3,;10.23,-2.29,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C28H33F3N4O2/c29-28(30,31)22-6-4-5-21(16-22)26(37)33-27-32-24-15-20(17-34-13-2-1-3-14-34)9-12-25(24)35(27)23-10-7-19(18-36)8-11-23/h4-6,9,12,15-16,19,23,36H,1-3,7-8,10-11,13-14,17-18H2,(H,32,33,37)/t19-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499205
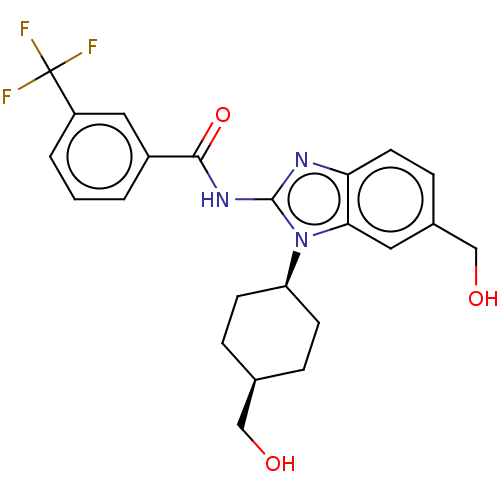
(CHEMBL3734814)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2ccc(CO)cc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.81,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;9,-2.3,;10.23,-2.29,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-3.72,-1.53,;-3.72,-2.76,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C23H24F3N3O3/c24-23(25,26)17-3-1-2-16(11-17)21(32)28-22-27-19-9-6-15(13-31)10-20(19)29(22)18-7-4-14(12-30)5-8-18/h1-3,6,9-11,14,18,30-31H,4-5,7-8,12-13H2,(H,27,28,32)/t14-,18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499208
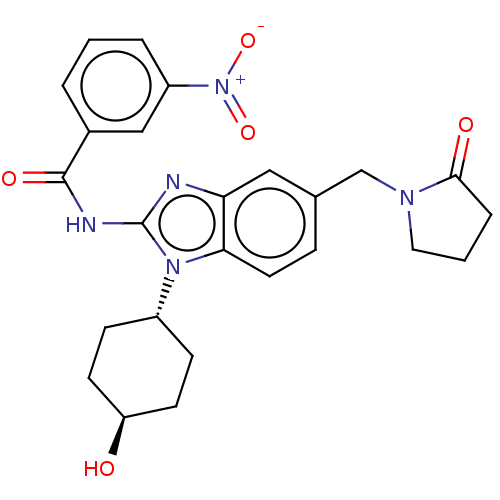
(CHEMBL3734872)Show SMILES O[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCC3=O)ccc12 |r,wU:4.7,wD:1.0,(3.28,-6.88,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-5.17,-.78,;-6.68,-1.11,;-7.46,.21,;-6.44,1.37,;-6.7,2.57,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C25H27N5O5/c31-20-9-7-18(8-10-20)29-22-11-6-16(15-28-12-2-5-23(28)32)13-21(22)26-25(29)27-24(33)17-3-1-4-19(14-17)30(34)35/h1,3-4,6,11,13-14,18,20,31H,2,5,7-10,12,15H2,(H,26,27,33)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499194

(CHEMBL3734854)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCCC3)ccc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C27H33N5O4/c33-18-19-7-10-22(11-8-19)31-25-12-9-20(17-30-13-2-1-3-14-30)15-24(25)28-27(31)29-26(34)21-5-4-6-23(16-21)32(35)36/h4-6,9,12,15-16,19,22,33H,1-3,7-8,10-11,13-14,17-18H2,(H,28,29,34)/t19-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50272598
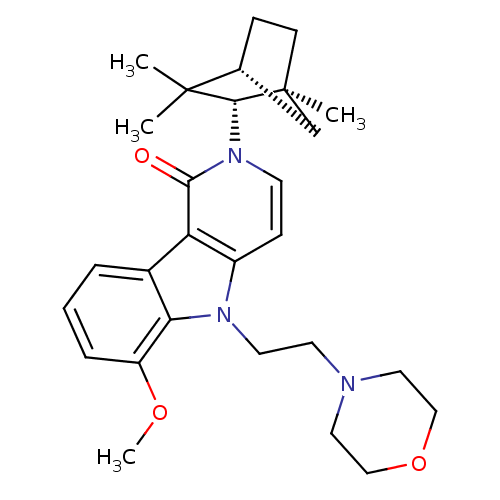
(6-Methoxy-5-(2-morpholin-4-yl-ethyl)-2-(1,3,3-trim...)Show SMILES COc1cccc2c1n(CCN1CCOCC1)c1ccn([C@H]3[C@@]4(C)CC[C@H](C4)C3(C)C)c(=O)c21 |r| Show InChI InChI=1S/C28H37N3O3/c1-27(2)19-8-10-28(3,18-19)26(27)31-11-9-21-23(25(31)32)20-6-5-7-22(33-4)24(20)30(21)13-12-29-14-16-34-17-15-29/h5-7,9,11,19,26H,8,10,12-18H2,1-4H3/t19-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | CHEMBL5267188

Show SMILES COc1cc(Cc2cnc(N)nc2N)cc2C(CF)=CC(C)(CF)Nc12 |c:19| Show InChI InChI=1S/C18H21F2N5O/c1-18(9-20)6-12(7-19)13-4-10(5-14(26-2)15(13)25-18)3-11-8-23-17(22)24-16(11)21/h4-6,8,25H,3,7,9H2,1-2H3,(H4,21,22,23,24) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human carbonic anhydrase II (0.1 nM). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM466728

(US10800761, Example 47 | US11731958, Example 47)Show SMILES COc1cccc(F)c1-c1nccc(n1)C(=O)Nc1ccc(cc1N1C[C@@H](N)C[C@H]1CO)-c1c(cnn1C)C#N |r| Show InChI InChI=1S/C28H27FN8O3/c1-36-26(17(12-30)13-33-36)16-6-7-21(23(10-16)37-14-18(31)11-19(37)15-38)35-28(39)22-8-9-32-27(34-22)25-20(29)4-3-5-24(25)40-2/h3-10,13,18-19,38H,11,14-15,31H2,1-2H3,(H,35,39)/t18-,19-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human carbonic anhydrase II (0.1 nM). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM466724

(US10800761, Example 43 | US11731958, Example 43)Show SMILES COc1cccc(F)c1-c1nccc(n1)C(=O)Nc1ccc(cc1N1C[C@@H](N)C[C@H]1CO)-c1cccnc1C |r| Show InChI InChI=1S/C29H29FN6O3/c1-17-21(5-4-11-32-17)18-8-9-23(25(13-18)36-15-19(31)14-20(36)16-37)35-29(38)24-10-12-33-28(34-24)27-22(30)6-3-7-26(27)39-2/h3-13,19-20,37H,14-16,31H2,1-2H3,(H,35,38)/t19-,20-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human carbonic anhydrase II (0.1 nM). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM466705
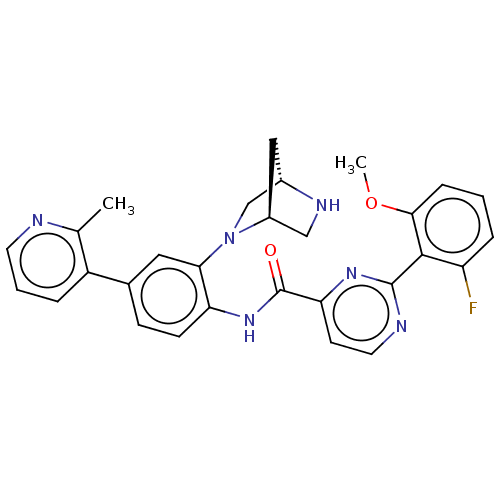
(US10800761, Example 24)Show SMILES COc1cccc(F)c1-c1nccc(n1)C(=O)Nc1ccc(cc1N1C[C@@H]2C[C@H]1CN2)-c1cccnc1C |r| Show InChI InChI=1S/C29H27FN6O2/c1-17-21(5-4-11-31-17)18-8-9-23(25(13-18)36-16-19-14-20(36)15-33-19)35-29(37)24-10-12-32-28(34-24)27-22(30)6-3-7-26(27)38-2/h3-13,19-20,33H,14-16H2,1-2H3,(H,35,37)/t19-,20-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human carbonic anhydrase II (0.1 nM). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | CHEMBL5273201

Show InChI InChI=1S/C16H17N5O/c1-9-3-4-19-14-12(9)6-10(7-13(14)22-2)5-11-8-20-16(18)21-15(11)17/h3-4,6-8H,5H2,1-2H3,(H4,17,18,20,21) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human carbonic anhydrase II (0.1 nM). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | CHEMBL5284132
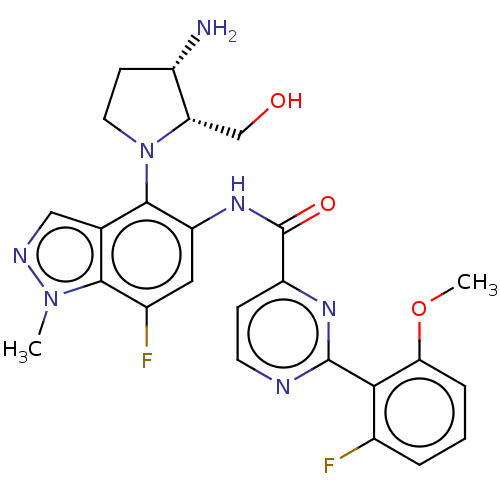
Show SMILES CCc1cc(Cc2cnc(N)nc2N)cc2C(C)=CC(C)(C)Nc12 |c:18| Show InChI InChI=1S/C19H25N5/c1-5-13-6-12(7-14-10-22-18(21)23-17(14)20)8-15-11(2)9-19(3,4)24-16(13)15/h6,8-10,24H,5,7H2,1-4H3,(H4,20,21,22,23) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human carbonic anhydrase II (0.1 nM). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | CHEMBL5274764
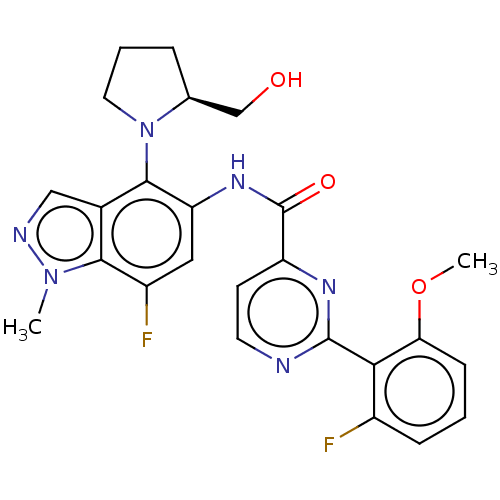
Show SMILES CCCNCCCCCC(CC)SC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C21H36N6O3S/c1-3-9-23-10-7-5-6-8-14(4-2)31-11-15-17(28)18(29)21(30-15)27-13-26-16-19(22)24-12-25-20(16)27/h12-15,17-18,21,23,28-29H,3-11H2,1-2H3,(H2,22,24,25)/t14?,15-,17-,18-,21?/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human carbonic anhydrase II (0.1 nM). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM414626

(US10435405, Example 43 | US10934288, Example 43)Show SMILES COc1cccc(F)c1-c1cc2c(n[nH]c2cn1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C24H24FN5O/c1-29-10-12-30(13-11-29)17-8-6-16(7-9-17)24-18-14-20(26-15-21(18)27-28-24)23-19(25)4-3-5-22(23)31-2/h3-9,14-15H,10-13H2,1-2H3,(H,27,28) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory concentration against rat liver dihydrofolate reductase(DHFR) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | CHEMBL5267747
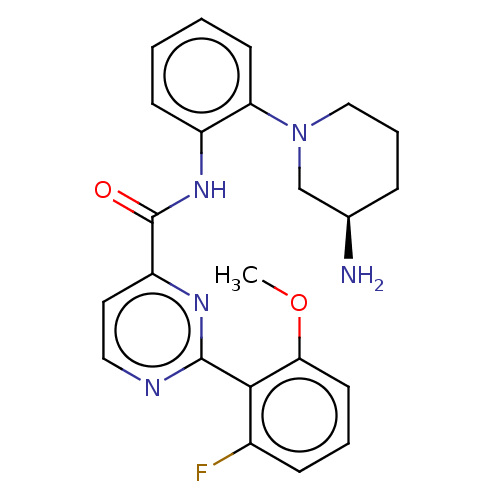
Show InChI InChI=1S/C13H16N2O3S2/c14-20(16,17)13-8-11-7-10(1-2-12(11)19-13)9-15-3-5-18-6-4-15/h1-2,7-8H,3-6,9H2,(H2,14,16,17) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM459443

(US10752635, Example 4, Peak 1 | US11492354, Exampl...)Show SMILES COc1cccc(F)c1-c1nccc(n1)C(=O)Nc1cc(F)c2n(C)ncc2c1N1CC[C@@H](N)C1 |r| Show InChI InChI=1S/C24H23F2N7O2/c1-32-21-14(11-29-32)22(33-9-7-13(27)12-33)18(10-16(21)26)31-24(34)17-6-8-28-23(30-17)20-15(25)4-3-5-19(20)35-2/h3-6,8,10-11,13H,7,9,12,27H2,1-2H3,(H,31,34)/t13-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM459444
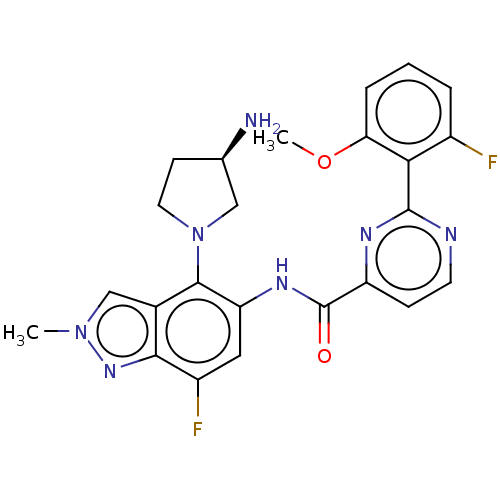
(US10752635, Example 4, Peak 2 | US11492354, Exampl...)Show SMILES COc1cccc(F)c1-c1nccc(n1)C(=O)Nc1cc(F)c2nn(C)cc2c1N1CC[C@@H](N)C1 |r| Show InChI InChI=1S/C24H23F2N7O2/c1-32-12-14-21(31-32)16(26)10-18(22(14)33-9-7-13(27)11-33)30-24(34)17-6-8-28-23(29-17)20-15(25)4-3-5-19(20)35-2/h3-6,8,10,12-13H,7,9,11,27H2,1-2H3,(H,30,34)/t13-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | CHEMBL5281003

Show InChI InChI=1S/C8H7NO3S2/c9-14(11,12)8-3-5-1-2-6(10)4-7(5)13-8/h1-4,10H,(H2,9,11,12) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Vasopressin V2 receptor antagonistic activity in vivo in anesthetized rats |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | CHEMBL5277241

Show InChI InChI=1S/C11H11NO4S2/c1-7(13)16-6-8-2-3-10-9(4-8)5-11(17-10)18(12,14)15/h2-5H,6H2,1H3,(H2,12,14,15) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Vasopressin V2 receptor antagonistic activity in vivo in anesthetized rats |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | CHEMBL5283973
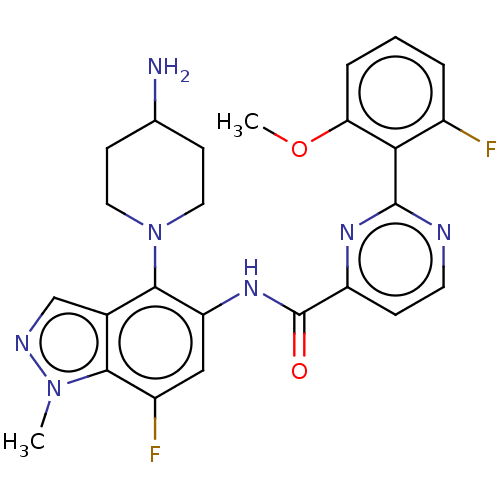
Show InChI InChI=1S/C11H14N2O2S2/c1-13(2)7-8-3-4-10-9(5-8)6-11(16-10)17(12,14)15/h3-6H,7H2,1-2H3,(H2,12,14,15) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Vasopressin V2 receptor antagonistic activity in vivo in anesthetized rats |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | CHEMBL5288420

Show InChI InChI=1S/C9H9NO3S2/c1-13-7-4-2-3-6-5-8(14-9(6)7)15(10,11)12/h2-5H,1H3,(H2,10,11,12) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Vasopressin V2 receptor antagonistic activity in vivo in anesthetized rats |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM459445

(US10752635, Example 5, Peak 1 | US11492354, Exampl...)Show SMILES COc1cccc(F)c1-c1nccc(n1)C(=O)Nc1cc(F)c2n(C)ncc2c1N1C[C@@H]2CC[C@H]1CN2 |r| Show InChI InChI=1S/C26H25F2N7O2/c1-34-23-16(12-31-34)24(35-13-14-6-7-15(35)11-30-14)20(10-18(23)28)33-26(36)19-8-9-29-25(32-19)22-17(27)4-3-5-21(22)37-2/h3-5,8-10,12,14-15,30H,6-7,11,13H2,1-2H3,(H,33,36)/t14-,15-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human carbonic anhydrase II (0.1 nM). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499197
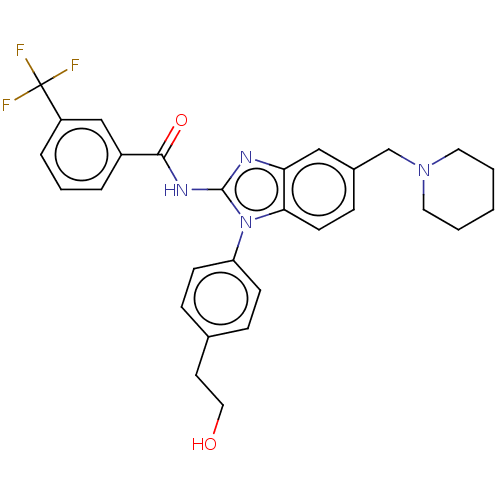
(CHEMBL3736465)Show SMILES OCCc1ccc(cc1)-n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C29H29F3N4O2/c30-29(31,32)23-6-4-5-22(18-23)27(38)34-28-33-25-17-21(19-35-14-2-1-3-15-35)9-12-26(25)36(28)24-10-7-20(8-11-24)13-16-37/h4-12,17-18,37H,1-3,13-16,19H2,(H,33,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | CHEMBL5276026

Show InChI InChI=1S/C17H23N5/c1-10-8-17(2,3)22-14-5-4-11(7-13(10)14)6-12-9-20-16(19)21-15(12)18/h4-5,7,9-10,22H,6,8H2,1-3H3,(H4,18,19,20,21) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human carbonic anhydrase II (0.1 nM). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
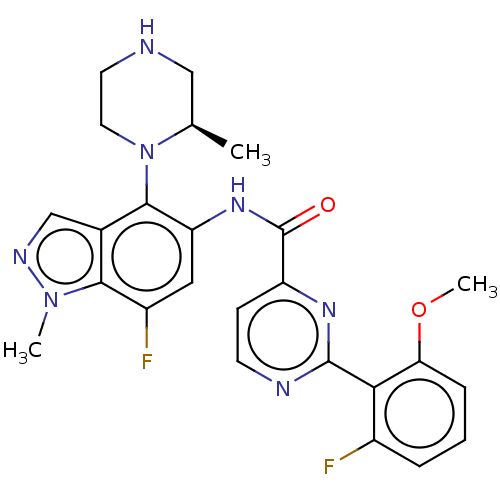
(Homo sapiens (Human)) | CHEMBL5268277
Show InChI InChI=1S/C13H18N2O2S3/c1-15(2)5-6-18-9-10-3-4-12-11(7-10)8-13(19-12)20(14,16)17/h3-4,7-8H,5-6,9H2,1-2H3,(H2,14,16,17) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
| | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Vasopressin V2 receptor antagonistic activity in vivo in anesthetized rats |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499206

(CHEMBL3735949)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(F)ccc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.45,1.38,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C21H21FN4O4/c22-15-6-9-19-18(11-15)23-21(25(19)16-7-4-13(12-27)5-8-16)24-20(28)14-2-1-3-17(10-14)26(29)30/h1-3,6,9-11,13,16,27H,4-5,7-8,12H2,(H,23,24,28)/t13-,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM466704
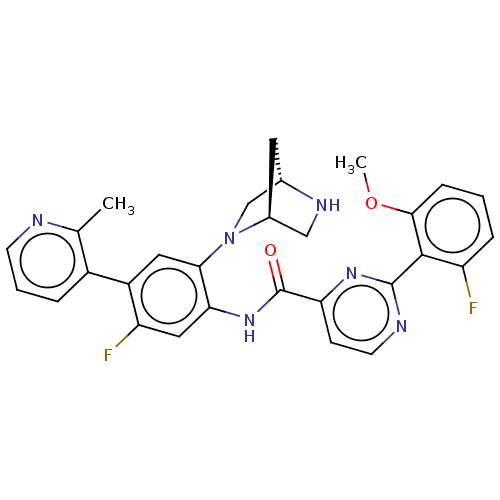
(US10800761, Example 23 | US11731958, Example 23)Show SMILES COc1cccc(F)c1-c1nccc(n1)C(=O)Nc1cc(F)c(cc1N1C[C@@H]2C[C@H]1CN2)-c1cccnc1C |r| Show InChI InChI=1S/C29H26F2N6O2/c1-16-19(5-4-9-32-16)20-12-25(37-15-17-11-18(37)14-34-17)24(13-22(20)31)36-29(38)23-8-10-33-28(35-23)27-21(30)6-3-7-26(27)39-2/h3-10,12-13,17-18,34H,11,14-15H2,1-2H3,(H,36,38)/t17-,18-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human carbonic anhydrase II (0.1 nM). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM459439

(US10752635, Example 1, Peak 2 | US11492354, Exampl...)Show SMILES COc1cccc(F)c1-c1nccc(n1)C(=O)Nc1cc(F)c2n(C)ncc2c1N1C[C@@H]2C[C@H]1CN2 |r| Show InChI InChI=1S/C25H23F2N7O2/c1-33-22-15(11-30-33)23(34-12-13-8-14(34)10-29-13)19(9-17(22)27)32-25(35)18-6-7-28-24(31-18)21-16(26)4-3-5-20(21)36-2/h3-7,9,11,13-14,29H,8,10,12H2,1-2H3,(H,32,35)/t13-,14-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Vasopressin V2 receptor antagonistic activity in vivo in anesthetized rats |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM466687
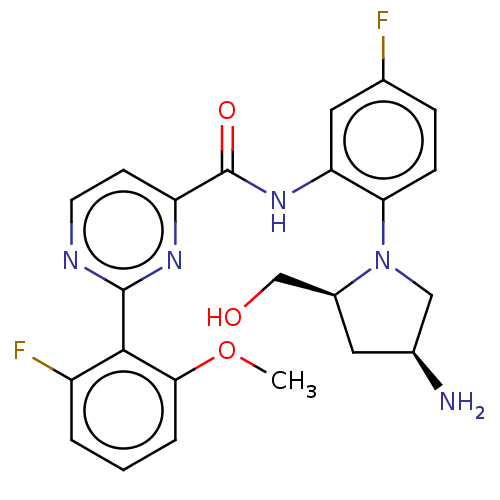
(US10800761, Example 6 | US11731958, Example 6 | US...)Show SMILES COc1cccc(F)c1-c1nccc(n1)C(=O)Nc1cc(F)ccc1N1C[C@@H](N)C[C@H]1CO |r| Show InChI InChI=1S/C23H23F2N5O3/c1-33-20-4-2-3-16(25)21(20)22-27-8-7-17(28-22)23(32)29-18-9-13(24)5-6-19(18)30-11-14(26)10-15(30)12-31/h2-9,14-15,31H,10-12,26H2,1H3,(H,29,32)/t14-,15-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human carbonic anhydrase II (0.1 nM). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | CHEMBL5277555
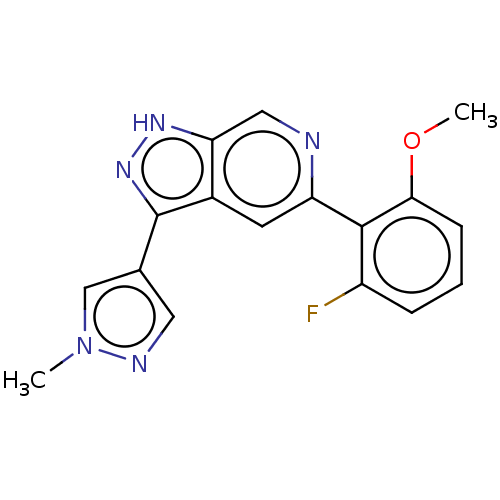
Show InChI InChI=1S/C13H18N6/c1-3-9-11(12(15)19-13(16)18-9)7-4-5-10(17-2)8(14)6-7/h4-6,17H,3,14H2,1-2H3,(H4,15,16,18,19) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory concentration against rat liver dihydrofolate reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM466727
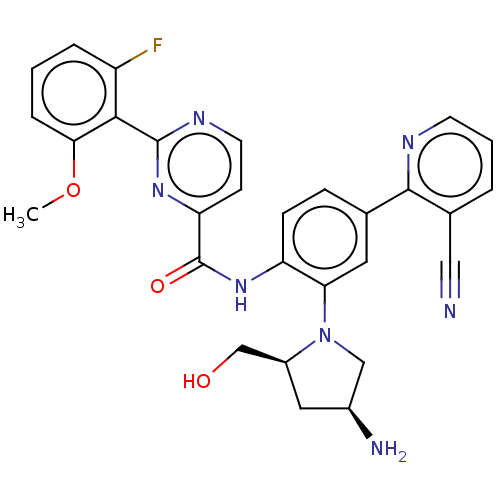
(US10800761, Example 46 | US11731958, Example 46)Show SMILES COc1cccc(F)c1-c1nccc(n1)C(=O)Nc1ccc(cc1N1C[C@@H](N)C[C@H]1CO)-c1ncccc1C#N |r| Show InChI InChI=1S/C29H26FN7O3/c1-40-25-6-2-5-21(30)26(25)28-34-11-9-23(35-28)29(39)36-22-8-7-17(27-18(14-31)4-3-10-33-27)12-24(22)37-15-19(32)13-20(37)16-38/h2-12,19-20,38H,13,15-16,32H2,1H3,(H,36,39)/t19-,20-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human carbonic anhydrase II (0.1 nM). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | CHEMBL5279971

Show InChI InChI=1S/C8H8N2O2S2/c9-6-2-1-5-3-8(14(10,11)12)13-7(5)4-6/h1-4H,9H2,(H2,10,11,12) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Vasopressin V2 receptor antagonistic activity in vivo in anesthetized rats |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM459484

(US10752635, Example 35 | US11492354, Example 35)Show SMILES COc1cccc(F)c1-c1nccc(n1)C(=O)Nc1cc(F)c2n(C)ncc2c1N1C[C@@H](O)C[C@H]1CN |r| Show InChI InChI=1S/C25H25F2N7O3/c1-33-22-15(11-30-33)23(34-12-14(35)8-13(34)10-28)19(9-17(22)27)32-25(36)18-6-7-29-24(31-18)21-16(26)4-3-5-20(21)37-2/h3-7,9,11,13-14,35H,8,10,12,28H2,1-2H3,(H,32,36)/t13-,14-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human carbonic anhydrase II (0.1 nM). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM466682
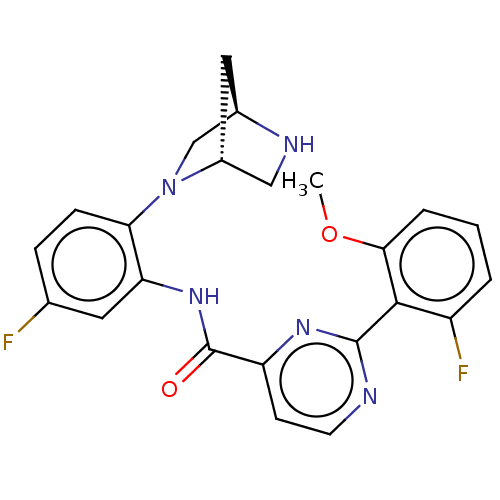
(US10800761, Example 1 | US11731958, Example 1)Show SMILES COc1cccc(F)c1-c1nccc(n1)C(=O)Nc1cc(F)ccc1N1C[C@H]2C[C@@H]1CN2 |r| Show InChI InChI=1S/C23H21F2N5O2/c1-32-20-4-2-3-16(25)21(20)22-26-8-7-17(28-22)23(31)29-18-9-13(24)5-6-19(18)30-12-14-10-15(30)11-27-14/h2-9,14-15,27H,10-12H2,1H3,(H,29,31)/t14-,15-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human carbonic anhydrase II (0.1 nM). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM459441

(US10752635, Example 3, Peak 1 | US11492354, Exampl...)Show SMILES COc1cccc(F)c1-c1nccc(n1)C(=O)Nc1cc(F)c2n(C)ncc2c1N1C[C@@H](N)C[C@H]1CO |r| Show InChI InChI=1S/C25H25F2N7O3/c1-33-22-15(10-30-33)23(34-11-13(28)8-14(34)12-35)19(9-17(22)27)32-25(36)18-6-7-29-24(31-18)21-16(26)4-3-5-20(21)37-2/h3-7,9-10,13-14,35H,8,11-12,28H2,1-2H3,(H,32,36)/t13-,14-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human carbonic anhydrase II (0.1 nM). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM498971

(US11014929, Example 11 | US11542265, Example 11)Show SMILES COc1cccc(F)c1-c1ncc2[nH]nc(-c3ccc(cc3)N3CCN(C)CC3)c2n1 Show InChI InChI=1S/C23H23FN6O/c1-29-10-12-30(13-11-29)16-8-6-15(7-9-16)21-22-18(27-28-21)14-25-23(26-22)20-17(24)4-3-5-19(20)31-2/h3-9,14H,10-13H2,1-2H3,(H,27,28) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory concentration against rat liver dihydrofolate reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | CHEMBL5271378

Show SMILES CC(C)(C)N1CC(COc2ccc3cc(sc3c2)S(N)(=O)=O)OC1=O Show InChI InChI=1S/C16H20N2O5S2/c1-16(2,3)18-8-12(23-15(18)19)9-22-11-5-4-10-6-14(25(17,20)21)24-13(10)7-11/h4-7,12H,8-9H2,1-3H3,(H2,17,20,21) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
| | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM414751
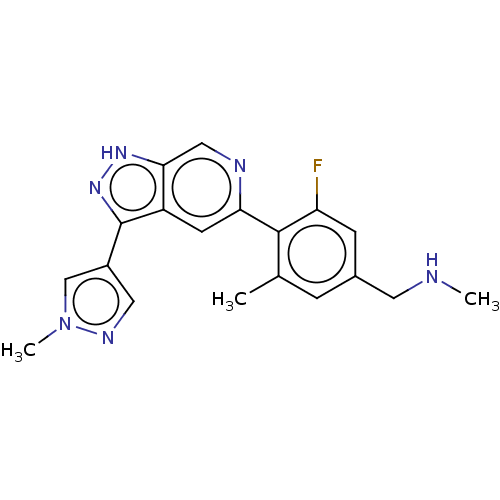
(US10435405, Example 168 | US10934288, Example 168)Show SMILES CNCc1cc(C)c(c(F)c1)-c1cc2c(n[nH]c2cn1)-c1cnn(C)c1 Show InChI InChI=1S/C19H19FN6/c1-11-4-12(7-21-2)5-15(20)18(11)16-6-14-17(9-22-16)24-25-19(14)13-8-23-26(3)10-13/h4-6,8-10,21H,7H2,1-3H3,(H,24,25) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory concentration against rat liver dihydrofolate reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499198
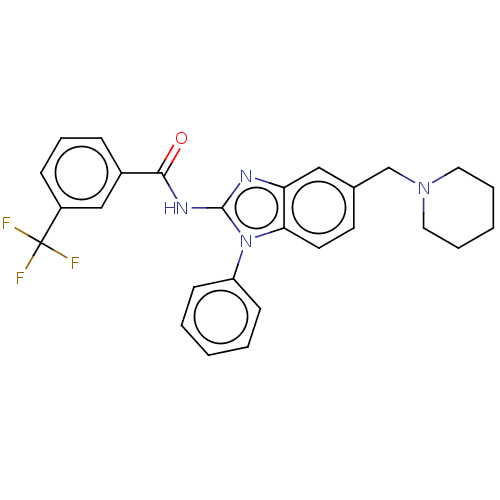
(CHEMBL3736278)Show SMILES FC(F)(F)c1cccc(c1)C(=O)Nc1nc2cc(CN3CCCCC3)ccc2n1-c1ccccc1 Show InChI InChI=1S/C27H25F3N4O/c28-27(29,30)21-9-7-8-20(17-21)25(35)32-26-31-23-16-19(18-33-14-5-2-6-15-33)12-13-24(23)34(26)22-10-3-1-4-11-22/h1,3-4,7-13,16-17H,2,5-6,14-15,18H2,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499199

(CHEMBL3735673)Show SMILES O[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCCC3)ccc12 |r,wU:4.7,wD:1.0,(3.28,-6.88,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C26H31N5O4/c32-22-10-8-20(9-11-22)30-24-12-7-18(17-29-13-2-1-3-14-29)15-23(24)27-26(30)28-25(33)19-5-4-6-21(16-19)31(34)35/h4-7,12,15-16,20,22,32H,1-3,8-11,13-14,17H2,(H,27,28,33)/t20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | CHEMBL5269770

Show InChI InChI=1S/C8H8NO6PS2/c9-18(13,14)8-3-5-1-2-6(4-7(5)17-8)15-16(10,11)12/h1-4H,(H2,9,13,14)(H2,10,11,12)/p-2 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
| | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM536101

(US11242343, Example 8)Show SMILES COc1cccc(F)c1-c1ccc2[nH]nc(-c3ccc(cc3)N3CCN(C)CC3)c2n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory concentration against rat liver dihydrofolate reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | CHEMBL5281791
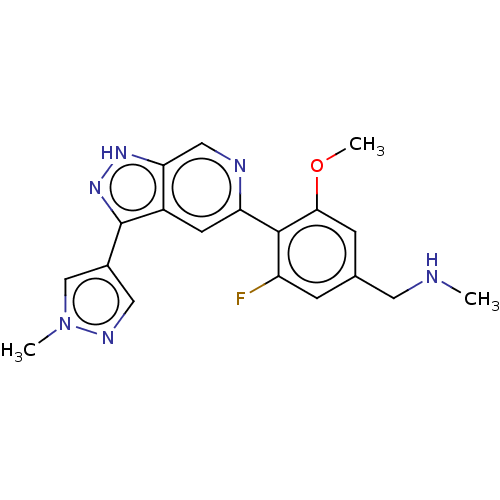
Show InChI InChI=1S/C17H24N6/c1-3-14-15(16(18)21-17(19)20-14)12-4-6-13(7-5-12)23-10-8-22(2)9-11-23/h4-7H,3,8-11H2,1-2H3,(H4,18,19,20,21) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory concentration against rat liver dihydrofolate reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | CHEMBL5279231

Show SMILES CCCCCC(CC)SC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C18H29N5O3S/c1-3-5-6-7-11(4-2)27-8-12-14(24)15(25)18(26-12)23-10-22-13-16(19)20-9-21-17(13)23/h9-12,14-15,18,24-25H,3-8H2,1-2H3,(H2,19,20,21)/t11?,12-,14-,15-,18?/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human carbonic anhydrase II (0.1 nM). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM414714
(US10435405, Example 131 | US10934288, Example 131)Show SMILES CNCc1cc(F)c(-c2cc3c(n[nH]c3cn2)-c2cnn(C)c2)c(c1)C(F)(F)F Show InChI InChI=1S/C19H16F4N6/c1-24-6-10-3-13(19(21,22)23)17(14(20)4-10)15-5-12-16(8-25-15)27-28-18(12)11-7-26-29(2)9-11/h3-5,7-9,24H,6H2,1-2H3,(H,27,28) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory concentration against rat liver dihydrofolate reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499202

(CHEMBL3735523)Show SMILES O[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCOCC3)ccc12 |r,wU:4.7,wD:1.0,(3.28,-6.88,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C25H29N5O5/c31-21-7-5-19(6-8-21)29-23-9-4-17(16-28-10-12-35-13-11-28)14-22(23)26-25(29)27-24(32)18-2-1-3-20(15-18)30(33)34/h1-4,9,14-15,19,21,31H,5-8,10-13,16H2,(H,26,27,32)/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | CHEMBL5267479

Show SMILES COc1cc(Cc2cnc(N)nc2N)cc2C(C)=CC(C)(C)N(C)c12 |c:18| Show InChI InChI=1S/C19H25N5O/c1-11-9-19(2,3)24(4)16-14(11)7-12(8-15(16)25-5)6-13-10-22-18(21)23-17(13)20/h7-10H,6H2,1-5H3,(H4,20,21,22,23) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human carbonic anhydrase II (0.1 nM). |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data