

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM29525 (3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5-HT4B receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 64: 629-37 (2013) Article DOI: 10.1016/j.ejmech.2013.03.060 BindingDB Entry DOI: 10.7270/Q27947NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256265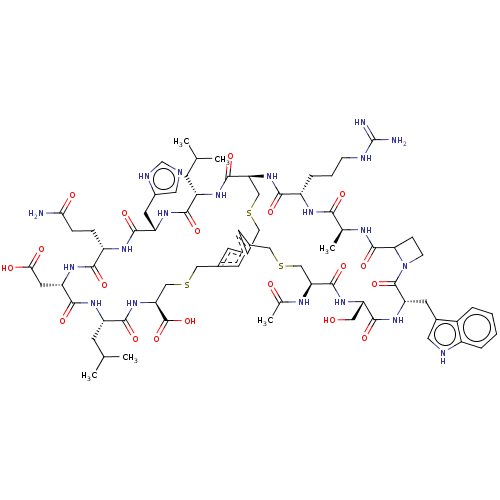 (CHEMBL4089486) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230846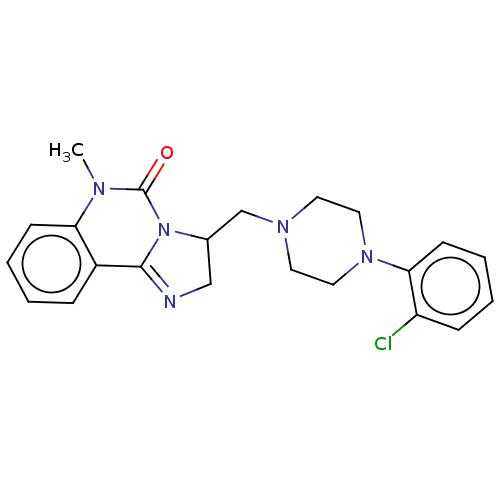 (CHEMBL2114071) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230859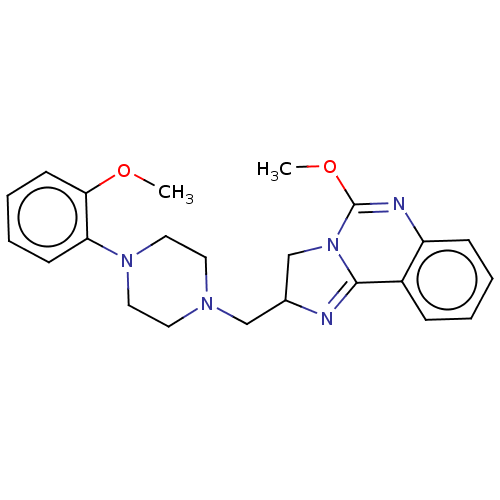 (CHEMBL309150) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256283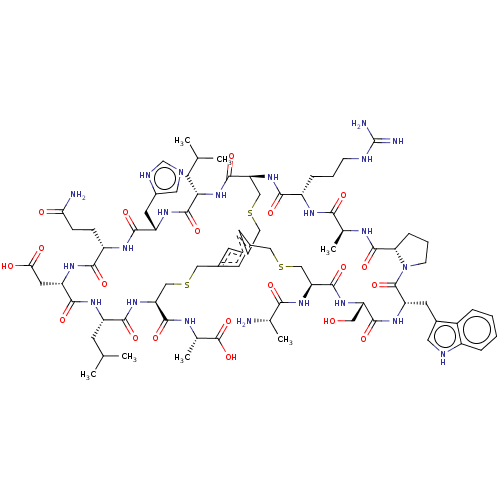 (CHEMBL4079711) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM466723 (US10800761, Example 42 | US10800761, Example 55 | ...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro inhibition of human carbonic anhydrase II (0.1 nM). | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM85026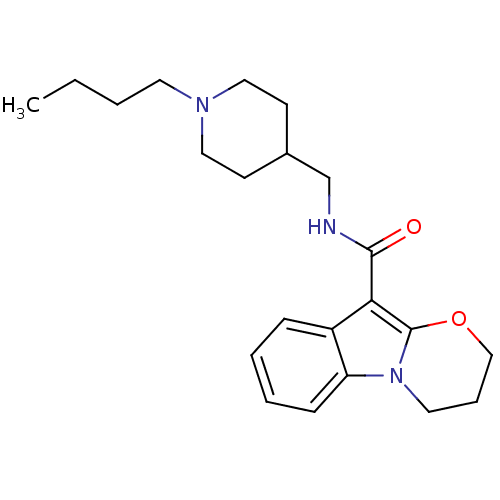 (N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5-HT4B receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 64: 629-37 (2013) Article DOI: 10.1016/j.ejmech.2013.03.060 BindingDB Entry DOI: 10.7270/Q27947NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230848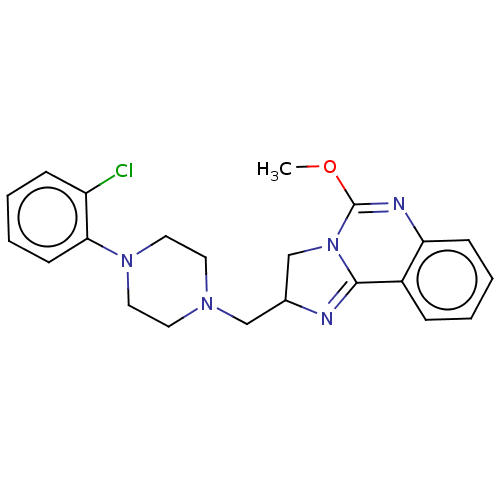 (CHEMBL70872) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50492064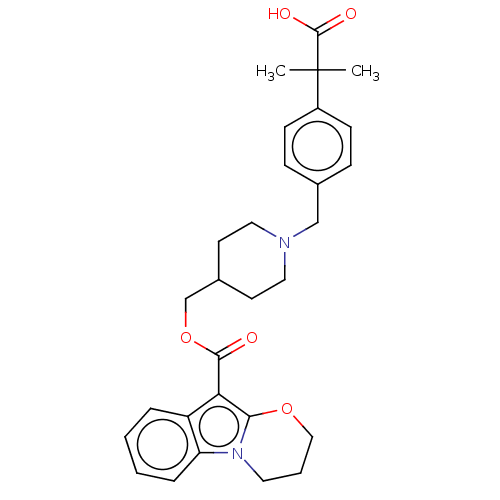 (CHEMBL2391994) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5-HT4B receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 64: 629-37 (2013) Article DOI: 10.1016/j.ejmech.2013.03.060 BindingDB Entry DOI: 10.7270/Q27947NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256264 (CHEMBL4099333) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256277 (CHEMBL4093698) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256286 (CHEMBL4076199) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50066109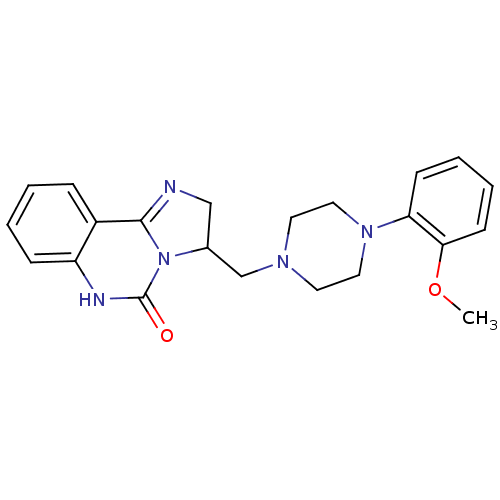 (3-[4-(2-Methoxy-phenyl)-piperazin-1-ylmethyl]-2,6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50369383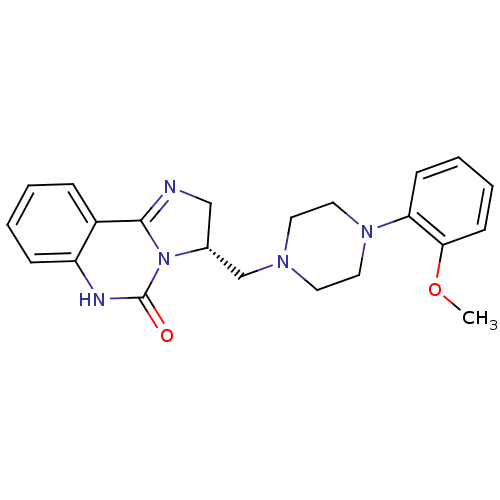 (CHEMBL1788222) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from Alpha-1A adrenergic receptorof rat submaxillary gland membranes | J Med Chem 41: 3128-41 (1998) Article DOI: 10.1021/jm970159v BindingDB Entry DOI: 10.7270/Q2GM880H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50040253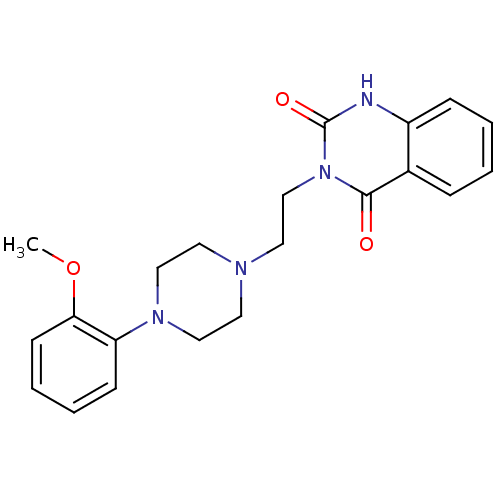 (3-{2-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-ethyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256262 (CHEMBL4070056) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50066109 (3-[4-(2-Methoxy-phenyl)-piperazin-1-ylmethyl]-2,6-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from Alpha-1A adrenergic receptorof rat submaxillary gland membranes | J Med Chem 41: 3128-41 (1998) Article DOI: 10.1021/jm970159v BindingDB Entry DOI: 10.7270/Q2GM880H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50261255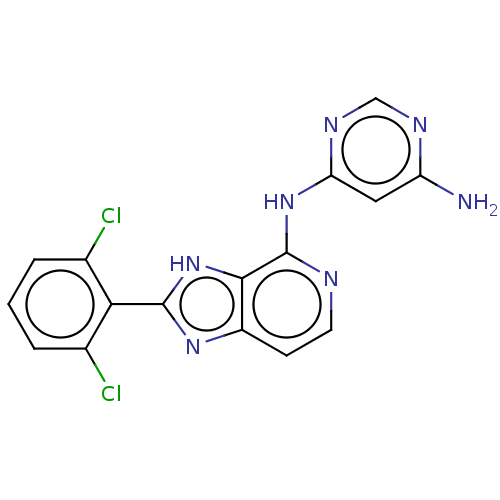 (CHEMBL4084436) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of TYK2 (unknown origin) by biochemical assay | Bioorg Med Chem Lett 27: 4370-4376 (2017) Article DOI: 10.1016/j.bmcl.2017.08.022 BindingDB Entry DOI: 10.7270/Q2RF5XG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM263346 (US9546162, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | 11 | n/a | n/a | 7.4 | n/a |
ALLERGAN, INC. US Patent | Assay Description Cells were seeded at a density of 5×104 cells per well in Biocoat® Poly-D-lysine-coated black-wall, clear-bottom 96-well plates (Becton-Dickinson) an... | US Patent US9546162 (2017) BindingDB Entry DOI: 10.7270/Q2P55QHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor | J Med Chem 51: 5019-34 (2008) Article DOI: 10.1021/jm800463f BindingDB Entry DOI: 10.7270/Q2W096VS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230870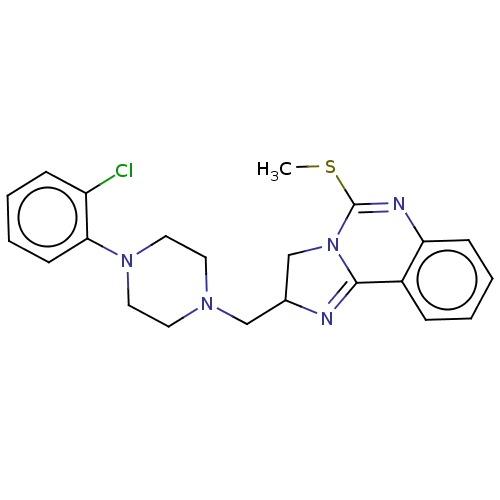 (CHEMBL70498) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230844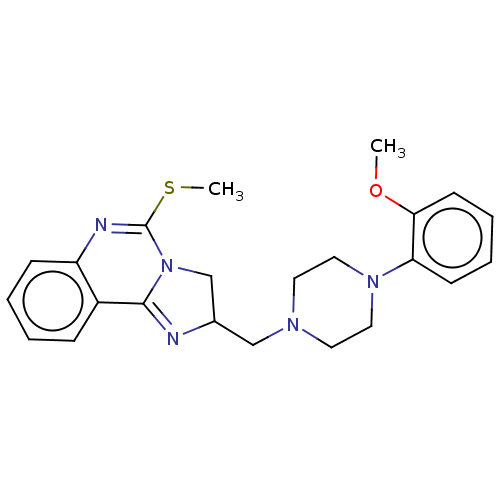 (CHEMBL71470) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256260 (CHEMBL4094403) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230861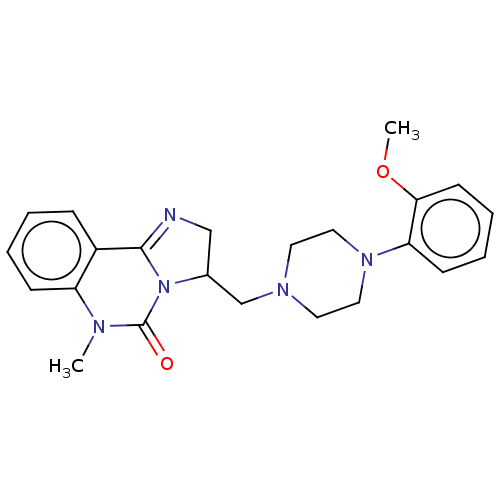 (CHEMBL2114072) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230853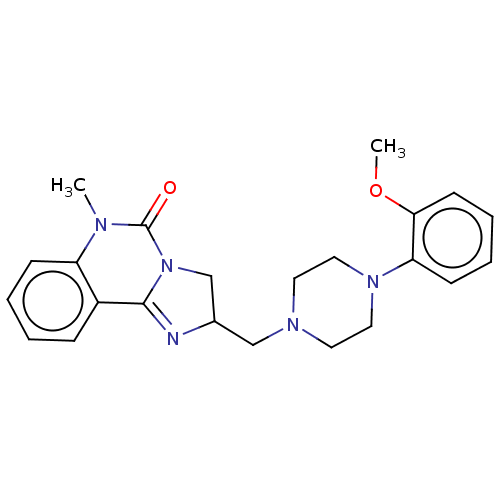 (CHEMBL304165) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256276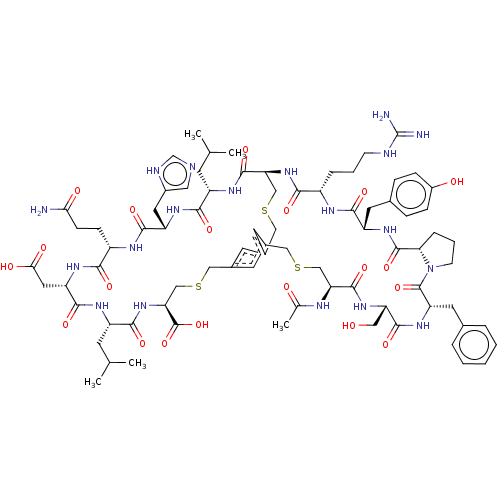 (CHEMBL4079260) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM50256284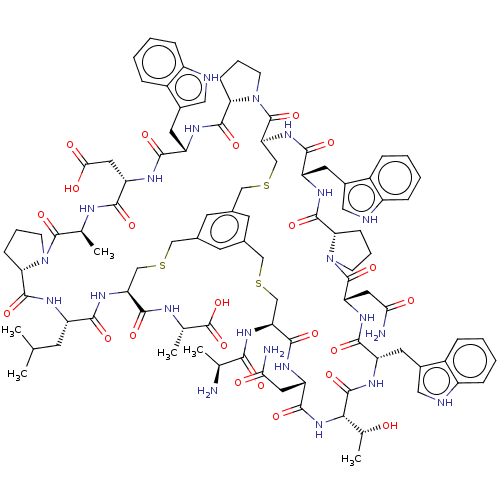 (CHEMBL4075544) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of recombinant Sprague-Dawley rat plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins foll... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50261268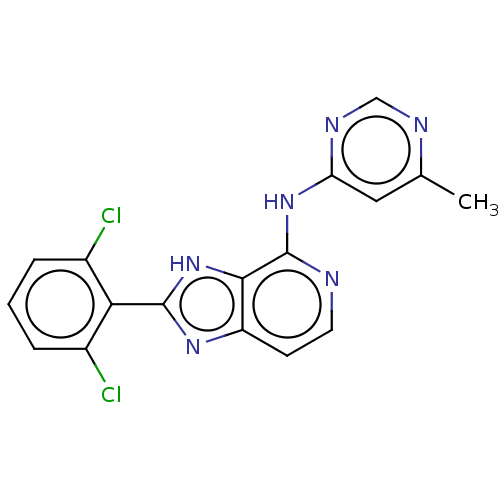 (CHEMBL4062758) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of TYK2 (unknown origin) by biochemical assay | Bioorg Med Chem Lett 27: 4370-4376 (2017) Article DOI: 10.1016/j.bmcl.2017.08.022 BindingDB Entry DOI: 10.7270/Q2RF5XG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM263361 (US9546162, 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | 2 | n/a | n/a | 7.4 | n/a |
ALLERGAN, INC. US Patent | Assay Description HEK-293 cells stably expressing the human or feline FP receptor, or EP1, EP2, or EP4 receptors were washed with TME buffer, scraped from the bottom o... | US Patent US9546162 (2017) BindingDB Entry DOI: 10.7270/Q2P55QHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Rattus norvegicus (rat)) | BDBM50369383 (CHEMBL1788222) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from Alpha-1B adrenergic receptor of rat liver membrane | J Med Chem 41: 3128-41 (1998) Article DOI: 10.1021/jm970159v BindingDB Entry DOI: 10.7270/Q2GM880H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256288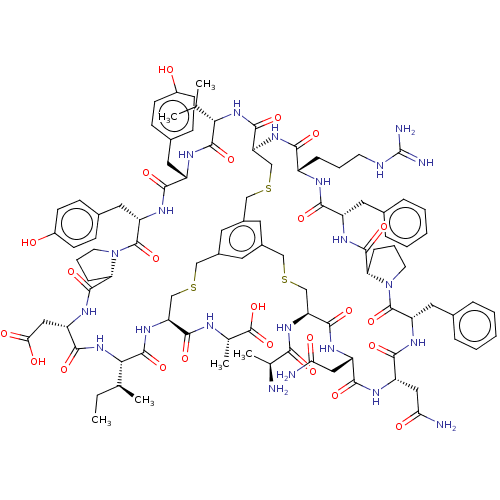 (CHEMBL4085408) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50261261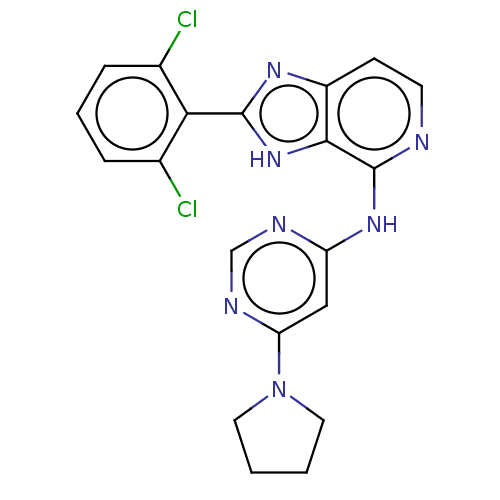 (CHEMBL4075453) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of TYK2 (unknown origin) by biochemical assay | Bioorg Med Chem Lett 27: 4370-4376 (2017) Article DOI: 10.1016/j.bmcl.2017.08.022 BindingDB Entry DOI: 10.7270/Q2RF5XG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM120128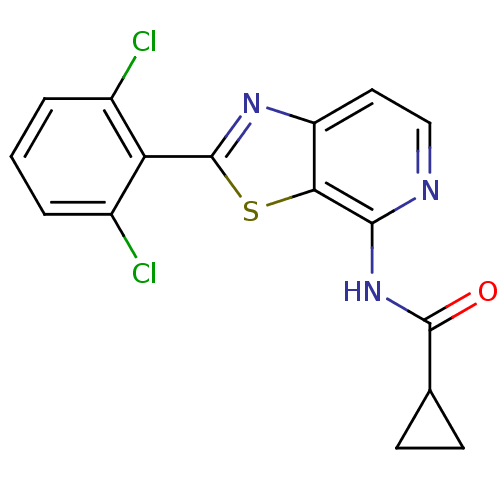 (US8697708, 2) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of TYK2 (unknown origin) assessed as reduction in IL-23 induced STAT3 phosphorylation by cell based ELISA | Bioorg Med Chem Lett 27: 4370-4376 (2017) Article DOI: 10.1016/j.bmcl.2017.08.022 BindingDB Entry DOI: 10.7270/Q2RF5XG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxycytidine kinase (Homo sapiens (Human)) | BDBM50440176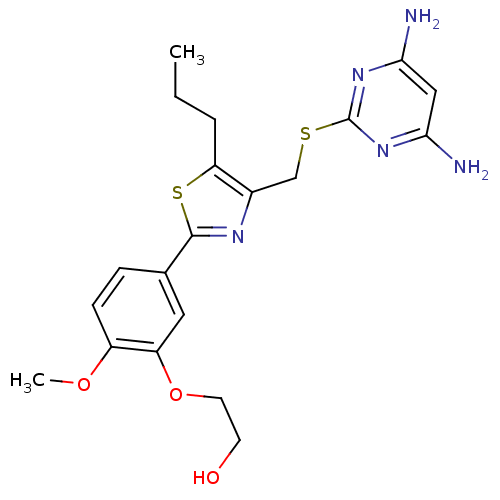 (CHEMBL2426570) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Apparent inhibition of human dCK by steady-state kinetic assay | J Med Chem 57: 9480-94 (2014) Article DOI: 10.1021/jm501124j BindingDB Entry DOI: 10.7270/Q29025DM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Deoxycytidine kinase (Homo sapiens (Human)) | BDBM50440172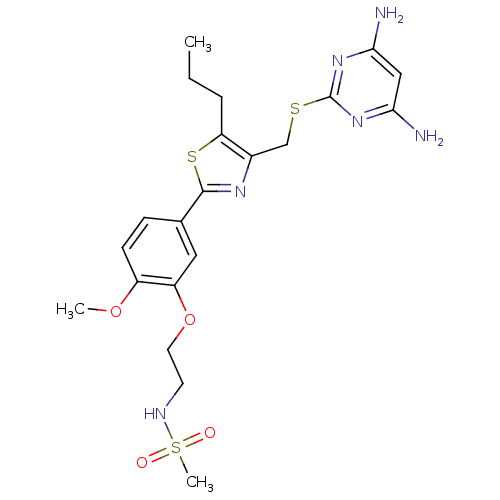 (CHEMBL2426574) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Apparent inhibition of human dCK by steady-state kinetic assay | J Med Chem 57: 9480-94 (2014) Article DOI: 10.1021/jm501124j BindingDB Entry DOI: 10.7270/Q29025DM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50261256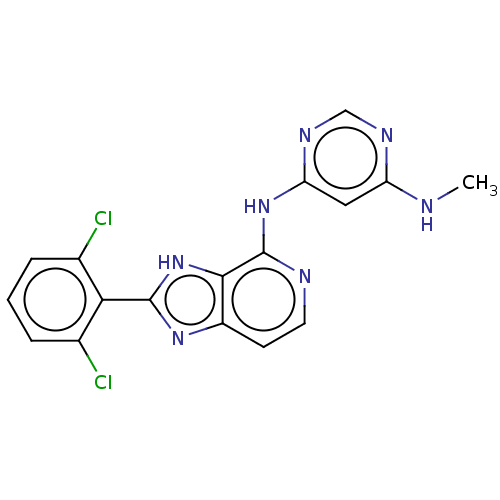 (CHEMBL4071399) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of TYK2 (unknown origin) by biochemical assay | Bioorg Med Chem Lett 27: 4370-4376 (2017) Article DOI: 10.1016/j.bmcl.2017.08.022 BindingDB Entry DOI: 10.7270/Q2RF5XG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50040253 (3-{2-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-ethyl}-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from Alpha-1A adrenergic receptorof rat submaxillary gland membranes | J Med Chem 41: 3128-41 (1998) Article DOI: 10.1021/jm970159v BindingDB Entry DOI: 10.7270/Q2GM880H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230847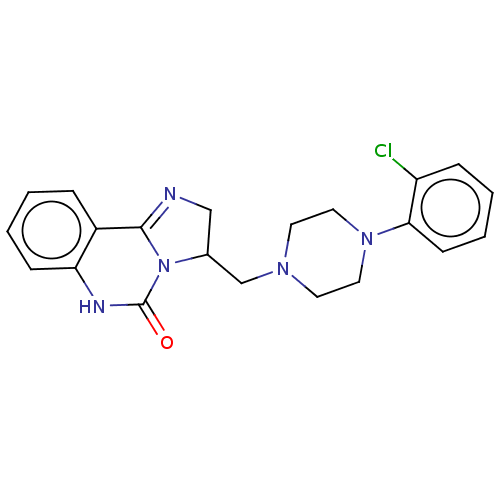 (CHEMBL2114069) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50261254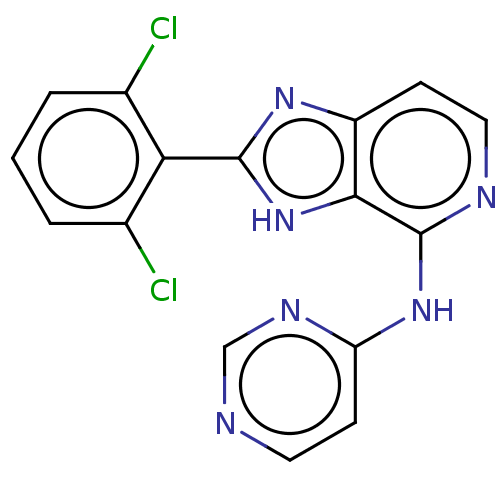 (CHEMBL4074130) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of TYK2 (unknown origin) assessed as reduction in IL-23 induced STAT3 phosphorylation by cell based ELISA | Bioorg Med Chem Lett 27: 4370-4376 (2017) Article DOI: 10.1016/j.bmcl.2017.08.022 BindingDB Entry DOI: 10.7270/Q2RF5XG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM36608 (Rapamycin C-7, analog 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals | Assay Description FKBP12 assay using rapamycin analogs. | Chem Biol 2: 471-81 (1995) Article DOI: 10.1016/1074-5521(95)90264-3 BindingDB Entry DOI: 10.7270/Q2CZ35J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50261276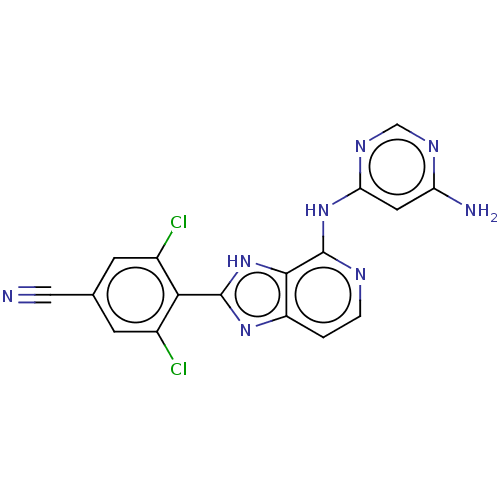 (CHEMBL4092116) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of TYK2 (unknown origin) by biochemical assay | Bioorg Med Chem Lett 27: 4370-4376 (2017) Article DOI: 10.1016/j.bmcl.2017.08.022 BindingDB Entry DOI: 10.7270/Q2RF5XG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50261274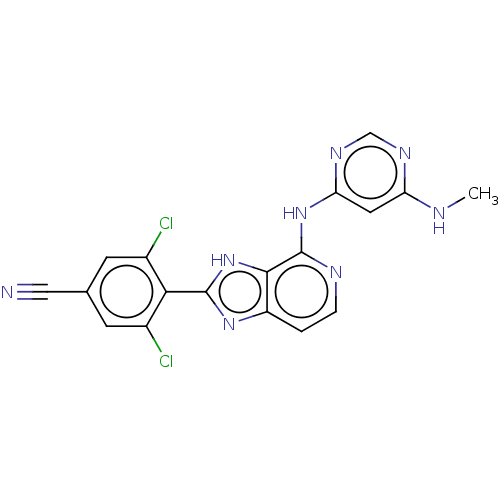 (CHEMBL4062680) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of TYK2 (unknown origin) by biochemical assay | Bioorg Med Chem Lett 27: 4370-4376 (2017) Article DOI: 10.1016/j.bmcl.2017.08.022 BindingDB Entry DOI: 10.7270/Q2RF5XG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50261260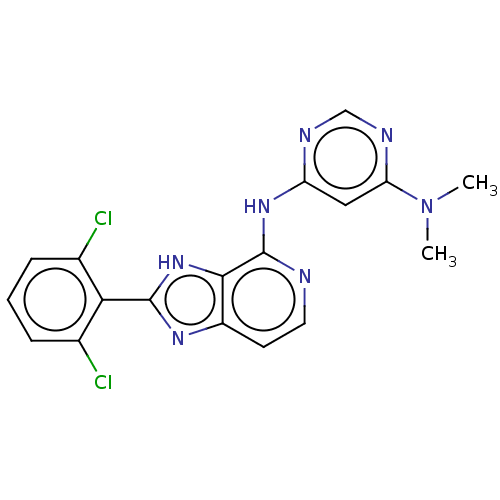 (CHEMBL4069942) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of TYK2 (unknown origin) assessed as reduction in IL-23 induced STAT3 phosphorylation by cell based ELISA | Bioorg Med Chem Lett 27: 4370-4376 (2017) Article DOI: 10.1016/j.bmcl.2017.08.022 BindingDB Entry DOI: 10.7270/Q2RF5XG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50261270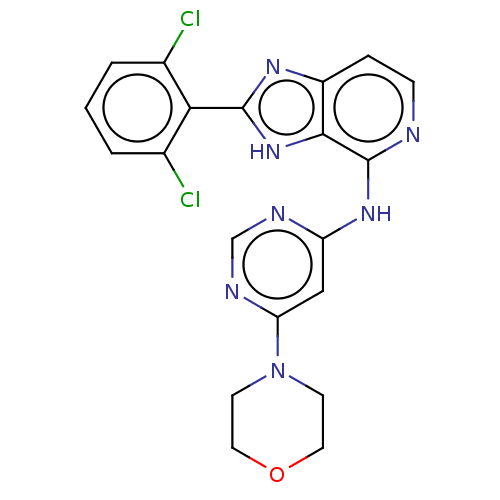 (CHEMBL4093872) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of TYK2 (unknown origin) by biochemical assay | Bioorg Med Chem Lett 27: 4370-4376 (2017) Article DOI: 10.1016/j.bmcl.2017.08.022 BindingDB Entry DOI: 10.7270/Q2RF5XG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50040260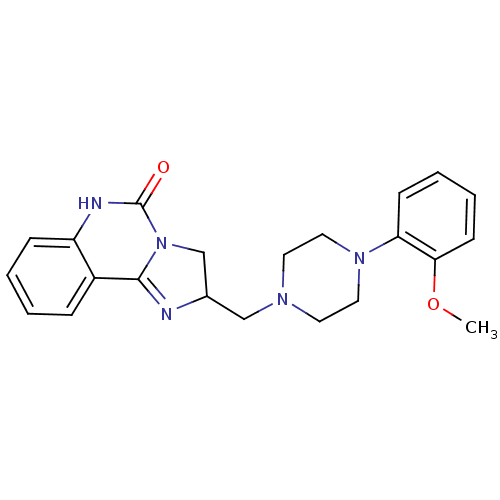 (2-[4-(2-Methoxy-phenyl)-piperazin-1-ylmethyl]-2,6-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from Alpha-1A adrenergic receptorof rat submaxillary gland membranes | J Med Chem 41: 3128-41 (1998) Article DOI: 10.1021/jm970159v BindingDB Entry DOI: 10.7270/Q2GM880H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Rattus norvegicus (rat)) | BDBM50066109 (3-[4-(2-Methoxy-phenyl)-piperazin-1-ylmethyl]-2,6-...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from Alpha-1B adrenergic receptor of rat liver membrane | J Med Chem 41: 3128-41 (1998) Article DOI: 10.1021/jm970159v BindingDB Entry DOI: 10.7270/Q2GM880H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50230851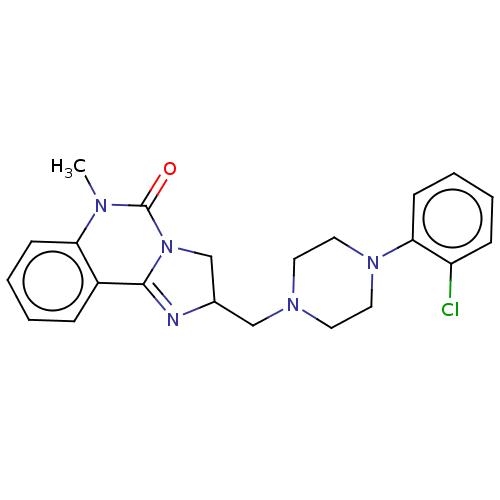 (CHEMBL70347) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor by measuring displacement of [3H]prazosin from rat brain cortex membranes (in vitro). | J Med Chem 36: 2196-207 (1993) Article DOI: 10.1021/jm00067a017 BindingDB Entry DOI: 10.7270/Q298896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50261269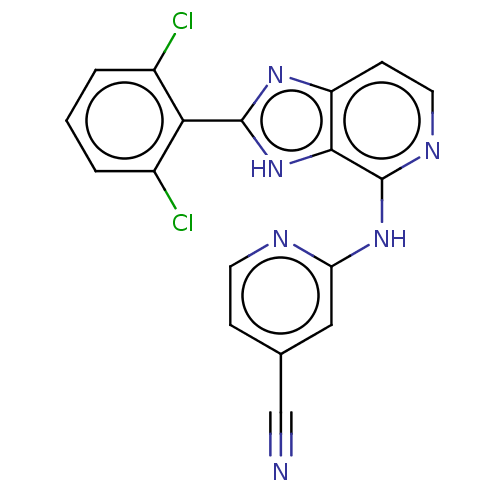 (CHEMBL4092191) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of TYK2 (unknown origin) by biochemical assay | Bioorg Med Chem Lett 27: 4370-4376 (2017) Article DOI: 10.1016/j.bmcl.2017.08.022 BindingDB Entry DOI: 10.7270/Q2RF5XG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50261257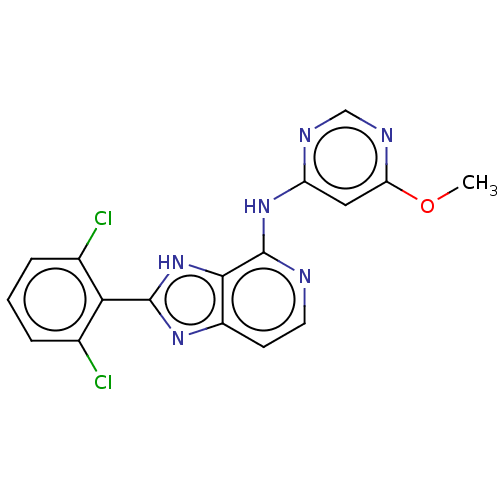 (CHEMBL4099854) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of TYK2 (unknown origin) by biochemical assay | Bioorg Med Chem Lett 27: 4370-4376 (2017) Article DOI: 10.1016/j.bmcl.2017.08.022 BindingDB Entry DOI: 10.7270/Q2RF5XG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 9820 total ) | Next | Last >> |